Review Article
Predicting Perioperative Fluid Responsiveness in Pediatric Patients; how it Differ from the Adults?
Waleed Elmatite1 and Surjya Upadhyay2*
1John R Oishei Children’s Hospital Buffalo, New York, USA
2NMC Hospital DIP, Dubai
*Address for Correspondence: Surjya Upadhyay, NMC hospital DIP, Dubai investment park, Dubai, UAE, Po box 7832, Tel: +000-971-554-078-445; E-mail: run77in@yahoo.com/surjya.upadhyay@nmc.ae
Dates: Submitted: 04 June 2018; Approved: 29 June 2018; Published: 04 July 2018
Citation this article: Elmatite W, Upadhyay S. Predicting Perioperative Fluid Responsiveness in Pediatric Patients; how it Differ from the Adults? Am J Anesth Clin Res. 2018;4(1): 008-014.
Copyright: © 2018 Upadhyay S., et al This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Fluid responsiveness; Pediatric surgery; Dynamic parameters; Static parameters
Abstract
Accurate Prediction of fluid responsiveness can be challenging, particularly in children. Although, fluid administration is the main stay of resuscitation during pediatric surgery associated with significant blood or third space loss, volume overload is frequently encountered in small children and is often associated with adverse outcomes. The parameters used to assess fluid responsive are basically classified into two categories, static and dynamic parameters. Static parameters like central venous pressure measurement are slowly becoming unpopular and are replaced by dynamic parameters which rely on the heart-lung interactions are more accurate predictors of fluid responsiveness. Unlike adults, there are insufficient data on the efficacy of dynamic variables for the prediction of fluid responsiveness in children. In this review article we discuss the strengths and limitations of both the static and dynamic parameters for assessing the fluid responsiveness in the perioperative period in pediatric patients.
Introduction
Intravenous fluid therapy is an essential part of intraoperative anesthetic and perioperative surgical care. However, inappropriate fluid therapy can have significant perioperative outcomes [1-5]. The goals of perioperative fluid management are to restore and maintain blood volume and cardiac output to provide adequate perfusion to pertinent tissues and prevent hypoperfusion and tissue injury. On the other hand hypervolemia due to excess iatrogenic fluid loading has been associated with increased length of hospital stay, significant morbidity and mortality [6-7]. Predicting fluid responsiveness can be challenging, particularly in children especially when there is ongoing blood loss where estimation of blood loss and third space loss may be difficult. Clinical assessment of hemodynamic status based on physical examination and routine monitoring is inadequate, particularly in small children. These finding underline the need for more reliable predictors of fluid responsiveness [8-10].
Numerous hemodynamic variables have been proposed as predictors of fluid responsiveness. Static variables are based on observation against static time. This includes clinical observations such as heart rate and arterial blood pressure, preload pressures such as Central Venous Pressure (CVP) and pulmonary artery occlusion pressure. Dynamic indices of preload, which reflect heart-lung interaction through ventilation-induced cyclic changes in left ventricular stroke volume over a period of time. In this review article we discuss the reliability of these parameters in perioperative setting to detect fluid responsiveness in pediatric patients.
Static variables
Clinical parameters (Systolic Blood Pressure (SBP) and Heart Rate(HR))
SBP and HR under anesthesia are affected by confounding factors like depth of anesthesia or surgical simulations, so they are not very sensitive indicators for volume status. Studies in paediatric patients during period using the heart rate and SBP variability as a clinical predictor of volume status was not significant (P > 0.5) in most of the studies [11-13].
Central venous and pulmonary arterial wedge pressure: Numerical values of Central Venous Pressure (CVP) and values of Pulmonary Artery Wedge Pressure (PAWP), do not accurately measure the circulating blood volume or responsiveness to fluid challenge (an increase in Cardiac Output, reflected by predictable change in CVP after a bolus of fluid). A sophisticated approach of measuring separately total blood volume and circulating blood volume, using a dye dilution or thermodilution technique, also did not demonstrate consistent correlation between values of CVP and blood volume, either total or circulating blood volume [14,15]. In addition, these invasive techniques are associated with complication during insertion such as pneumothorax or hematoma formation [16].
Echocardiography and Doppler assessment of ventricular function: Use of Left Ventricular End Diastolic Area (LVEDA) and aortic Velocity-Time Integral Aortic Output (VTI -AO) (figure 1) as measured by trans esophageal or transthoracic Echocardiographic measurement was looking promising initially, but on meticulous re-evaluation, it was found not that much useful tool to predict fluid responsiveness [11]; further, Echocardiography need special expertise and training for the operator and it may not be applicable in some surgical procedure limiting its usefulness as routine practice. Raux et al. [17] have shown Trans-Esophageal Doppler (TED)-derived parameters that included Stroke Volume Index (SVI); Corrected Flow Time (CFT); Peak Velocity (PV) during volatile anesthesia was useful to predict and follow Volume Expansion (VE) responsiveness in neonates and infants [17,18]. Trans esophageal Doppler has the advantage of being simple and relatively non-invasive technique unlike echocardiography, but, TED was not useful to detect FR (Fluid Responsiveness) in cardiac patients [19,20].
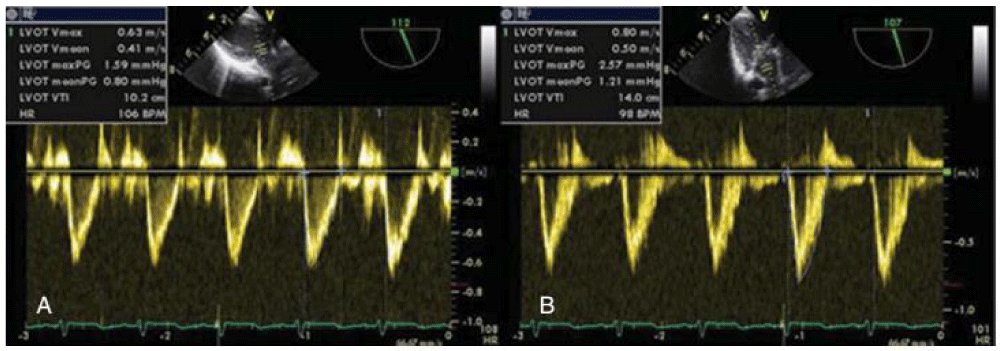 Figure 1: Measurement of Doppler velocity time integral in the LVOT (A) and in the ascending aorta (B) [44].
Figure 1: Measurement of Doppler velocity time integral in the LVOT (A) and in the ascending aorta (B) [44].
Thermodilution and Ultrasound Dilution technique: Saxena et al. [18] investigated the static variables (total end diastolic volume, active circulation volume, central blood volume, and total ejection fraction derived from trans pulmonary ultrasound dilution method (ultrasound transit time-based measurement of pulmonary blood flow) as predictors of FR defined by increase in SV by 15% [18]. The result of the study was that none of these markers discriminated adequately for volume responsiveness and all of them were comparable to CVP (AUC 0.41, 95% CI 0.26-0.55) [18].
Global End Diastolic Volume Index (GEDVI) measured by thermodilution failed to predict fluid responsive in patient with left to right shunt (VSD /ASA) (AUC: 0.59 P = 0.42) but its predictive value improved after repair of shunt (AUC: 0.77 P = 0.008 Cutoff: ≤ 400 ml) [20].
Laboratory based parameters
Hematocrit dilution: Hematocrit dilution is often based on changes in measured values from the baseline value and can only assess changes in the circulating part of the blood volume, ignoring a considerable non circulating portion of the plasma [21-23] but these changes take place over a certain period and do not immediately reflect acute changes in volume status. In addition, there is possibility of lag between the timing of result and timing of the blood sampling.
Urine Output (UO): Oliguria (UO < 0.5mL/kg per hour) is a commonly used general indicator of hypovolemia. However, perioperative oliguria alone is not a sufficient indication for fluid administration. Perioperative medication and anaesthetic drugs and techniques as well as surgical technique and manipulation may reduce UO. Administration of fluid to treat oliguria in a euvolemic state may lead to fluid overload [24,25].
Mixed venous (SVO2) and Central venous oxygen saturation (ScVO2): SVO2 and ScVO2 value can be obtained periodically from pulmonary artery catheter or central venous catheter through blood gas analysis or continuously using a fiber-optic catheter. Although SvO2 and ScVO2 are proportional to cardiac output, tissue perfusion, and tissue O2 delivery, these measurements are also inversely proportional to tissue O2 consumption. However, these changes may not reflect accurate changes in tissue perfusion during the perioperative period when regional perfusion and O2 consumption may vary [26,27].
Base Deficit (BD) and serum lactate: Lactic acidosis on sequential arterial blood gases can be a surrogate marker of reduced global tissue perfusion. However, these laboratory values do not provide information regarding contemporaneous clinical intravascular volume status since they are measured intermittently and do not immediately reflect acute changes. In addition; the BD can be caused by intravenous infusion predominately normal saline. Moreover, base deficit as a surrogate for serum lactate can be confounded by renal dysfunction and ketoacidosis [28].
Dynamic variables
Dynamic indices of circulating fluid volume based on ventilation-induced (cardiopulmonary interaction) cyclic changes in Left Ventricular (LV) stroke volume have been shown to reliably predict the FR in adults [29-32]. During positive pressure ventilation; on inspiration - increase in intrathoracic pressure- reduces venous return and thereby reducing right ventricular stroke volume which is proportional to the degree of hypovolemia and is transmitted to the left heart after two or three beats (pulmonary vascular transit time [31,33]. These variables can be calculated through the direct arterial pressure trace.
Variable derived from the arterial pressure: The variable derived from the arterial pressure are SPV (Systolic Arterial Pressure Variation) and PPV (Pulse Pressure Variation) (Figure (2).
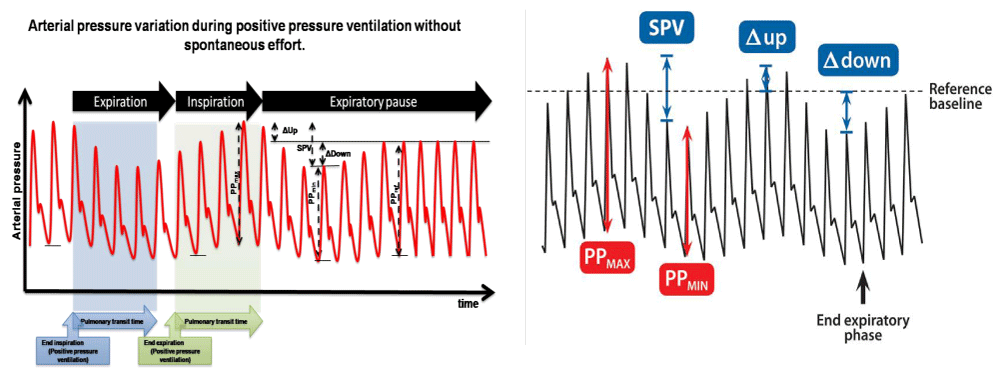 Figure 2: Arterial pressure variation, showing Systolic Pressure Variation, Pulse Pressure (PP) maximum and minimum.
Figure 2: Arterial pressure variation, showing Systolic Pressure Variation, Pulse Pressure (PP) maximum and minimum.
SPV is calculated using he maximal and minimal SAP (Systolic Arterial Pressure Variation) values measured in a single respiratory cycle.
SPV (%) = (SAP max - SPB min)/ [(SAP max + SAP min)/2] × 100 [34].
PPV was defined as the difference between Pulse Pressure (systolic minus diastolic pressure) Maximal (PP max) and minimal (PP min) values over mean pulse pressure were determined over the same respiratory cycle.
PPV = PP max-PP min/ PP mean
PPV (%) =100× (PP max - PP min)/ [(PP max +PP min)/2]]
PV is also calculated with PP using an analogous formula. An average is taken either over 3 consecutive down =SP ref- SP min, and up = SP max- SP ref. where is (SP ref) reference systolic pressure at the end expiratory pause (SP ref) at the end-expiratory pause [34,35].
In contrast to the strong evidence of predictive value of this variable in adult, the evidence in children is poor as almost all studies revealed that these dynamic parameters are not significant predictors of fluid responsiveness in children. There are several possible explanations for the difference in the predictive value of PPV between children and adults. Arterial elastance in children is low (compliance is high), therefore, pressures transmitted by an increase in stroke volume may be partially absorbed, and pressure variations caused by an increase in stroke volume may be absent from the arterial pressure waveform [35,36].
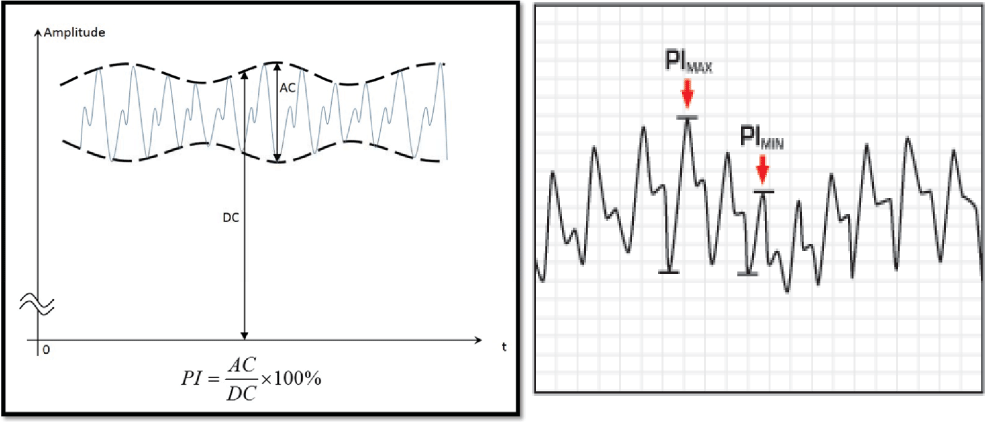
Variables derived of Plethysmography: Two variables derived from Plethysmography ∆POP = Pulse Oximeter Plethysmography amplitude variation and PVI = Plethysmography Variability Index.
Red and infrared light are used by a pulse oximeter to measure oxygen saturation. A constant amount of light (DC) from the pulse oximeter is absorbed by skin, other tissues, and non-pulsatile blood, whereas a variable amount (AC) is absorbed by pulsatile arterial inflow. The PI (Perfusion Index) is calculated from the absorptions of red and infrared light, using: PI (%) = (AC/DC) ×100. AC and DC signals are extracted from the amplitude of the plethysmographic waveform. The dynamic change in perfusion index PI during a respiratory cycle results in the PVI, which was automatically calculated using: PVI =100 × (PI max-PI min)/PI max. PVI reflects respiratory variations in PI (Figure 3) [35]. The plethysmographic waveform amplitude is measured beat to beat as the vertical distance between peak and preceding valley in the output waveform. ∆POP is then calculated using a formula analogue to that of SPV and PPV. Similar to the calculation of PPV, an average is usually taken over 3 consecutive respiratory cycles [36,37]. There is no evidence ∆POP is predictor of fluid responsive in children but for PVI the results of the studies are variable [12,16,34,39].
Flow Based Variables
These variables include respiratory variation in aortic blood flow peak velocity (∆Peak), the aortic velocity, Time Integral (∆VTI) and Inferior Vena Cava Diameter Variation (∆IVCD). Unlike pressure based variable and Plethysmography based variable, flow based variable are not affected by high arterial compliance in children. ∆V peak is measured either transthoracic or Trans esophageal echo by Doppler at LVOT or ascending aorta. (Figure 1).
∆V peak = (V peak max-V peak min)/ [(V peak max + V peak min)/2],
Where V is peak max and V peak min are the maximum and minimum aortic flow peak velocities over a single respiratory cycle, respectively.
∆V peak has the strongest evidence as a predictor VR among all other dynamic and static variable. As the Area under the Curve (AUC) or ROC for all studies ranges from 0.8 to 1.00. [11,13,20]. However, only one study found ∆IVCD as reliable predictor of FR in pediatric intensive care unit PICU, [40] but other subsequent study failed to usefulness of vana caval diameter (IVCD) change as predictor of FR [13]. IVCD was measured from the subcostal view (2 cm from the right atrium) using M-mode. The maximum and minimum IVCD (IVCD max and IVCD min) over a single respiratory cycle are recorded. DIVCD was calculated as DIVCD (%) = 100 × (IVCD max + IVCD min) / [(IVCD max + IVCD min)/2] [13]. These technique have limitations that skilled operator is required for accurate measurements and interpretation.
Passive Leg Raising (PLR)
PLR induced changes in cardiac output reliably predicted the fluid responsiveness regardless of ventilation mode, underlying cardiac rhythm, and technique of measurement. PLR-induced changes in pulse pressure variation could be a viable alternative, but in children it has a lower predictive ability as Children have a lower leg-length to body-length ratio than adults, so the effect of PLR may be smaller [42]. Moreover, PLR may be not convenient or practical in Operating Room due to possibility of interruption of surgery. Lukito et al. [43] used PLR test to predict fluid responsiveness in pediatric intensive care unit patients and to get a cutoff value of cardiac index in predicting fluid responsiveness in pediatric patients. The study has shown that Cardiac Index (CI) increase by ≥ 10% induced by passive leg raising predicted preload-dependent status with sensitivity of 55% and specificity of 85% (area under the curve 0.71 ± 0.084, 95% confidence interval 0.546-0.874 [43].
Limitations of Dynamic Parameters in Paediatric Age Group
Unlike Adult, there are several factors that may play important role in paediatric population because of which many variables may not accurately reflect the FR in paediatric age group [44]. Dynamic parameters are more accurate in adults compared to static ones. There are many different types of dynamic parameters based cardio-pulmonary interaction, these dynamic variables are
1. Arterial pressure related variable
2. Plethysmography derived variable
3. Left ventricular outflow - aortic flow dependent variable
4. Passive leg raising - based on clinical parameters.
Arterial pressure trace is dependent on stroke volume and arterial elastance, as the arterial elastance is different in paediatric age group, arterial pressure trace and pulse wave velocity may not reflect like in adults. The pulse wave velocity increase non-linearly with age in arteries of arms and legs [45]. The non-linearity is due to change in both arterial wall thickness and change in elastance. Because of these changes may explained why pulse pressure and PPV may not predict fluid responsiveness in children between aged 6-14 years of age [45]. The basic of dynamic parameters is heart -lung interaction or Cardio-pulmonary interaction, which paediatric age group may be different than adults, lung compliance and chest wall rigidity, airway resistance, respiratory rate and tidal volume which may have difference impacts on the preload and after load unlike adults. Recently, it was reported that the significance of dynamic parameters in a ventilated may be influenced not only by tidal volume, but also by other ventilator settings. Kang et al. [46] observed significant difference in Stroke Volume Variation (SVV) in same patient with same volume status with different level of Peak Inspiratory Pressure (PIP) [46].
Obtaining accurate Pulse oxymetry trace may be challenging in small pediatric patients and is influenced by many factors like regional blood flow, temperature, electro-cautery, use of inotropes etc. Plethysmography derived parameters may not be as useful as PPV or SPV [47].
Unlike pressure based variable and Plethysmography based variable, flow based variable are not affected by high arterial compliance in children. ∆V peak is measured either transthoracic or Trans esophageal echo by Doppler at LVOT or ascending aorta, this may not be practical or feasible to obtained the image during intraoperative period. Moreover, use of echocardiography require special experience and training making its usefulness limited to few center with expertise in using it.
Passive Leg Raising (PLR) maneuver is a dynamic test to detect fluid responsiveness based on shifting of significant amount of blood to central compartment and thereby reflected by changes in cardiac output. This maneuver is not very popular because the blood volume shift with this maneuver may be small and variable depending on the ratio between leg and thorax, size and age of the patient. Other alternative dynamic tests such as end-expiratory occlusion may be of interest in this setting, which require further testing [48].
Authors Preferred Techniques to Detect Volume Responsive in Pediatric Surgery
Clearly, dynamic parameters are superior over the static variable to assess the fluid status and fluid responsiveness. Among the dynamic parameters, apart from flow variables which require special skills and special facilities, there is no evidence that any pressure variable dynamic parameters are reliable predictor of volume responsive if each used alone. Instead of relying on single variable, use of multiple variable significantly improved the sensitivity and specificity. Clinical assessment of intravascular volume begins with knowledge of age-related norms for heart rate and BP and then answer the following questions: Is the heart rate persistently increased, or does it vary with surgical stimulation? Is the pulse pressure narrow, or more ominously, is the BP reduced for age? Does it vary with positive-pressure breaths? Are there SAP (Systolic Arterial Pressure variation or ∆POP (Pulse Oximeter Plethysmography amplitude variation)? Are the extremities warm? Is capillary refill brisk? What is the urine output? How low CVP? How low the hematocrit and hemoglobin level? Are these variables changing? What is the rate of the change? Is there evidence of blood loss in the surgical field? What is the result of blood gases? When hypovolemia is suspected, observing the response to a 10 to 20 mLkg bolus of isotonic crystalloid or colloid may test the hypothesis of hypovolemia and re check the change in this parameters.
Conclusion
Dynamic parameters are definitely better predictor of FR over static variables. So far, we don’t have a single predictor to have highly sensitive and specific for fluid responsiveness. Instead of relying on single parameters, combing these multiple parameter along with clinical scenario may help to improve their sensitivity and specificity.
References
- Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, LindorffLarsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens. Ann Surg. 2003; 238: 641-648. https://goo.gl/LcNZwj
- Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002; 89: 622-632. https://goo.gl/wECZPB
- Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005; 103: 25-32. https://goo.gl/Q1afpL
- Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D,
DeBoisblanc B, et al. Comparison of two fluid-management strategies in
acute lung injury. N Engl J Med. 2006; 354: 2564-2575. https://goo.gl/ELz6wK - Jacob M, Chappell D, Rehm M. Clinical update: perioperative fluid
management. Lancet. 2007; 369: 1984-1986. https://goo.gl/WRX7MA - Brandstrup B. Fluid therapy for the surgical patient. Best Pract Res Clin Anaesthesiol. 2006; 20: 265-283. https://goo.gl/SzLrF 9
- Lowell JA, Schifferdecker C, Driscoll DF, Benotti PN, Bistrian BR. Postoperative fluid overload: not a benign problem. Crit Care Med. 1990; 18: 728. https://goo.gl/U5EnpT
- Tibby SM, Hatherill M, Marsh MJ, Murdoch IA. Clinicians abilities to estimate cardiac index in ventilated children and infants. Arch Dis Child. 1997; 77: 516518. https://goo.gl/K2LbqX
- Egan JR, Festa M, Cole AD, Nunn GR, Gillis J, Winlaw DS. Clinical assessment of cardiac performance in infants and children following cardiac surgery. Intensive Care Med. 2005; 31: 568-573. https://goo.gl/X5apXo
- Vincent JL, Weil MH. Fluid challenge revisited. Crit Care Med. 2006; 34: 1333-1337. https://goo.gl/reycWf
- Pereira de Souza Neto E, Grousson S, Duflo F, Ducreux C, JolyH, Convert J, et al. Predicting fluid responsiveness in mechanically ventilated children under general anaesthesia using dynamic parameters and transthoracic echocardiography. Br J Anaesth. 2011; 106: 856-864. https://goo.gl/g2EBPr
- Renner J, Broch O, Gruenewald M, Scheewe J, FrancksenH, Jung O, et al. Non-invasive prediction of fluid responsiveness in infants using pleth variability index. Anaesthesia. 2011; 66: 582-589. https://goo.gl/MTAhtw
- Byon HJ, Lim CW, Lee JH, Park YH, Kim HS, et al. Prediction of fluid responsiveness in mechanically ventilated children undergoing neurosurgery. Br J Anaesth. 2013; 110: 586-591. https://goo.gl/HZnXk5
- Oohashi S, Endoh H. Does central venous pressure or pulmonary capillary wedge pressure reflect the status of circulating blood volume in patients after extended transthoracic esophagectomy. J Anesth. 2005; 19: 21-25. https://goo.gl/o1JqM8
- Wiesenack C, Flegl C, Keyser A, Prasser C, Keyl C. Assessment of fluid responsiveness in mechanically ventilated cardiac surgical patients. Eur J Anaesth. 2005; 22: 658-665. https://goo.gl/wHW6B7
- Kornbau C, Lee KC, Hughes GD, Firstenberg MS. Central line complications. International Journal of Critical Illness and Injury Science. 2015; 5: 170-178. https://goo.gl/QY6zr4
- Raux O, Spencer A, Fesseau R, Mercier G, Rochette A, Bringuier S, et al. Intraoperative use of transoesophageal Doppler to predict response to volume expansion in infants and neonates. Br J Anaesth. 2012; 108: 100-107. https://goo.gl/KHfqv5
- Tibby SM, Hatherill M, Durward A, Murdoch IA. Are transoesophageal Doppler parameters a reliable guide to paediatric haemodynamic status and fluid management. Intensive Care Med. 2001; 27: 201-205. https://goo.gl/oTbaao
- Saxena R, Durward A, Puppala NK, Murdoch I, Tibby S. A comparison between novel static and dynamic markers of fluid responsiveness: preliminary data from 47 children. Proceeding of the 22nd Annual Congress of the ESPNIC. 2011; 37: 315-442
- Renner J, Broch O, Duetschke P, Scheewe J, Hocker J, MosebyM, et al. Prediction of fluid responsiveness in infantsand neonates undergoing congenital heart surgery. Br J Anaesth. 2012; 108: 108-115. https://goo.gl/fYr5Db
- Rehm M, Haller M, Orth V, Kreimeier U, Jacob M, Dressel H, et al. Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology. 2001; 95: 849-856. https://goo.gl/sAU64S
- Norberg A, Hahn RG, Li H, Olsson J, Prough DS, Borsheim E, et al. Population volume kinetics predicts retention of 0.9% saline infused in awake and isoflurane-anesthetized volunteers. Anesthesiology. 2007; 107: 24-32. https://goo.gl/HfcFji
- Norberg, A Hahn, RG Li, H Olsson, J Prough, DS Borsheim, E Wolf, S Minton, RK Svensen, CH Sjostrand F, Hahn RG. Volume kinetics of glucose 2.5% solution during laparoscopic cholecystectomy. Br J Anaesth. 2004; 92: 485-925.
- Brauer KI, Svensen C, Hahn RG, Traber LD, Prough DS. Volume kinetic analysis of the distribution of 0.9% saline in conscious versus isoflurane-anesthetized sheep. Anesthesiology. 2002; 96: 44. https://goo.gl/1hLnYy
- Connolly CM, Kramer GC, Hahn RG, Chaisson NF, Svensen CH, Kirschner RA, et al. Isoflurane but not mechanical ventilation promotes extravascular fluid accumulation during crystalloid volume loading. Anesthesiology. 2003; 98: 670-681. https://goo.gl/dsKzYr
- Renner J, Scholz J, Bein B. Monitoring fluid therapy. Best Pract Res Clin Anaesthesiol. 2009; 23: 159-171. https://goo.gl/eFBN1w
- Knotzer H, Hasibeder WR. Microcirculatory function monitoring at the bedside-a view from the intensive care. Physiol Meas. 2007; 28: 65-86. https://goo.gl/ED22JF
- Lakhmir S Chawla, Amirali Nader, Todd Nelson, Trusha Govindji, Ryan Wilson, Sonia Szlyk, et al. Utilization of base deficit and reliability of base deficit as a surrogate for serum lactate in the peri-operative setting. BMC Anesthesiology. 2010; 10: 16. https://goo.gl/VbhQv4
- Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002; 121: 2000-2008. https://goo.gl/RGUHqW
- Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009; 37: 2642-2647. https://goo.gl/oTjrUQ
- Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005; 103: 419-428. https://goo.gl/w8H3DA
- Hofer CK, Muller SM, Furrer L, Klaghofer R, Genoni M, Zollinger A. Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest. 2005; 128: 848-854. https://goo.gl/XrSTj8
- Cavallaro F, Sandroni C, Antonelli M. Functional hemodynamicmonitoring and dynamic indices of fluid responsiveness. Minerva Anestesiol. 2008; 74: 123-135. https://goo.gl/Y5pyBu
- Byon HJ, Lim CW, Lee JH, Park YH, Kim HS, Kim CS, Kim JT. Prediction of fluid responsiveness in mechanically ventilated children undergoing neurosurgery. Br J Anaesth. 2013; 110: 586-591. https://goo.gl/B7DTXa
- Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000; 162: 134-138. https://goo.gl/4Qgs1V
- Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P,et al. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol. 1998; 274: 500-505. https://goo.gl/7FG2QV
- Cannesson M, Besnard C, Durand PG, Bohe J, Jacques D. Relation between respiratory variations in pulse oximetry plethysmographic waveform amplitude and arterial pulse pressure in ventilated patients. Crit Care. 2005; 9: 562-568. https://goo.gl/uMrzjd
- Cannesson M, Attof Y, Rosamel P, Desebbe O, Joseph P,Metton O, et al. Respiratory variations in pulseoximetry plethysmographic waveform amplitude to predictfluid responsiveness in the operating room. Anesthesiology. 2007; 106: 1105-1111. https://goo.gl/ULQSc3
- Chandler JR, Cooke E, Hosking M, Froese N, Karlen W, Ansermino JM. Volume responsiveness in children, a comparison of static and dynamic variables. Proceedings of the IARS 2011 Annual Meeting. 2011; 200.
- Choi DY, Kwak HJ, Park HY, Kim YB, Choi CH, Lee JY. Respiratory variation in aortic blood flow velocity as a predictor of fluid responsiveness in children after repair of ventricular septal defect. Pediatr Cardiol.2010; 31: 1166-1170. https://goo.gl/Cs7NBn
- Cavallaro F, Sandroni C, Marano C, La Torre G, Mannocci A, DeWaure C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010; 36: 1475-1478. https://goo.gl/yiwLkr
- Fredriks AM, van Buuren S, van Heel WJ, Dijkman-Neerincx RH, Verloove-Vanhorick SP, Wit JM. Nationwide age references for sitting height, leg length, and sitting height/height ratio, and their diagnostic value for disproportionate growth disorders. Arch Dis Child. 2005; 90: 807-812. https://goo.gl/6fKvhU
- Lukito V, Djer MM, Pudjiadi AH, Munasir Z. The role of passive leg raising to predict fluid responsiveness in pediatric intensive care unit patients. Pediatr Crit Care Med. 2012; 13: 155-160. https://goo.gl/rfqKbX
- Renner J, Broch O, Duetschke P, Scheewe J, Hocker J, Moseby M, et al. Prediction of fluid responsiveness in infants and neonates undergoing congenital heart surgery. Br J Anaesth. 2012; 108: 108-115. https://goo.gl/pd5CL4
- Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF, et al. Effects of aging on changing arterial compliance and left ventricular load in northern Chinese urban community. Circulation. 1983; 68: 50-58. https://goo.gl/7ffTzC
- Kang WS, Kim JY, Woo NS, Yoon TG. The influence of different mechanical ventilator settings of peak inspiratory pressure on stroke volume variation in pediatric cardiac surgery patients. Korean journal of anesthesiology. 2014; 66: 358-363. https://goo.gl/UVoUhy
- Monnet X, Guerin L, Jozwiak M, Bataille A, Julien F, Richard C, et al. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. British Journal of Anaesthesia. 2013; 110: 207-213. https://goo.gl/PqRA4m
- Monnet, X, Osman, D, Ridel, C, Lamia, B, Richard, C, and Teboul, JL. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med. 2009; 37: 951-956. https://goo.gl/hkVYzW
Authors submit all Proposals and manuscripts via Electronic Form!