Research Article
Association of Neuropsychiatric Symptoms with Temporopolar Cortex Atrophy in Cognitive Deficits
Ana M. Insausti, Pedro Abizanda, Leonor Maicas, Beatriz L. Ramos, Francisco Mansilla, Emilio A. Perula* , Maria del Mar Ubero and Ricardo Insausti
Ana M. Insausti1, Pedro Abizanda2, Leonor Maicas2, Beatriz L. Ramos2, Francisco Mansilla3, Emilio A. Perula4*, Maria del Mar Ubero4 and Ricardo Insausti4
1Department of Health, Physical Therapy School, Public University of Navarra, Tudela, Spain
2Department of Geriatrics, University Hospital Complex of Albacete (CHUA), Albacete, Spain
3Department of Radiology, University Hospital Complex of Albacete (CHUA), Albacete, Spain
4Human Neuroanatomy Laboratory, School of Medicine, Department of Health, Sciences and CRIB, University of Castilla-La Mancha, Spain
*Address for Correspondence: Emilio Artacho Perula, Human Neuroanatomy Laboratory, Human Anatomy and Embryology Area, Department of Health Sciences and C.R.I.B, School of Medicine, University of Castilla-La Mancha, C/ Almansa, 14, 02006, Albacete, Spain, Tel: +34967599200 #2960; E-mail: [email protected]
Dates: Submitted: 21 July 2017; Approved: 30 August 2017; Published: 07 September 2017
Citation this article: Insausti AM, Abizanda P, Maicas L, Ramos BL, Mansilla F, et al. Association of Neuropsychiatric Symptoms with Temporopolar Cortex Atrophy in Cognitive Deficits. Int J Brain Disord Ther. 2017;1(1): 006-013.
Copyright: © 2017 Insausti AM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Temporal pole volume; MRI; Atrophy; Neuropsychiatric symptoms; Alzheimer´s disease (AD); Mild cognitive impairment (MCI)
Abstract
Background: The temporopolar cortex is an extensive cortical region strongly interconnected with different cortical and subcortical structures. The medial temporal lobe atrophy, including the temporopolar cortex changes, represents a clear indication of cognitive dysfunction, often present in neurodegenerative and psychiatric illnesses. Thus, neuropsychiatric symptoms are common in Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD) patients, which worsen the quality of life and the burden of caregivers.
Methods: Twenty-three patients with MCI (n = 9) and AD (n = 14), and age and sex matched controls (n = 12) were examined with a neuropsychological evaluation. The MRI parcellation of the temporopolar cortex, in conjunction with the stereological assessment of the temporopolar cortex volume was used for correlation analysis with neuropsychiatric symptoms in patients with MCI and AD.
Results: Temporopolar cortex volume decrease in MCI (-6%), and AD (-11%) relative to control cases. Delusions, agitation, apathy, disinhibition and irritability correlated with smaller temporopolar cortex volumes. The volume of the temporopolar cortex decreases when there are neuropsychiatric symptoms, with statistical significance in psychotic and behavioral symptoms, while affective symptoms are not.
Conclusions: The correlation between neuropsychiatric symptoms and temporopolar cortex volume show an association of neuroanatomical changes and symptoms which have clinical relevance. In addition, the temporopolar cortex volume assessment could be contribute to the differential diagnosis of cognitive deficits in MCI and AD.
Introduction
The Medial Temporal Lobe (MTL) of human and nonhuman primates is made up of different neuroanatomical structures that comprise the hippocampal formation, amygdala, and the surrounding cortical strip (the so-called Parahippocampal Region, PHR).The PHR is made up of Temporopolar Cortex (TPC), Perirhinal Cortex (PRC), and the posterior Parahippocampal Cortex (PPH). In rostrocaudal direction, the PHR is continued by retrosplenial cortex [1]. Research in neuropsychology, clinical neuroscience and neuroimaging has established that human MTL structures play an important role in memory consolidation [2-4] and language [5]. Among the MTL cortices, TPC has been shown to be involved in neurological diseases as Alzheimer´s Disease (AD) [6], Parkinson´s disease [7], schizophrenia [8] and temporal lobe epilepsy [9-11]. The TPC is a cortical region the roughly corresponds to BA 38, although recent studies [12,13] point to a more reduced extension of TPC than represented by Brodmann [14]. The TPC is strongly interconnected with different cortical and subcortical structures such as the amygdala, the orbitofrontal cortex, PRC and PPH of the PHR, the insular, auditory association and rostral inferotemporal cortices, as shown in human and nonhuman primates [11,15-17]. Moreover, experimental studies with damage of the TPC in nonhuman primates have resulted in aKlüver-Bucy syndrome [18]. TPC damage is responsible for socioemotional disorders in humans [19]. Notwithstanding the considerable interest of this region, and the variety of functions in which it is involved, few reports exist, to the best of our knowledge, on the correlation of changes in TPC volume and neuropsychiatric symptoms (NPS) in Mild Cognitive Impairment (MCI) and AD patients [20,21].
NPS are behavioral disturbances, which are common in AD and MCI patients. Studies in different countries and different settings have reported a prevalence between 35-75% in MCI patients and between 49-89% in dementia patients [22-29]. These disturbances are associated with increased mortality [30], lower quality of live, greater institutionalization [30,31], caregiver burden [32] as well as with a greater use of medication and health services. NPS are, in many cases, more troublesome than cognitive deficits. However, the specific etiological factors and pathophysiologic mechanisms involved remain unclear. The association between delusional thoughts in AD patients with higher density of neurofibrillary tangles and neuronal cell loss [33,34] or neurochemical abnormalities in specific cortical and subcortical regions in autopsy brain tissue have been both described [35,36]. Some studies using structural and functional neuroimaging techniques have suggested that focal structural alterations (ventricular enlargement or white matter lesions) may be associated with the presence of psychosis in AD [37-39]. Paranoid delusions in mild stages of AD have been associated with right MTL atrophy in CT scans [20,21]. Patients with moderate to severe AD show some types of delusions, which are associated with greater atrophy in the right anterior temporal regions, while the left side is relatively intact [34]. Atrophy on the right (but not in the left) TPC correlate with changes in appropriate behavior in the temporal variant of frontotemporal dementia [40]. Irritability, apathy and rapid change in mood plus anxiety symptoms have been also described in TPC lesions [11,41,42]. Different authors, using functional neuroimaging techniques, have reported an association between delusional thoughts in AD and altered function in temporal cortical areas [43,44], frontal [45-48] and other cortical regions [45,49].
The purpose of this study is the evaluation of changes in TPC volume in MCI and AD patients with NPS by segmenting the TPC on MR images according to our own previously reported criteria [50], and by estimating differences in the right and left hemispheres to explore the association with specific NPS in cognitive deficits.
Materials and Methods
Subjects and study design
Our study included a total of 35 subjects grouped as: Control (CO), n = 12; MCI, n = 9; and AD, n = 14. All subjects were recruited at the Department of Geriatrics, Hospital of Albacete (Complejo Hospitalario Universitario de Albacete, CHUA); all cases were age-matched, and gave informed consent for their participation in the study. Basic notations including demographic data, medical records, physical and neurological examination was obtained during the baseline visit. In all cases, a complete neuropsychological evaluation, as well as a structural cerebral MR scan were performed. Blood samples were collected for hematology, biochemistry, thyroid hormones, vitamin B12 and folic acid to exclude other causes of dementia. The study was approved by the clinical investigation ethical committee of the CHUA, Albacete, Spain (http://www.chospab.es/investigacion/ceic/intro.htm).
Diagnostic evaluation of all subjects was performed by a geriatrician; the study included a detailed neuropsychological evaluation that incorporated medical records, physical and neurological examinations. The diagnosis of dementia was based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4thed. (DSM-IV) and the diagnosis of AD on the National Institute of Neurologic and Communicative Disorders and Stroke and Alzheimer´s Disease and Related Disorders Associations (NINCDS/ADRDA) criteria. MCI patients were diagnosed using the modified criteria proposed by the Mayo Clinic Alzheimer´s Disease Research Center. The severity of cognitive decline was graded according to the Global Deterioration Scale of Reisberg.
The following battery of tests was used for a comprehensive neuropsychological evaluation of different cognitive domains.
1. Memory: immediate and delayed word list recall subtest from ADAS-cog, Benton test C (application B) and D (application D).
2. Language: verbal and written comprehension and verbal expression subtests from the Barcelona test.
3. Attention and executive function: verbal fluency test, Trail Making Test, part A, direct and inverse digit span, attention and abstraction subtests from Barcelona test.
4. Visuospatial skills: Constructional praxis subtest from Barcelona test.
5. Ideomotor praxis: subtest from Barcelona test.
6. Global functioning: Mini-Mental State Examination, Clock Drawing Test (Schulmann).
7. Depression: Geriatric Depression Scale from Yesavage (GDS).
8. Neuropsychiatric evaluation: Neuropsychiatric Inventory (NPI).
The presence or absence of each NPS were considered as dependent variables, and were also grouped in four domains for analysis:
1. Psychotic symptoms (delusions and hallucinations).
2. Affective symptoms (depression, anxiety and dysphoria).
3. Behavioral symptoms (irritability, agitation and abnormal motor behaviors).
4. Frontal symptoms (apathy and disinhibition).
Measurements
MRI acquisition was performed on all patients under equal conditions using a 1.5T Phillips®Gyroscan Interascanner applying a protocol of three-dimensional magnetization prepared for rapid acquisition with a gradient echo sequence TR/TE 9.7/4 and a 13.5/5.7, matrix 256 x 512, one acquisition (Magnetic Resonance Unit, Radiology Service, CHUA, and Albacete, Spain). The images were re-aligned to correct the undesirable effects for head tilt and rotation. The anterior-posterior commissure line (ac-pc line) was used as neuroanatomical landmark, orthogonal to which the scans were obtained, as 2.0 mm thick, contiguous coronal slices. The intracranial area at the level of the anterior commissure was measured in coronal sections, and used for normalization of the volumetric data to the intracraneal area, as described in Free, et al. [51]. Segmentation criteria of the TPC were described by Insausti, et al. [13,50]. Shortly, the TPC starts at the first coronal section containing temporal pole cortex at the middle cranial fossa, and extends for about 6 mm (first three sections in our study) in all the cross sectional area. Further caudally, the TPC borders dorsally the neocortex of the superior temporal gyrus. The limit lies at the lateral bank of the polar sulcus (or the most lateral one if more than one polar sulcus is present; in some cases no polar sulci are present, in which case the limit corresponds to the midpoint of the dorsal surface of the temporal pole). Ventrally, the transition between the TPC and the inferotemporal cortex takes place at the lateral bank of the superior temporal sulcus. When the inferior temporal sulcus becomes visible, the border is placed at its lateral bank (fusiform gyrus). The posterior limit of TPC is usually placed 2 mm in front of the limen insulae (frontotemporal junction). The presence of the collateral sulcus indicates the ventromedial limit of the TPC.
The TPC volume was assessed through manual tracing of the region of interest by an experienced neuroanatomist (AM.I.). The intra-rater variability was 3.1% (mean error, range 1.3% - 5.8%). Volume estimates were obtained according to the principle of Cavalieri [52] that allows the calculation of the volume of a structure of arbitrary shape and size. The Cavalieri theorem of systematic sampling in combination with point counting is considered a reliable and efficient method for estimating volumes in MRI. The object under study is intercepted by a series of parallel planes at a given distance (t); the cross-sectional area is estimated by counting the number of points that hit the structure under study, thereby the method is unbiased [53]. Point counts are converted into section areas by multiplying the total number of counted points ∑P by the area per test point a/p. Each hemisphere was estimated separately. For each brain, the target parameter was the total volume of TPC est (VTPC) in each hemisphere. Thus, the Cavalieri volume of brain compartments is finally estimated by multiplying the distance between sections t by their total cross-sectional area:
est (VTPC) = t•(a/p)•∑PTPC
A basis scheme for quantifying the TPC is shown in the figure 1 with the rostrocaudal outlining of the region and the superimposition of a system of test points that allows to count the total number of points hitting the TPC.
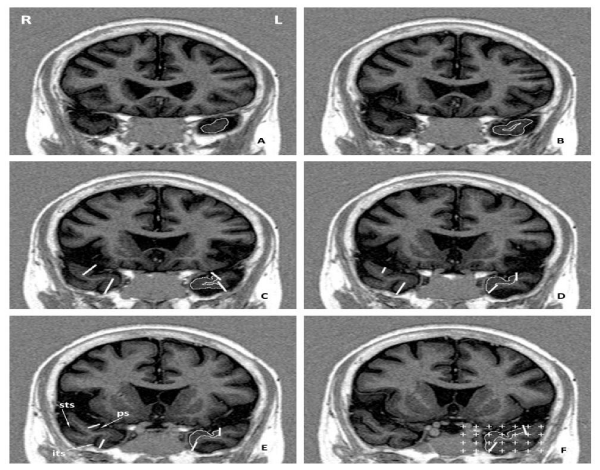 Figure 1: MRI scans of the rostral part of the human temporal lobe ordered from mostrostral (A) to caudal (F) ina representative control case showing the segmentation of the TPC. Note the outline of TPC on the left side.Image in F showsthe last section in which TPC can be discerned, 2mm in front of the limen insulae. Note in F the overlay of the grid used in the stereological quantification of the TPC volume. The location of the Superior Temporal Sulcus (STS), Inferior Temporal Sulcus (ITS) and Polar Sulcus (PS) are indicated in panel (E).
Figure 1: MRI scans of the rostral part of the human temporal lobe ordered from mostrostral (A) to caudal (F) ina representative control case showing the segmentation of the TPC. Note the outline of TPC on the left side.Image in F showsthe last section in which TPC can be discerned, 2mm in front of the limen insulae. Note in F the overlay of the grid used in the stereological quantification of the TPC volume. The location of the Superior Temporal Sulcus (STS), Inferior Temporal Sulcus (ITS) and Polar Sulcus (PS) are indicated in panel (E).
Statistical analysis
All data were analyzed with the SPSS/PC® program (Statistical Package for the Social Sciences, Chicago, IL) v. 19. All the volumetric data were analyzed using normalized values. Differences in TPC volumes in CO, MCI and AD subjects were analyzed with the Kruskal-Wallis test. Spearman correlations were used to find associations between TPC volumes and NPI scores (frequency x severity), while U-Mann Whitney test was used to find group differences in volumes between subjects with or without each of the symptoms included in the NPI, as well as groups of symptoms. Multiple regression analysis was used to describe the association between NPI scores and TPC volumes adjusted by confounding variables as age, sex, amount of years of education, MMSE and cognitive status in MCI or AD patients.
Results
Table 1 shows the data of the patients for the three studied groups (CO, MCI, and AD cases), and normalized TPC volumes are also included. TPC volume reduction was 11% in AD and 6% in MCI cases relative to CO cases. Smaller TPC volume was observed among AD relative to MCI or Co-participants; however this difference did not reach statistical significance. Quartile volumes of right TPC are 1.72/2.47/2.62 mm3 in patients with AD, 2.01/2.53/2.66 mm3 in patients with MCI, and 2.15/2.48/2.76 mm3 for CO patients. Quartile volumes of left TPC are 2.01/2.23/2.36 mm3 in patients with AD, 1.73/2.36/2.81 mm3 in patients with MCI, and 2.13/2.40/2.82 mm3 for CO patients. In addition, none of the cognitive test used in the neuropsychologic assessment correlated with TPC volumes. Neither, age, sex, education, MMSE or functional tests (Barthel and Lawton) were associated with TPC volumes.
| Table 1: Demographic characteristics of the studied population. | |||
| CO | MCI | AD | |
| n | 12 | 9 | 14 |
| Age (years) | 73.2 ± 4.8 | 72.6 ± 4.7 | 75.0 ± 4.0 |
| Female / male (n) | 8 / 4 | 7 / 2 | 10 / 4 |
| Education (years) | 5.8 ± 2.9 | 5.6 ± 2.9 | 4.9 ± 3.1 |
| MMSE | 26 ± 3.4 | 25 ± 2.9 | 18.4 ± 6.2 |
| GDS Reisberg (4 / 5 / 6) | --- | --- | 9 / 4 / 1 |
| Barthel index | 98.8 ± 2.3 | 95.6 ± 3.9 | 92.9 ± 6.4 |
| Lawton index | 7.1 ± 1.4 | 6.4 ± 1.6 | 2.9 ± 1.6 |
| GDS Yesavage | 3.7 ± 4.2 | 4.1 ± 3.4 | 2.9 ± 1.7 |
| NPI (frecuency x severity) | 3.0 ± 2.3 | 7.6 ± 6.4 | 7.6 ± 8.1 |
| Right TPC volume (mm3) | 2.51 ± 0.49 | 2.32 ± 0.48 | 2.24 ± 0.61 |
| Left TPC volume (mm3) | 2.44 ± 0.40 | 2.32 ± 0.51 | 2.18 ± 0.47 |
| Data are mean ± SD. | |||
Data from the neuropsychological assessment are presented in table 2. These values showed a continuous scoring fall in most tests from CO subjects, to MCI, and AD patients. As expected, inverse relation is found for immediate word list recall, the delayed word list recall, and for trail making test A. The changes were manifest between MCI to AD with a slight variation between CO and MCI.
| Table 2: Neuropsychological assessment. | |||
| CO | MCI | AD | |
| Direct digit span (n) | 4 ± 1.2 | 3 ± 0.9 | 3 ± 1.0 |
| Inverse digit span (n) | 3 ± 0.6 | 2 ± 0.5 | 2 ± 0.6 |
| Gnosis (0-20) | 19.2 ± 1.2 | 19.1 ± 0.9 | 16.7 ± 3.7 |
| Verbal comprehension (0-16) | 15.8 ± 0.4 | 15.1 ± 1.1 | 13.6 ± 2.8 |
| Written expression (0-13) | 11.1 ± 2.6 | 11.4 ± 2.7 | 8.3 ± 5.2 |
| Sentence repetition (0-2) | 1.5 ± 0.5 | 1.4 ± 0.5 | 1.1 ± 0.3 |
| Written comprehension (0-10) | 9.2 ± 0.8 | 7.7 ± 1.6 | 6.5 ± 2.5 |
| Benton C (0-10) | 4.3 ± 1.8 | 4.3 ± 2.4 | 1.9 ± 1.4 |
| Benton D (0-10) | 3.8 ± 2.6 | 2.7 ± 1.0 | 0.8 ± 0.9 |
| Immediate word list recall (10-0) | 5.2 ± 0.9 | 5.7 ± 1.4 | 7.6 ± 1.2 |
| Delayed word list recall (0-10) | 5.2 ± 1.7 | 5.6 ± 2.1 | 8.9 ± 1.6 |
| Verbal fluency (n) | 18.1 ± 4.3 | 14.3 ± 1.0 | 8.9 ± 3.2 |
| Trail Making Test A (sec) | 115 ± 43 | 130 ± 39 | 213 ± 87 |
| Verbal expression (0-15) | 12.4 ± 2.1 | 12.3 ± 1.9 | 7.6 ± 2.5 |
| Ideomotor praxis (0-20) | 18.6 ± 1.2 | 18.6 ± 1.8 | 16.0 ± 3.0 |
| Constructional praxis (0-18) | 12.6 ± 0.9 | 13.3 ± 2.7 | 9.4 ± 2.7 |
| Abstraction subtest (0-12) | 7.5 ± 2.2 | 7.6 ± 2.0 | 3.2 ± 2.7 |
| Clock drawing (0-10) | 9.1 ± 1.4 | 8.2 ± 2.0 | 5.9 ± 2.6 |
| In brackets are presented minimum and maximum scores for each test. (n): number of digits or words. (Sec): seconds. Data are mean ± SD. |
|||
The association between TPC volumes and the presence or absence of NPS in MCI and AD patients are shown in table 3. MCI and AD patients with delusions, agitation, apathy, disinhibition and irritability have significant smaller TPC volumes. Results of grouped NPS are summarized in figure 2, in which it is shown a clear decrease of the TPC volumes for all symptoms, and in both sides (right and left); however, only psychotic and behavioral symptoms showed statistical significance (p < 0.01 and p < 0.05, respectively).
| Table 3: TPC volume data by hemisphere in subjects with MCI and AD correlated with the neuropsychiatric symptoms. | ||||||||||
| * | MCI | AD | MCI + AD | |||||||
| n | Right | Left | n | Right | Left | n | Right | Left | ||
| Delusions | Yes | 1 | 1.67 | 1.54 | 4 | 1.62 | 1.74 | 5 | 1.63 | 1.70 |
| No | 8 | 2.43 | 2.39 | 10 | 2.49* | 2.36* | 18 | 2.46* | 2.37Ϯ | |
| Hallucinations | Yes | 0 | - | - | 3 | 1.90 | 2.01 | 3 | 1.90 | 2.01 |
| No | 9 | 2.35 | 2.29 | 11 | 2.33 | 2.23 | 20 | 2.34 | 2.26 | |
| Agitation | Yes | 3 | 1.80 | 1.89 | 5 | 1.95 | 2.01 | 8 | 1.89 | 1.97 |
| No | 6 | 2.62* | 2.49 | 9 | 2.40 | 2.27 | 15 | 2.49* | 2.36* | |
| Depression | Yes | 6 | 2.20 | 2.07 | 8 | 2.17 | 2.20 | 14 | 2.18 | 2.15 |
| No | 3 | 2.65 | 2.73 | 6 | 2.32 | 2.15 | 9 | 2.43 | 2.34 | |
| Anxiety | Yes | 7 | 2.28 | 2.21 | 9 | 2.18 | 2.19 | 16 | 2.22 | 2.19 |
| No | 2 | 2.57 | 2.60 | 5 | 2.35 | 2.17 | 7 | 2.41 | 2.29 | |
| Euphoria | Yes | 0 | - | - | 2 | 1.97 | 1.97 | 2 | 1.97 | 1.97 |
| No | 9 | 2.35 | 2.29 | 12 | 2.28 | 2.22 | 21 | 2.31 | 2.25 | |
| Apathy | Yes | 4 | 2.19 | 2.13 | 5 | 1.82 | 1.97 | 9 | 1.99 | 2.04 |
| No | 5 | 2.47 | 2.42 | 9 | 2.47 | 2.30 | 14 | 2.47* | 2.34 | |
| Disinhibition | Yes | 2 | 2.10 | 1.62 | 2 | 2.07 | 2.04 | 4 | 2.09 | 1.83 |
| No | 7 | 2.42 | 2.49* | 12 | 2.27 | 2.20 | 19 | 2.32 | 2.31 | |
| Irritability | Yes | 7 | 2.28 | 2.21 | 6 | 1.75 | 1.88 | 13 | 2.04 | 2.06 |
| No | 2 | 2.57 | 2.60 | 8 | 2.60* | 2.41* | 10 | 2.60 | 2.44* | |
| All data are means (mm3). † p < 0.01. *p < 0.05. | ||||||||||
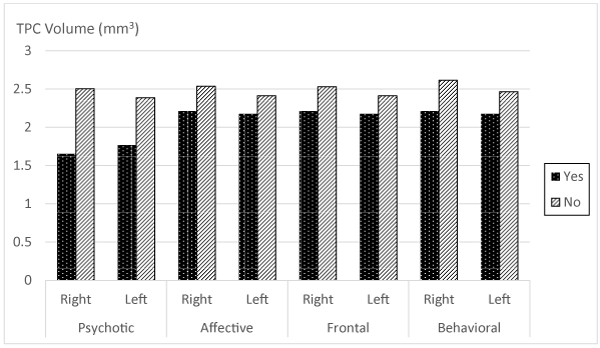 Figure 2: Histograms of TPC volume and presence or absence of grouped NPS in each hemisphere. Significant differences were observed for psychotic and behavioral symptoms in both right and left hemispheres (p < 0.01 and p < 0.05, respectively).
Figure 2: Histograms of TPC volume and presence or absence of grouped NPS in each hemisphere. Significant differences were observed for psychotic and behavioral symptoms in both right and left hemispheres (p < 0.01 and p < 0.05, respectively).
NPI scores for the four TPC volumes quartiles in MCI or AD patients were respectively: right TPC 15.0/5.8/9.3/1.3 (global difference p < 0.01) with statistically significant differences between the first and fourth quartile (p < 0.01); left TPC were 15.6/5.7/8.0/2.3 (global difference p < 0.05), also with statistically significant differences between the first and fourth quartile (p < 0.01) (Figure 3).
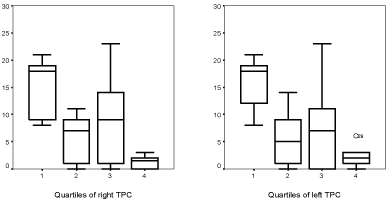 Figure 3: Box-plot showing NPI scores (severity x frequency) depending on the quartile of right and left TPC volumes, in patients with MCI or AD.
Figure 3: Box-plot showing NPI scores (severity x frequency) depending on the quartile of right and left TPC volumes, in patients with MCI or AD.
Multiple regression analysis in AD or MCI patients resulted in an association of right TPC volume with NPI scores (B = -6.85; 95%CI -12.39 to -1.31; r2 = 0.465; p < 0.05). Likewise, the left TPC also showed an association (B = -8.41; 95% CI -14.16 to –2.65; r2 = 0.521; p < 0.01). Also, both right TPC (B = -2.33; 95%CI -4.10 to -0.55; r2 = 0.446; p < 0.05) and left TPC (B = -2.34; 95%CI -4.37 to -0.31; r2 = 0.402; p < 0.05) were associated with the number of NPS. All these analyses are adjusted by age, sex, years of education, MMSE and Reisberg Global Deterioration Scale.
Discussion
NPS are very common in patients with dementia and MCI, and are responsible of many adverse events associated with these conditions. Moreover, NPS in MCI are often accompanied with cognitive and functional ability decline [54], and those which show depression, apathy and agitation are at a higher risk of later conversion to AD [22], although the specific etiology and pathophysiologic mechanisms involved remain unclear. The importance and clinical relevance of TPC prompted us to find the association between NPS and the degree of atrophy of the TPC in both MCI and AD. The present study is a complementary study using quantitative estimates of the TPC volume that are interrelationships with NPS; previous studies [20] have only used an indirect measure of medial temporal cortical and hippocampal atrophy based on the width measurement of the anterior temporal horn of the lateral ventricle. Although the MTL is involved in important neural systems, including memory, each of its components (cortical and subcortical) are involved in specific memory functions [55]. In this context, atrophy of the hippocampal formation, including the entorhinal cortex have mostly been associated with a decline in memory function [56-59], while the amygdala has been associated with emotional memory processes [60].
TPC damage coincides with socioemotional disorders under different neurological conditions such as herpetic encephalitis with temporal lobe damage [19], unilateral anterior temporal lobe damage [61], drug-refractory temporal lobe epilepsy [62], post-ictal temporal lobe epilepsy [63], and temporal variant of frontotemporal dementia [19]. More specifically, patients with the temporal variant of frontotemporal dementia and atrophy on the right (but not in the left) TPC exhibit changes in personality and social behavior [40]. Depression, irritability, apathy and emotional blunting [11] have also been described. Other deficits or disorders after TPC damage include amnesic associative prosopagnosia [64] and impairment in the theory of mind [65]. In this context, the possibility arises that TPC lesions on the right side produce an impairment to couple emotional responses to highly processed sensory stimuli and of personal semantic memory [11].
Cortical changes have been linked to NPS. Thus, delusions have been associated with atrophy of the right MTL [20,21], as well as atrophy in the right frontal [34], and temporal cortices [43,44]. In contrast, agitation, irritability and abnormal motor movements have not been correlated with atrophy in any specific brain region. Our findings show the association between right and left TPC atrophy (quantified by TPC volume) and psychotic and behavioral symptoms in patients with cognitive deficit. Specific effects in our study are the association between TPC atrophy with the agitation and disinhibition symptoms in MCI patients while delusions and irritability did so in AD patients; moreover, a significant right TPC atrophy is related with the occurrence of apathy in patients with cognitive deficits. As shown in table 3, the mean TPC volumes of patients with NPS were smaller than those of patients without symptoms although it only reached statistical significance in agitation, disinhibition, delusions and irritability. Our series of patients, especially those with AD, did not show statistical significance in TPC volume, what may be explained by generalized brain atrophy in AD, rather than meaning a lack of involvement of the TPC in the remainder NPS.
The clinical importance of the neuroanatomical changes derived from the decreased TPC volume associated to NPS remains uncertain, although contributes to the knowledge of the brain areas implicated in cognitive deficits. The presence of NPS is a complex process involving many structures of the limbic system, and TPC volume change cannot bear the weight of all NPS. However, the TPC may play an important role in the pathogenesis of the symptoms, mainly psychotic, by a disruption of more complex neural systems that include this structure, as association between delusions and a higher volume of the right anterior temporal horn of the lateral ventricle in AD patients has been reported [20], along with abnormal social behavior in semantic dementia (with predominant right TPC atrophy) [40]. Our results indicate a tendency towards higher atrophy on the right side of the TPC associated with apathy and agitation, in agreement of the lateralization towards the right side reported above. However, disinhibition showed a preference for the left side, while other symptoms (delusions and irritability) did not show any side preference.
Acknowledgement
This study was supported by Grants PAI-02-022, and PPII-2014-013 of the Consejería de Sanidadand Consejería de Educación, Cultura y Deportes of the Junta de Comunidades de Castilla-La Mancha, and projects BFI2003-09581, BFU2006-12964 and HF2007-0016 from the Ministry of Science and Technology to RI.
References
- Insausti R, Amaral DG. Chapter 24 - Hippocampal Formation A2 - Mai, Jürgen K. In: Paxinos G, eds. The Human Nervous System (Third Edition). San Diego: Academic Press. 2012; 896-942. https://goo.gl/w75oDm
- Buchanan TW, Tranel D, Adolphs R. Memories for emotional autobiographical events following unilateral damage to medial temporal lobe. Brain. 2006; 129: 115-127. https://goo.gl/2aBrqF
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999; 9: 7-24. https://goo.gl/cXAX8y
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957; 20: 11-21. https://goo.gl/qS7Gvr
- Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001; 13: 199-212. https://goo.gl/bKL3vU
- Mori E, Yoneda Y, Yamashita H, Hirono N, Ikeda M, Yamadori A. Medial temporal structures relate to memory impairment in Alzheimer's disease: an MRI volumetric study. J Neurol Neurosurg Psychiatry. 1997; 63: 214-221. https://goo.gl/1Mi6V3
- Double KL, Halliday GM, McRitchie DA, Reid WG, Hely MA, Morris JG. Regional brain atrophy in idiopathic Parkinson’s disease and diffuse Lewy body disease. Dementia. 1996; 7: 304-313. https://goo.gl/7DBKDw
- Crespo FB, Nopoulos PC, Chemerinski E, Kim JJ, Andreasen NC, Magnotta V. Temporal pole morphology and psychopathology in males with schizophrenia. Psychiatry Res. 2004; 132: 107-115. https://goo.gl/6Yvc2t
- Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology. 2005; 65: 223-228. https://goo.gl/89DUMu
- Dupont S, Baulac M. Contribution of MRI to the exploration of partial refractory epilepsy. Rev Neurol (Paris). 2002; 158: 19-26. https://goo.gl/bQXJBv
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007; 130: 1718-1731. https://goo.gl/ucbZyb
- Ding SL, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. J Comp Neurol. 2009; 514: 595-623. https://goo.gl/MkHG49
- Insausti R, Annese J, Amaral DG, Squire LR. Human amnesia and the medial temporal lobe illuminated by neuropsychological and neurohistological findings for patient E.P. Proc Natl Acad Sci USA. 2013; 110: 1953-1962. https://goo.gl/oXPmyK
- Brodmann K. Vergleichende Lokalisationslehre der Groshirnrinde. Barth, Leipzig. 1909. https://goo.gl/VEfr1N
- Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. J Comp Neurol. 1960; 115: 333-369. https://goo.gl/QffYMp
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2003; 465: 499-523. https://goo.gl/PPLRD2
- Moran MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. J Comp Neurol. 1987; 256: 88-103. https://goo.gl/DqdPa7
- Kling AS, Tachiki K, Lloyd R. Neurochemical correlates of the Kluver-Bucy syndrome by in vivo microdialysis in monkey. Behav Brain Res. 1993; 56: 161-170. https://goo.gl/uuxD2J
- Lilly R, Cummings JL, Benson DF, Frankel M. The human Kluver-Bucy syndrome. Neurology. 1983; 33: 1141-1145. https://goo.gl/EHysBF
- Geroldi C, Akkawi NM, Galluzzi S, Ubezio M, Binetti G, Zanetti O, et al. Temporal lobe asymmetry in patients with Alzheimer's disease with delusions. J Neurol Neurosurg Psychiatry. 2000; 69: 187-191. https://goo.gl/JD7e9z
- Geroldi C, Bresciani L, Zanetti O, Frisoni GB. Regional brain atrophy in patients with mild Alzheimer's disease and delusions. Int Psychogeriatr. 2002; 14: 365-378. https://goo.gl/Hvjjiw
- Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008; 25: 115-126. https://goo.gl/idA4nK
- Ikeda M, Fukuhara R, Shigenobu K, Hokoishi K, Maki N, Nebu A, et al. Dementia associated mental and behavioural disturbances in elderly people in the community: findings from the first Nakayama study. J Neurol Neurosurg Psychiatry. 2004; 75: 146-148. https://goo.gl/ZctDjb
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002; 288: 1475-1483. https://goo.gl/3WwtEx
- Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000; 157: 708-714. https://goo.gl/wYF9FX
- Peters KR, Rockwood K, Black SE, Bouchard R, Gauthier S, Hogan D, et al. Characterizing neuropsychiatric symptoms in subjects referred to dementia clinics. Neurology. 2006; 66: 523-528. https://goo.gl/WbFT5H
- Tatsch MF, Bottino CM, Azevedo D, Hototian SR, Moscoso MA, Folquitto JC, et al. Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: prevalence and relationship with dementia severity. Am J Geriatr Psychiatry. 2006; 14: 438-445. https://goo.gl/jCeiRT
- Xie HG, Wang LN, Yu X, Wang W, Yang LJ, Ma TX, et al. Neuropsychiatric symptoms in dementia and elderly people in the community: results from the Beijing Dementia Cooperative Study. Zhonghua Liu Xing Bing Xue Za Zhi. 2004; 25: 829-832. https://goo.gl/36i3Xw
- Van Dam D, Vermeiren Y, Dekker AD, Naude PJ, Deyn PP. Neuropsychiatric Disturbances in Alzheimer's Disease: What Have We Learned from Neuropathological Studies? Curr Alzheimer Res. 2016; 13: 1145-1164. https://goo.gl/2rBfQf
- Stern Y, Tang MX, Albert MS, Brandt J, Jacobs DM, Bell K, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA. 1997; 277: 806-812. https://goo.gl/gm4Gnm
- Steele C, Rovner B, Chase GA, Folstein M. Psychiatric symptoms and nursing home placement of patients with Alzheimer's disease. Am J Psychiatry. 1990; 147: 1049-1051. https://goo.gl/zHK79Y
- Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer's disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998; 46: 210-215. https://goo.gl/3XzZAe
- Farber NB, Rubin EH, Newcomer JW, Kinscherf DA, Miller JP, Morris JC, et al. Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Arch Gen Psychiatry. 2000; 57: 1165-1173. https://goo.gl/Ef3Ux5
- Forstl H, Besthorn C, Burns A, Geiger-Kabisch C, Levy R, Sattel A. Delusional misidentification in Alzheimer's disease: a summary of clinical and biological aspects. Psychopathology. 1994; 27: 194-199. https://goo.gl/EV7Fj6
- Lai MK, Lai OF, Keene J, Esiri MM, Francis PT, Hope T, et al. Psychosis of Alzheimer's disease is associated with elevated muscarinic M2 binding in the cortex. Neurology. 2001; 57: 805-811. https://goo.gl/xayoqu
- Zubenko GS, Moossy J, Martinez AJ, Rao G, Claassen D, Rosen J, et al. Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol. 1991; 48: 619-624. https://goo.gl/AGBZXt
- Binetti G, Padovani A, Magni E, Bianchetti A, Scuratti A, Lenzi GL, et al. Delusions and dementia: clinical and CT correlates. Acta Neurol Scand. 1995; 91: 271-275. https://goo.gl/wWkZ8q
- Sultzer DL, Chen ST, Brown CV, Mahler ME, Cummings JL, Hinkin CH, et al. Subcortical hyperintensities in Alzheimer's disease: associated clinical and metabolic findings. J Neuropsychiatry Clin Neurosci. 2002; 14: 262-269. https://goo.gl/mcwc6W
- Weamer EA, DeMichele-Sweet MA, Cloonan YK, Lopez OL, Sweet RA. Incident Psychosis in Subjects With Mild Cognitive Impairment or Alzheimer's Disease. J Clin Psychiatry. 2016; 77: 1564-1569. https://goo.gl/vNMzXp
- Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003; 61: 1196-1203. https://goo.gl/eQtLLt
- Glosser G, Zwil AS, Glosser DS, O'Connor MJ, Sperling MR. Psychiatric aspects of temporal lobe epilepsy before and after anterior temporal lobectomy. J Neurol Neurosurg Psychiatry. 2000; 68: 53-58. https://goo.gl/ct81yM
- Murai T, Fujimoto S. Rapid cycling bipolar disorder after left temporal polar damage. Brain Inj. 2003; 17: 355-358. https://goo.gl/WmY4zs
- Hirono N, Mori E, Ishii K, Kitagaki H, Sasaki M, Ikejiri Y, et al. Alteration of regional cerebral glucose utilization with delusions in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 1998; 10: 433-439. https://goo.gl/vqH6nw
- Starkstein SE, Vazquez S, Petracca G, Sabe L, Migliorelli R, Teson A, et al. A SPECT study of delusions in Alzheimer's disease. Neurology. 1994; 44: 2055-2059. https://goo.gl/AcWnb6
- Mega MS, Lee L, Dinov ID, Mishkin F, Toga AW, Cummings JL. Cerebral correlates of psychotic symptoms in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2000; 69: 167-171. https://goo.gl/gtyXtk
- Staff RT, Shanks MF, Macintosh L, Pestell SJ, Gemmell HG, Venneri A. Delusions in Alzheimer's disease: spet evidence of right hemispheric dysfunction. Cortex. 1999; 35: 549-560. https://goo.gl/gMbk32
- Sultzer DL, Brown CV, Mandelkern MA, Mahler ME, Mendez MF, Chen ST, et al. Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer's disease. Am J Psychiatry. 2003; 160: 341-349. https://goo.gl/TWVZZ6
- Sultzer DL, Mahler ME, Mandelkern MA, Cummings JL, Van Gorp WG, Hinkin CH, et al. The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 1995; 7: 476-484.
- Mentis MJ, Weinstein EA, Horwitz B, McIntosh AR, Pietrini P, Alexander GE, et al. Abnormal brain glucose metabolism in the delusional misidentification syndromes: a positron emission tomography study in Alzheimer disease. Biol Psychiatry. 1995; 38: 438-449. https://goo.gl/jXG1vk
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998; 19: 659-671. https://goo.gl/SC75jE
- Free SL, Bergin PS, Fish DR, Cook MJ, Shorvon SD, Stevens JM. Methods for normalization of hippocampal volumes measured with MR. AJNR Am J Neuroradiol. 1995; 16: 637-643. https://goo.gl/EEngh8
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987; 147: 229-263. https://goo.gl/dxN4z6
- Jelsing J, Rostrup E, Markenroth K, Paulson OB, Gundersen HJ, Hemmingsen R, et al. Assessment of in vivo MR imaging compared to physical sections in vitro--a quantitative study of brain volumes using stereology. Neuroimage. 2005; 26: 57-65. https://goo.gl/fb3bnw
- Feldman H, Scheltens P, Scarpini E, Hermann N, Mesenbrink P, Mancione L, et al. Behavioral symptoms in mild cognitive impairment. Neurology. 2004; 62: 1199-1201. https://goo.gl/DPFTLE
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004; 27: 279-306. https://goo.gl/ocakXT
- Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, et al. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain. 2006; 129: 2867-2873. https://goo.gl/JvUP3d
- Di Paola M, Macaluso E, Carlesimo GA, Tomaiuolo F, Worsley KJ, Fadda L, et al. Episodic memory impairment in patients with Alzheimer's disease is correlated with entorhinal cortex atrophy. A voxel-based morphometry study. J Neurol. 2007; 254: 774-781. https://goo.gl/aoHi3F
- Juottonen K, Laakso MP, Insausti R, Lehtovirta M, Pitkanen A, Partanen K, et al. Volumes of the entorhinal and perirhinal cortices in Alzheimer's disease. Neurobiol Aging. 1998; 19: 15-22. https://goo.gl/2DeL4P
- Philippi N, Noblet V, Botzung A, Despres O, Renard F, Sfikas G, et al. MRI-based volumetry correlates of autobiographical memory in Alzheimer's disease. PLoS One. 2012; 7: 46200. https://goo.gl/MJnLVu
- Horinek D, Varjassyova A, Hort J. Magnetic resonance analysis of amygdalar volume in Alzheimer's disease. Curr Opin Psychiatry. 2007; 20: 273-277. https://goo.gl/QbXhz4
- Ghika-Schmid F, Assal G, De Tribolet N, Regli F. Kluver-Bucy syndrome after left anterior temporal resection. Neuropsychologia. 1995; 33: 101-113. https://goo.gl/QvZZVk
- Jutila L, Ylinen A, Partanen K, Alafuzoff I, Mervaala E, Partanen J, et al. MR volumetry of the entorhinal, perirhinal, and temporopolar cortices in drug-refractory temporal lobe epilepsy. AJNR Am J Neuroradiol. 2001; 22: 1490-1501. https://goo.gl/D2QPQh
- Anson JA, Kuhlman DT. Post-ictal Kluver-Bucy syndrome after temporal lobectomy. J Neurol Neurosurg Psychiatry. 1993; 56: 311-313. https://goo.gl/vV8gD4
- Damasio AR, Tranel D, Damasio H. Face agnosia and the neural substrates of memory. Annu Rev Neurosci. 1990; 13: 89-109. https://goo.gl/j179Ve
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006; 50: 655-663. https://goo.gl/v8S6Fq
Authors submit all Proposals and manuscripts via Electronic Form!




























