Hussein S. Hussein*, Lamyaa E. Allam, Amr Mohab, Nadia M. Elbarghouty and Salwa Y. Mohammed
Hussein S. Hussein1*, Lamyaa E. Allam2, Amr Mohab1, Nadia M. Elbarghouty3 and Salwa Y. Mohammed3
1Department of Nephrology
2Department Cardiology
3Internal Medicine Division, Nuovo Ospedale S.Agostino–Estense, Modena, Italy
4Radiology Cairo, Ain Shams University
*Address for Correspondence: Hussein S. Hussein, Department of Cardiology, Cairo Egypt, Tel: +201-111-385-899; E-mail: hsayedh@hotmail.com
Dates: Submitted: 11 November 2017; Approved: 24 November 2017; Published: 27 November 2017
Citation this article: Hussein HS, Allam LE, Mohab A, Elbarghouty NM, Mohammed SY. Fibroblast Gross Factor - 23 and Arterial Stiffness in Hemodialysis Patients. Int J Clin Cardiol Res. 2017;1(2): 067-071.
Copyright: © 2017 Hussein HS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: FGF - 23; Carotid Artery; Atherosclerosis; Hemodialysis
Abstract
Background: Increased FGF23 levels have been shown to be independently associated with a variety of adverse renal and cardiovascular outcomes in Hemodialysis (HD) patients although the mechanisms are not fully understood. The association between FGF - 23 and arterial stiffness among HD patients remains still controversial with conflicting data.
Methods: In this cross - sectional study, serum FGF - 23 concentrations were measured in 30 HD patients without known CVD, in hemodialysis unit of Ain Shams University. Plasma FGF - 23 concentrations were measured using two -site enzyme - linked immunosorbent assay. Assessment of arterial stiffness was through assessing radial artery Pulse Pressure (PP) using sphygmomanometer, both Carotid Arteries Intima - Media Thickness (CIMT) and presence of Carotid Plaques (CP) measured by B-Mode Doppler ultrasound.
Results: Patients had elevated serum levels of FGF - 23 ranging from 100 up to 700 pg/ml. Duration of arterial Hypertension had a strong positive correlation with serum FGF - 23 (r is 0.754, p value is 0.007). Statistically significant positive correlation was recorded between marked elevations of FGF - 23 and CIMT (r = 0.776, p value < 0.001) as well as presence of CP, while no significant correlation was found between FGF - 23 and PP (r = 0.334, p value 0.071).
Conclusions: Higher serum FGF - 23 is strongly associated with CIMT and presence of CP as markers of arterial stiffness which could possibly explain its role in pathogenesis of atherosclerosis among HD patients without known CVD.
Introduction
Fibroblast Growth Factor - 23 (FGF - 23) is a circulating osteocyte-derived hormone 9 [1]. It is a major systemic regulator of phosphate homeostasis. In kidney cells, FGF23 binds to complex of FGF23 receptor and klotho receptor [2]. Several studies demonstrated that elevated FGF23 levels were associated with arterial stiffness, increased left ventricular mass index and increased prevalence of left ventricular hypertrophy in patients with Chronic Kidney Disease (CKD) [3]. Several clinical trials have shown that high FGF23 level was an independent predictor of mortality in both incident and prevalent dialysis patients independently of serum phosphate levels [4], although the mechanistic causes remain unknown [5]. Increased FGF23 levels also have been shown to be independently associated with a variety of adverse renal and cardiovascular outcomes [4], which still remain the main cause of mortality in hemodialysis patients especially when associated with Vascular Calcification (VC) [6]. Aberrant calcium and phosphorous homeostasis has consistently been linked with arterial calcification and stiffness. Because FGF - 23 is integral in regulating phosphorous and vitamin D metabolism, it may also induce arterial calcification and stiffness. However, there is considerable controversy [7] because some, not all, previous studies have observed associations of FGF - 23 with arterial calcification and stiffness [8]. On the contrary, animal studies demonstrated that FGF - 23 null mice develop VC and FGF - 23 is protective against VC [9]. Also, there are clinical studies that have demonstrated the lack of any correlation between FGF - 23 and VC [10]. Therefore, it is important to provide more evidence to distinguish whether FGF - 23 is associated with cardiovascular risk or protection. The purpose of this study was to assess the links between FGF - 23and arterial stiffness in End Stage Renal Disease (ESRD) patients on regular Hemodialysis (HD).
Materials and Methods
This was a cross sectional study that was carried out on a cohort of randomly selected from HD unit at Ain Shams University hospitals during the period from 2016-2017. Thirty ESRD patients were recruited on regular Hemodialysis (HD). Eligibility criteria: patients with HD vintage more than 6 months (3 sessions per week, 4 hours for each session), age > 18 years, acceptance to the study protocol. Exclusion criteria: life expectancy less than 6 months, patients having cardiovascular or peripheral vascular disease, Arterio-Venous fistulas (A-V fistula) on both arms or their age below 18 or above 50 years. We recorded relevant data regarding history and physical examination, etiology of CKD, drugs taken by patients, time and duration of dialysis.
Hemodialysis Protocol
All patients were receiving 4 - hours HD schedule with polysulphone dialysers F6HPS and F7HPS (Fresenius AG, Bad Homburg, Germany), with bicarbonate dialysate and unfractionated heparin using a standard regimen, a 2000 unit initial bolus followed by infusion of 1000 U/h for standard anticoagulation. The mean blood flow rate was 300 mL/min during the HD session (range 250-340 mL/min). Dialysate fluid composition included sodium 140 mmol/L, potassium 1-3 mmol/L, calcium 1.5 mmol/L and bicarbonate 33 mmol/L. Dry weight was considered optimal when the patients had no residual symptoms of orthopnea, dyspnea and edema during the inter-dialytic period. Blood Pressure (BP) of the patients was measured with a conventional mercury manometer prior to each HD session.
Laboratory investigations
Venous blood samples were drawn after an overnight fast. The blood samples were obtained directly through an Arterio-venous fistula or central catheter on a mid-week dialysis day. Serum biochemical parameters (serum creatinine, serum urea, corrected serum calcium, serum phosphorous, complete blood count) and intact parathormone levels were studied by means of a computerized auto-analyzer (Hitachi 717; Boehringer-Mannheim). Urea Reduction Ratio (URR) was measured to quantify dialysis adequacy and was calculated as the following (urea predialsis – urea post dialysis /urea predialsis) × 100% [11]. Estimated glomerular filtration rate (eGFR) was measured by using MDRD (modified of diet in renal disease) equation = 186 x (serum creatinine /88.4) - 1.154 x (age) - 0.203x (0.742 if female) x (1.210 if black) [12].
FGF-23 Test
Serum FGF-23 was measured by two-site enzyme-linked immunosorbent assay (ELISA) (DRG® FGF-23; EIA-4737 International Inc., USA) and measurement of FGF-23 concentration was done using EDTA plasma. A morning, 12-h fasting sample was drawn. Samples were assayed immediately or stored frozen at -20°C or below. Intra-assay and inter-assay variations were 5% and 6.4%, respectively.
Assessment of arterial stiffness
Pulse Pressure (PP): Seated, resting Blood Pressure (BP) was measured 3 times in each participant using mercury sphygmomanometer, and the average of the last two measurements were used in analysis. Hypertension was defined as Systolic Blood Pressure (SBP) ≥ 140 mm Hg or Diastolic BP (DBP) ≥ 90 mm Hg. PP was defined as SBP minus DBP (13).
Carotid Duplex: Carotid duplex on both common carotid arteries was performed for all subjects using an Auto-IMT, Log i’q 7 ultrasound system General Electric Healthcare equipped with a 10 - 12 MHz (megahertz) linear transducer. A standard study was performed for all subjects by an accredited sonographer. During the scan, the patient was positioned supine with the head resting flat on the bed with a rolled-up towel under the neck. The carotid arteries were imaged in the transverse and longitudinal planes. The Common Carotid Arteries (CCA) on each side were examined by experienced operator for measuring the intima media thickness (CIMT) and detecting presence of any Carotid Plaques (CP). Caliper measurements were used to estimate the reduction in vessel diameter caused by the plaque. The pulsed Doppler sample volume was swept continuously throughout the length of the vessels to search for areas of increased velocity or flow disturbance in the longitudinal orientation. CP was defined as presence of plaque with < 50% vessel diameter reduction and a focal thickening that encroached into the lumen by 50% of the surrounding intima-media thickness. CIMT is considered abnormal if > 0.9 mm (14).
Statistical analysis
All data were revised and statistically analyzed using PASW version 18 (IBM© Corp., Armonk, NY, USA). Normality of data was tested using D’ Agostino-Pearson test, normally distributed numerical variables were presented as mean ± Standard Deviation (SD). Numerical data were compared using unpaired student t test for parametric data, and Mann-Whitney test for non-parametric data, qualitative data were compared using chi-square t test. Paired testing was done using Paired t test. Correlations were done using Pearson’s product-moment coefficient.
Results
The overall HD population was 30 patients. There were 17 males (56.7%) and 13 females (43.3%) ranging in age from 34 to 50 years with a mean age of 47.47 ± 4.26 years. The etiology of ESRD among study participants was heterogonous but hypertension was the most common etiology in 11 (36.7%) patients followed by Diabetes Mellitus (DM) affecting 7 (23.3%) patients, chronic glomerulonephritis in 3 (10 %) patients, analgesic nephropathy in 3 (10 %) patients, chronic pyelonephritis in 2 (6.7%) , lupus nephritis in 2 (6.7%) patients and polycystic kidney disease in 2 (6.7%) patients. Dialysis interval range was 4 -18 years. In hypertensive patients, the duration of hypertension was 8.35 ± 4.4 years while in diabetic patients; the duration of DM was 8.29 ± 2.43 years. As regards arterial stiffness among studied participants, the average CIMT detected by carotid arterial duplex was 1.03 ± 0.54 mm (range 0.08 - 1.80 mm) among the patients. CPs were found in 16 (53.3%) patients while the rest of patients (46.7%) had no CP. While pulse pressure among the patients was 44.07 ± 18.27 mmHg (range 10 - 87.5 mmHg).
Correlations between serum FGF - 23 with demographic data and common carotid intimal thickness
Serum FGF-23 showed no significant correlation with age or gender within study groups. There was high positive correlation with duration of hypertension (r = 0.754, p value 0.007) while there was no significant correlation with duration of diabetes mellitus (r = 0.471, p value 0.062). Serum FGF - 23 showed highly significant positive correlation with CIMT (r = 0.776, p value < 0.001) while no significant correlation between serum FGF - 23 and pulse pressure (r = 0.334, p value 0.071).
Correlations between serum FGF-23 and laboratory investigations
Serum FGF - 23 showed moderate positive correlation of high significance with serum urea (r = 0.405, P value: 0.026), while it showed strong negative correlation of high significance with serum phosphorus (r = -0.881, p value: < 0.001), moderate negative correlation with (eGFR) (r = -0.520, p value: 0.026) and corrected serum Calcium(r = -0.474, p value: 0.037). Serum FGF -23 levels among studied participants were 100 - 199 pg/mL in 5 patients (16.7%) , 200 - 299 pg/mL in 10 patients (33.3%), 300 - 400 pg/mL in 11 patients (36.7%) and > 400 pg/mL in 4patients (13.3%) respectively. Also there was significant correlation between elevated serum levels of FGF-23 and presence of CP in carotid arterial duplex, where in patients whose serum FGF - 23 was < 100 - 199 pg/mL, no CP were detected, while in patients with serum FGF - 23 > 300 pg/mL, CP were detected in all patients (p value < 0.001).
Discussion
In our study, we explored whether higher FGF - 23 concentrations were associated with markers of arterial stiffness in HD patients. We chose a cohort without prevalent CVD. Existing data on the relationship of FGF - 23 with arterial calcification and stiffness are mixed; some studies demonstrated a positive and independent association with aortic [15], peripheral [16] or coronary calcification in HD patients [17]. Other studies reported negative correlations. Tamei et al. [18] had studied the relation of FGF - 23 with the progression of VC; they demonstrated that FGF - 23 level in repressors was significantly higher than in non-progressors and progressors. In this study, patients had elevated levels of FGF - 23 ranging from 100 to 700pg/ml where 16.7% had FGF23 ranging from 100 to < 200 pg/ml and 33.3% patients had FGF23 serum level ranging from > 200 to < 300 pg/ml, 36.7% had FGF23 serum level ranging from 300 to 400 pg/ml and 13.3% had FGF23 serum level > 400 pg/ml. These results agree with Mirza et al [3], where FGF23 levels were elevated among HD patients. Regarding etiology of ESRD, there was a strong positive association between FGF-23 and duration of hypertension (which was not present with DM) among study populations which coincides with the study done by Gutierrez et al. [19] where higher FGF - 23 levels were independently associated with hypertension. In the current study, there was statistically non-significant negative correlation between FGF23 and serum iPTH levels ( r = -0.194, p value 0.305) and this result didn’t coincide with the study done by Shimada et al, [20], and with the study done by Ben Dov et al, [21] stating that there was statistical significant negative correlation between FGF23 and iPTH. In this study, serum FGF - 23 showed highly significant positive correlation with (CIMT) (r = 0.776, p value < 0.001) as well as presence of CP detected by carotid arterial duplex as patients whose serum FGF-23 > 300 pg/mL, CP were detected in all patients while FGF-23 was < 100 - 199 pg/mL, there was no CP at all (p value < 0.001, where the highest percentage of present CP was 68.8% in 11 participants whose serum FGF-23 were > 300 Pg/l (p value is < 0.001). Similar findings were reported in several studies like Zeng et al [22] who concluded that there is a positive correlation between serum FGF-23 and CIMT suggesting the role of serum FGF-23 in pathogenesis of atherosclerosis among HD patients and Balci et al, (23) found strong association between serum FGF-23 and presence of CP in addition to increased CIMT among HD patients. But in contrast to Moldovan et al, [24] who found a lack of correlation of FGF-23 with vascular calcification and arterial stiffness. Also our result coincides with Nirav et al, [25] study where FGF - 23 elevated serum levels were associated with CP so higher FGF - 23 associated with greater likelihood and burden of carotid atherosclerosis independent of CKD, also they stated that atherosclerosis may be a mechanism through which FGF - 23 increases cardiovascular events and stroke, also they stated that these associations suggest FGF - 23 may be a risk factor for large vessel atherosclerosis. The current study showed non-significant positive correlation between serum level of FGF - 23 and pulse pressure (p value is 0.071, r = 0.334), association between higher FGF - 23 and larger pulse pressure. In the present study there was statistical significant positive correlation between serum level of FGF - 23 in relation to intimal media thickness of common carotid artery (p value is < 0.001; r = 0.776). This agreed with Rebeca Reyes [27] study that found direct significant relationship between elevated serum FGF - 23 levels and changes in intima media thickness. So higher FGF-23, even within the normal range, is independently associated with impaired vaso reactivity and increased arterial stiffness in the general population. There were a few limitations of our study, such as study population, being observational and its cross-sectional nature and the need to be correlated with other markers of chronic inflammation in HD like hs -CRP, Fetuin A, hepatitis c virus, homocystiene and comparing our results to peritoneal dialysis patients. In conclusion, higher FGF - 23 elevations are present among prevalent HD patients possibly playing a role in pathogenesis of increased arterial stiffness which would eventually be associated with poor cardiovascular outcomes.
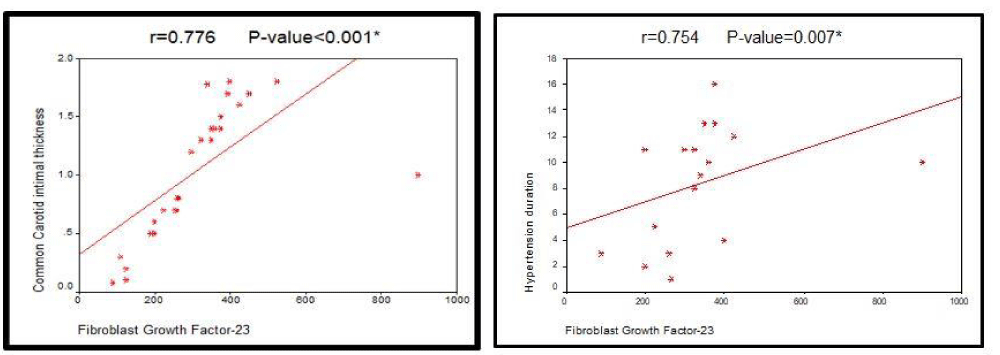 Figure 1: The left panel showing the correlation between Fibroblast growth factor 23(FGF - 23) level and carotid intimal thickness while the right panel shows the correlation between FGF-23 and hypertension duration.
Figure 1: The left panel showing the correlation between Fibroblast growth factor 23(FGF - 23) level and carotid intimal thickness while the right panel shows the correlation between FGF-23 and hypertension duration.
| Table 1: Results of laboratory investigations among studied patients. | ||
| Laboratory investigations | Range | Mean ± SD |
| Serum FGF - 23 concentration (pg/ml) | 90 - 700 | 304.6 ± 129.5 |
| Estimated glomerular filtration rate (e GFR) (ml/min) | 6.9 - 30.7 | 16.7 ± 5.6 |
| Urea reduction ratio (URR) (mg/ml) | 44 - 89 | 66.1 ± 11.3 |
| Serum Creatinine (mg/ml) | 4 - 9.1 | 5.9 ± 1.66 |
| Serum Phosphorous (mg/ml) | 1.9 - 7 | 4.08 ± 1.4 |
| Corrected serum Calcium (mg/ml) | 7.3 - 11.2 | 8.9 ± 0.79 |
| Intact parathyroid hormone (iPTH) (pg/ml) | 19 - 4733 | 1026.4 ± 1289.6 |
| Serum Hemoglobin (mg/ml) Urea Reduction Ratio (URR) (mg/dl) Estimated glomerular filtration rate (eGFR) (ml/min) |
6.9 - 14.9 44 - 89 6.9 - 30.7 |
10.5 ± 1.73 66.1 ± 11.3 16.7 ± 5.76 |
| FGF - 23: Fibroblast growth factor - 23; ml: milliliter, min: minute; mg: milligram; Pg: Picogram. | ||
| Table 2: Correlation of serum FGF - 23 with baseline characteristics of study population. | ||
| Variable | Pearson coefficient ( r ) | P value |
| Age | 0.155 | 0.413 |
| Hypertension duration | 0.754 | 0.007 |
| Diabetes Mellitus | 0.417 | 0.062 |
| Pulse pressure | 0.334 | 0.071 |
| Common carotid intimal thickness | 0.776 | < 0.001 |
| Table 3: Relation between Fibroblast Growth Factor - 23 (FGF -23) and laboratory investigations. | ||
| Variable | Serum FGF - 23 | |
| Pearson coefficient ( r ) | Pvalue | |
| Serum Hemoglobin | 0.007 | 0.973 |
| Intact PTH | - 0.194 | 0.305 |
| Corrected serum Calcium (S.Ca) | - 0.474 | 0.037 |
| Serum Phosphorous (S.Po4) | - 0.881 | < 0.001 |
| Serum. Creatinine (S.Creat.) | 0.112 | 0.556 |
| Serum Urea | 0.405 | 0.026 |
| Urea Reduction Rate (URR) | - 0.095 | 0.616 |
| Estimated glomerular filtration rate (eGFR) | - 0.520 | 0.026 |
| Table 4: Relation between Fibroblast Growth Factor-23 (FGF-23) level and presence of carotid plaques in the common carotid artery. | ||||||
| Carotid plaques | Fibroblast growth factor – 23 (FGF - 23) (pg/mL) | |||||
| < 100 - 200 | < 200 - 299 | 300 - 400 | > 400 | Total | ||
| Present | Numbers | 0.0 | 1.0 | 11.0 | 4.0 | 16.0 |
| Percent (%) | 0.0 | 6.3 | 68.8 | 25.0 | 100 | |
| Absent | Numbers | 5.0 | 9.0 | 0.0 | 0.0 | 14.0 |
| Percent (%) | 35.7 | 64.3 | 0.0 | 0.0 | 100 | |
| Total | Numbers | 5.0 | 10.0 | 11.0 | 4.0 | 30.0 |
| Percent (%) | 16.7 | 33.3 | 36.7 | 13.3 | 100 | |
| Chi - square | X2 | 26.384 | ||||
| P value | < 0.001 | |||||
References
- Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary Phosphate in men and women. Bone Miner Res. 2006; 21: 1187-1196. https://goo.gl/XVXnzL
- Kuroo M. Klotho and the aging process. Korean J Intern Med. 2011; 26: 113-122. https://goo.gl/GJHkPq
- Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009; 207: 546-51. https://goo.gl/Tkqp5Y
- Evenepoel P, Viaene L , Meijers B. PTH, FGF23, and calcium: it takes three to tango? Kidney Int. 2011; 80: 1377. https://goo.gl/xz4Nw1
- Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008; 359: 584-592. https://goo.gl/pnCUU6
- Adragao T, Pires A, Lucas C, Birne R, Magalhaes L, Gonçalves M, et al. A simple vascular calcification score predicts cardiovascular risk in hemodialysis patients. Nephrol Dial Transplant. 2004; 19: 1480-1488. https://goo.gl/Y5xfJQ
- Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, et al. FGF23 is independently associated with vascular calcification but not bone mineral density inpatients at various CKD stages. Osteoporos Int. 2012; 23: 2017-2025. https://goo.gl/R9q6r3
- Scialla JJ, Lau WL, Reilly MP, Isakova T, Yang HY, Crouthamel MH, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013; 83: 1159-1168. https://goo.gl/993tFf
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004; 113: 561-568. https://goo.gl/ZeDSgg
- Roos M, Lutz J, Salmhofer H, Luppa P, Knauss A, Braun S, et al. Relation between plasma fibroblast growth factor-23, serum fetuin-A levels and coronary artery calcification evaluated by multislice computed tomography in patients with normal kidney function. Clin Endocrinol. 2008; 68: 660-665. https://goo.gl/FduA6P
- Chijioke A, Aderibigbe A, Rafiu MO, Olanrewaju TO, Makusidi AM. The Assessment of Hemodialysis Adequacy among ESRD Patients in Ilorin using Urea Reduction Ratio. Tropical Journal of Nephrology. 2009; 4: 115-119. https://goo.gl/GGdL6Z
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150: 604-612. https://goo.gl/2URgPm
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the joint National committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003; 289: 2560-2572. https://goo.gl/1ebyGX
- Mancia G, Fagard R, Narkiewicz K, Josep Redon, Alberto Zanchetti, Michael Böhm, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. European Heart Journal. 2013; 34: 2159-2219. https://goo.gl/HTRfgN
- Nasrallah MM, El-Shehaby AR, Salem MM, Osman NA, El Sheikh E, Sharaf El Din UA. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant. 2010; 25: 2679-2685. https://goo.gl/ZAsj3N
- Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, et al. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant. 2009; 24: 948-955. https://goo.gl/g4dMMp
- Coen G, De Paolis P, Ballanti P, Pierantozzi A, Pisanò S, Sardella D, et al. Peripheral artery calcifications evaluated by histology correlate to those detected by CT: relationships with fetuin-A and FGF-23. J Nephrol. 2011; 24: 313–321. https://goo.gl/V1NC6B
- Tamei N, Ogawa T, Ishida H, Ando Y, Nitta K. Serum fibroblast growth factor-23 levels and progression of aortic arch calcification in non-diabetic patients on chronic hemodialysis. J Atheroscler Thromb. 2011; 18: 217-223. https://goo.gl/kfQNh3
- Gutierrez OM, Wolf M and Taylor EN. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals’ follow-up study. Clin J Am Soc Nephrol. 2011; 6: 2871-2878. https://goo.gl/ZXwnr8
- Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005; 289: 1088-1095. https://goo.gl/RL4XbG
- Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007; 117: 4003-04008. https://goo.gl/MGn2Vi
- Zeng Y, Feng S, Han OY, Shen HY, Jin DH, Shi YB. Role of fibroblast growth factor-23 in the pathogenesis of atherosclerosis in peritoneal dialysis patients. Genet Mol Res. 2015; 14: 719-729. https://goo.gl/Dn41HS
- Balci M, Kirkpantur A, Gulbay M, Gurbuz OA. Plasma fibroblast growth factor-23 levels are independently associated with carotid artery atherosclerosis in maintenance hemodialysis patients. Hemodial Int. 2010; 14: 425-432. https://goo.gl/egYcPR
- Moldovan D, Moldovan I, Rusu C, Kacso I, Patiu IM, Gherman-Caprioara M. FGF-23, vascular calcification, and cardiovascular diseases in chronic hemodialysis patients. Int Urol Nephrol. 2014; 46: 121-128. https://goo.gl/b8o4Lf
- Shah NH, Dong C, Elkind MS, Sacco RL, Mendez AJ, Hudson BI1, et al. Fibroblast growth factor 23 is associated with carotid plaque presence and area: the northern manhattan study. Arterioscler Thromb Vasc Biol. 2015; 35: 2048-2053. https://goo.gl/YxiY2H
- Hsu JJ, Katz R, Ix JH, de Boer IH, Kestenbaum B, Shlipak MG. Association of fibroblast growth factor-23 with arterial stiffness in the Multi-Ethnic Study of Atherosclerosis. Nephrol Dial Transplant. 2014; 29: 2099-2105. https://goo.gl/g1uwjU
- Rebeca RG, Antonia GM, In, Beatriz GF, Sonia MS, Pedro RM, Manuel MT. FGF23 in type 2 diabetic patients: relationship with bone metabolism and vascular disease. Diabetes Care. 2014; 37: 89-90. https://goo.gl/tRjGJB
Authors submit all Proposals and manuscripts via Electronic Form!




























