Case Report
Giant Esophageal Liposarcoma – Multimodal Treatment Results in Long Term Survival: Case Report and Review of the Literature
Steffen Pistorius1,2#*, Daniel Kupper1,3#, Nino Kiria4, Jessica Pablik5 and Christina Jentsch2,6
1Department of Visceral-, Thoracic- and Vascular Surgery, University of Dresden, Germany
2University Cancer Center (UCC/NCT), University of Dresden, Germany
3Department of General-, Visceral- and Vascular Surgery, Evangelic Hospital Paul Gerhardt Stift Wittenberg, Germany
4Institute of Pathology, University of Dresden, Germany
5Institute of Diagnostic Radiology, University of Dresden, Germany, Institute of Pathology, University of Dresden, Germany
6Department of Radiation Oncology, University of Dresden, Germany
#Authors contributed equally to this work
*Address for Correspondence: Steffen Pistorius, Department of Visceral, Thoracic and Vascular Surgery, University of Dresden, University Cancer Center Dresden (UCC), Fetscherstr. 74, 01307 Dresden, Germany Tel: +49 351 458 2742, Fax: +49 351 458 4395, E-mail: steffen.pistorius@uniklinikum-dresden.de
Dates: 03 September, 2017; Approved: 10 October, 2017; Published: 11 October, 2017
Citation this article: Pistorius S, Kupper D, Kiria N, Pablik J, Jentsch C, et al. Giant Esophageal Liposarcoma – Multimodal Treatment Results in Long Term Survival: Case Report and Review of the Literature. Int J Case Rep Short Rev. 2017;3(4): 060-066.
Copyright: © 2017 Pistorius S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Liposarcoma; Esophagus; Radiation; Resection
Abstract
Background: Esophageal liposarcomas are notably rare mesenchymal malignant tumours. Due to their slow progress, well-differentiated sarcomas of the esophagus may remain asymptomatic for a long time resulting in an advanced diagnosed stage and, therefore, represent a significant challenge for treatment.
Case presentation: We report the case of a 66-year old male patient who presented with the second largest esophageal liposarcoma reported to date with involvement of the upper aerodigestive tract and the posterior mediastinum. Clinical, radiological and pathological findings, a detailed description of the multimodal treatment and long-term follow up data are presented. In detail, palliative tumour debunking with final histological confirmation and conformal radiation therapy resulted in tumour response with control of symptoms and a 55 months survival.
Conclusion: Multimodal treatment in patients with not curatively resectable esophageal liposarcoma may result in long-term survival, even in patients with very advanced tumours.
Background
Although liposarcoma is the most common malignant soft tissue tumour in adults, it rarely develops in the hypopharynx, the gastrointestinal tract, and the mediastinum. The case of a liposarcoma of the esophagus [1]. Thus far, a total number of only 46 single case reports have been published in literature. Here, we report the case of a very advanced tumour. Clinical, radiological, endoscopic and pathological findings, a detailed description of the multimodal treatment and long-term follow up data are presented.
Case Report
Patient 1
A 66-year-old male patient was admitted to our department because of dysphagia, odynophagia and loss of body weight of 7 kg within one year.
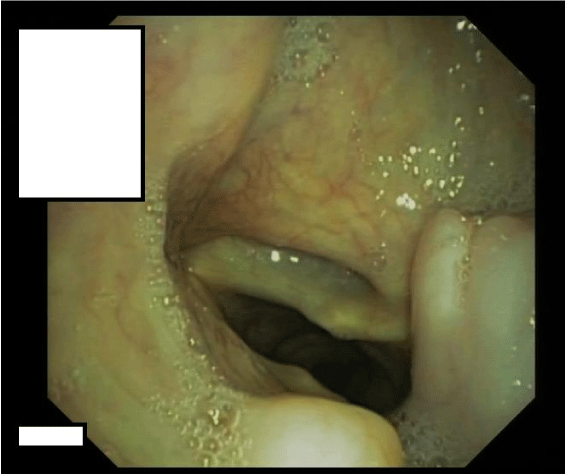
On flexible esophagogastroduodenoscopy, a large partially pedunculated submucosal soft tissue tumour, whitish-yellow in color, commencing at the proximal esophageal sphincter, and continuing down to the distal esophagus (Figure 1) was found. Flexible bronchoscopy revealed the proximal border of the tumour infiltrating both aryepiglottic folds (Figure 2) and a tracheal compression resulting in a tracheal stenosis of 30%. The right upper lobe bronchus appeared compressed with mucosal inflammation. However, the mucosa was not affected. Cytological examination of the bronchoalveolar lavage and repeated biopsies by flexible endoscopy failed to determine the diagnosis. Therefore, three consecutive rigid hypopharyngo-, microlaryngo- and esophagoscopies with multiple deep biopsies in different locations (hypopharynx, aryepiglottic folds and proximal esophagus) containing a total volume of more than 5 cc were performed. Pathological examination of these biopsies revealed a myxoid lesion, possibly a myxoid liposarcoma.
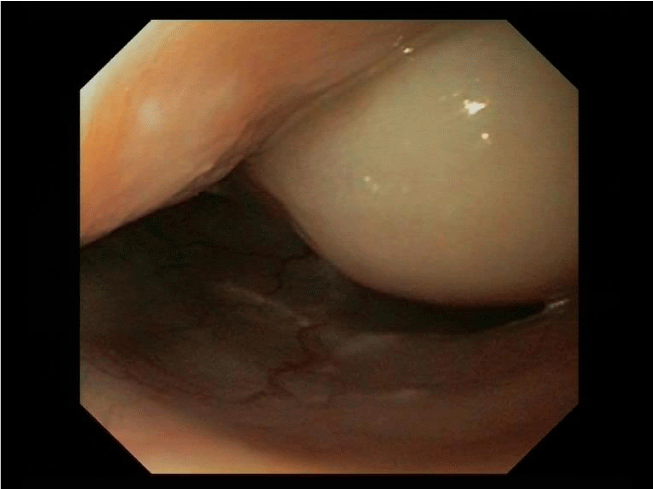 Figure 1: Esophagoscopic view: Submucosal soft tissue tumour of whitish-yellow color of the esophagus.
Figure 1: Esophagoscopic view: Submucosal soft tissue tumour of whitish-yellow color of the esophagus.
Diagnostic imaging was performed including a Computed Tomography (CT), a cine esophagography and a Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET). The multi-slice CT of the chest and abdomen was performed in 2 x 64 x 0, 6 mm Collimation (Siemens Somatom Definition AS +), with oral and i. V contrast medium application. CT scan revealed a polylobulated mass (tumour size of 330x85x65 mm) of the esophagus with fatty and soft tissue density, extending from the thoracic inlet to the distal esophagus and causing a tracheal compression as well as a compression of the right main stem bronchus (Figure 3). There were no metastatic lesions detected in the CT. In the PET only the tumour masses in the middle part of the mediastinum showed a FDG uptake with a maximal Standard Uptake Value (SUV) of 7,4 (Figure 4).
 Figure 3: Computed tomography scan of the chest: large tumour mass with multiple circumscribed lobulations with fat and soft tissue density extending from the thoracic inlet to the lower mediastinum.
Figure 3: Computed tomography scan of the chest: large tumour mass with multiple circumscribed lobulations with fat and soft tissue density extending from the thoracic inlet to the lower mediastinum.
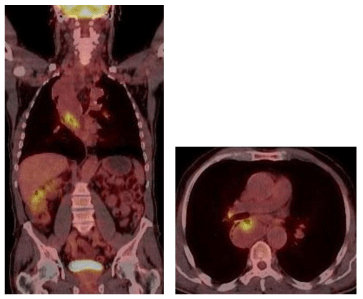 Figure 4: PET/CT: FDG positive mass in the middle part of mediastinum with a maximum standardized uptake value (SUVmax) of 7, 4.
Figure 4: PET/CT: FDG positive mass in the middle part of mediastinum with a maximum standardized uptake value (SUVmax) of 7, 4.
A cine-esophagogram showed a dilated esophagus with a large, irregularly circumscribed contrast media filling defect mainly throughout the upper two-thirds of esophagus (Figure 5).
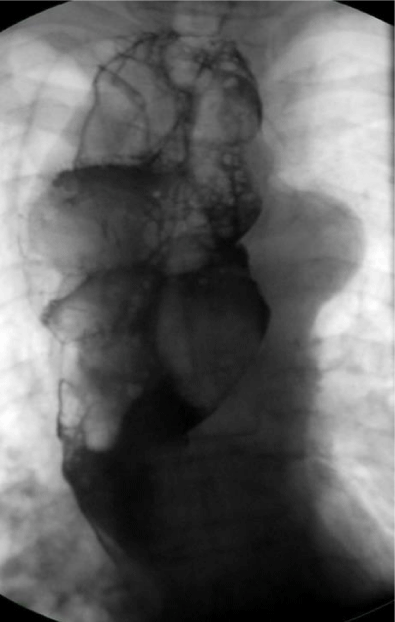 Figure 5: Cine esophagography: dilated esophagus with large contrast media filling defect througout the esophagus.
Figure 5: Cine esophagography: dilated esophagus with large contrast media filling defect througout the esophagus.
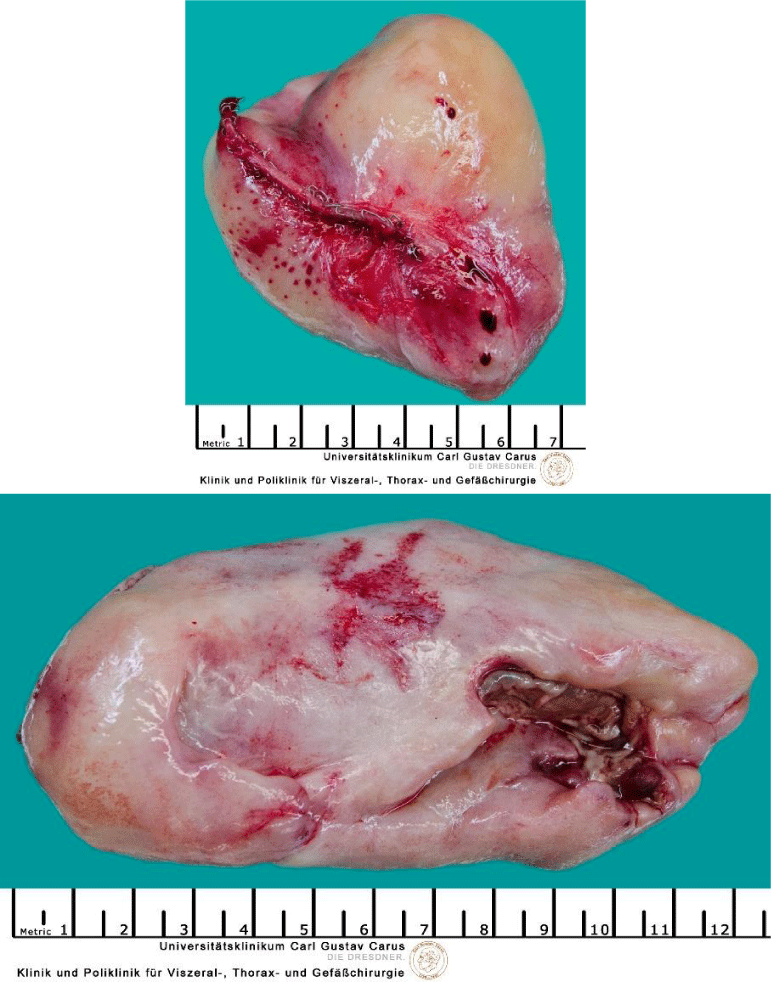
After repeated discussion of this patient in the interdisciplinary tumour board of the University Cancer Center (UCC), we performed an explorative right-sided dorsolateral thoracotomy in order to prove the malignant histology of this tumour and to relieve the patient of his symptoms. While technicaly feasible, a laryngo-pharyngo-esophagectomy as treatment with curative intent was not favoured by the patient. Intraoperatively, we discovered gross dilatation of the thoracic esophagus up to 8 cm in diameter caused by the intraluminal tumour mass (Figure 6). Via longitudinal esophagotomy, two large pedunculated tumours originating from the cervical esophagus and hypopharynx were protruded and (Figure 7) debulking of these intraluminal tumour masses was achieved with an Endo-GIA-Stapler device (Figure 8). The postoperative course was uneventful, and the patient was discharged from the hospital on day 13.
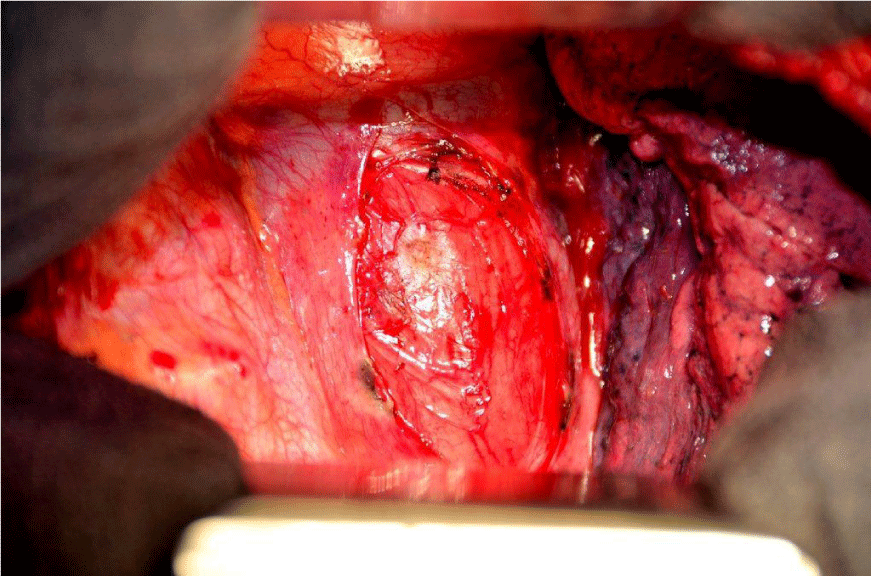 Figure 6: Right-sided thoracotomy: dilatation of the esophagus up to 8 cm in diameter due to intraluminal tumour mass.
Figure 6: Right-sided thoracotomy: dilatation of the esophagus up to 8 cm in diameter due to intraluminal tumour mass.
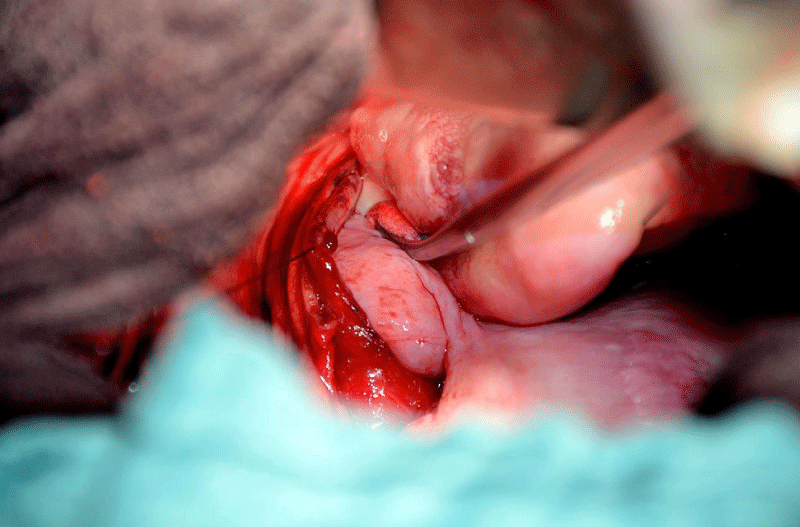 Figure 7: Right-sided thoracotomy and esophagotomy: large pedunculated tumors originating with broad base in the cervical esophagus and hypopharynx protruding out of the esophageal lumen.
Figure 7: Right-sided thoracotomy and esophagotomy: large pedunculated tumors originating with broad base in the cervical esophagus and hypopharynx protruding out of the esophageal lumen.
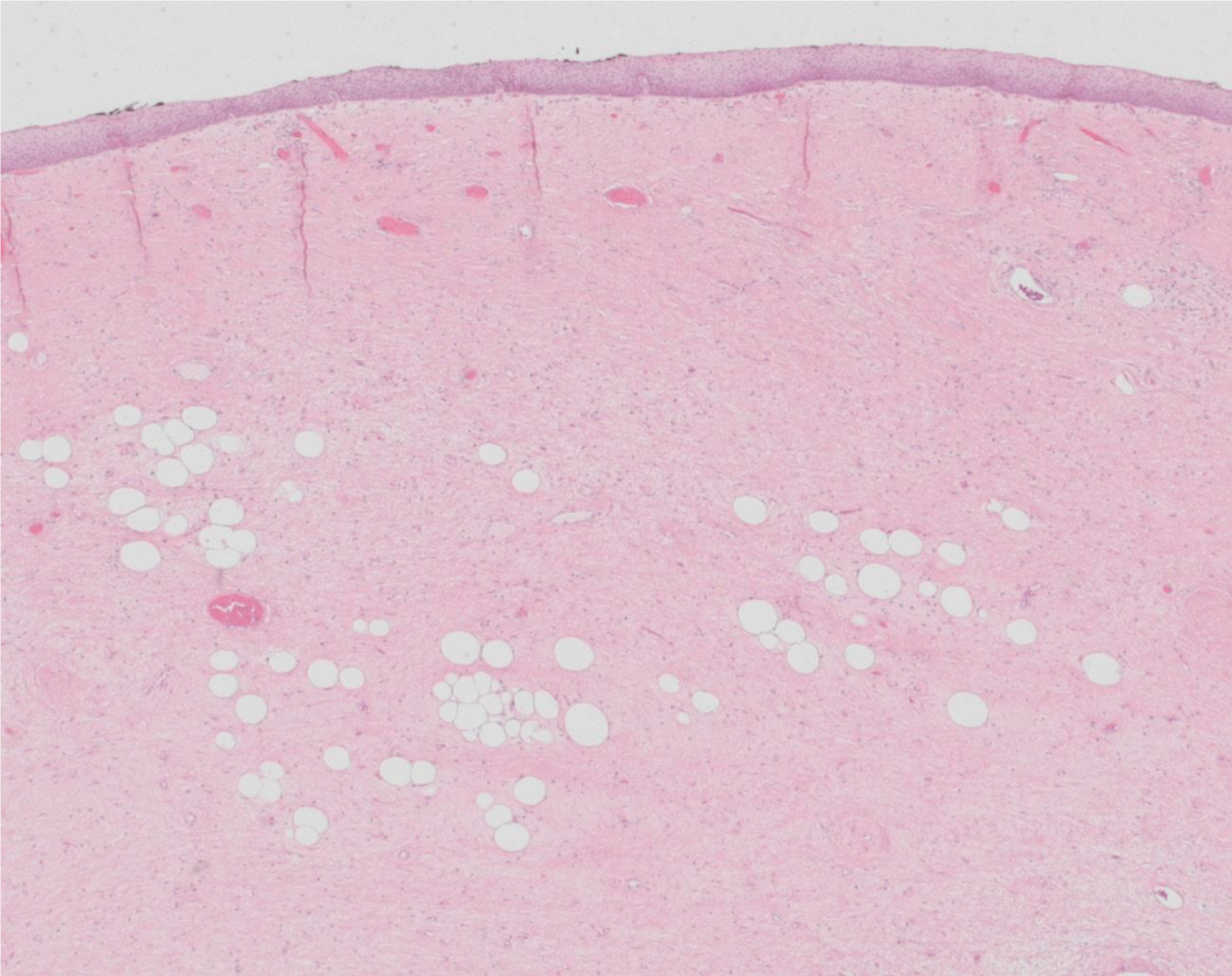
Histologically, the two resected lesions were composed of a paucicellular collagenous tissue with uniform spindle cells. In the two specimen mild nuclear atypia and scattered bizarre stromal cells associated with rare multivacuolated lipoblasts and adipocytes could be identified (Figure 9). The adipocytic component showed a significant variation in cell size with focal nuclear atypia and hyperchromasia. No mitotic activity was found. The tumour was covered by the esophageal epithelium (Figure 10). Although the fibrous component represented the majority of the lesion, the diagnosis of a well-differentiated liposarcoma was determined based on the presence of the scattered adipocytes and lipoblasts. This diagnosis was confirmed by a Fluorescence in Situ Hybridization (FISH), which revealed amplification in MDM2 (data not shown).
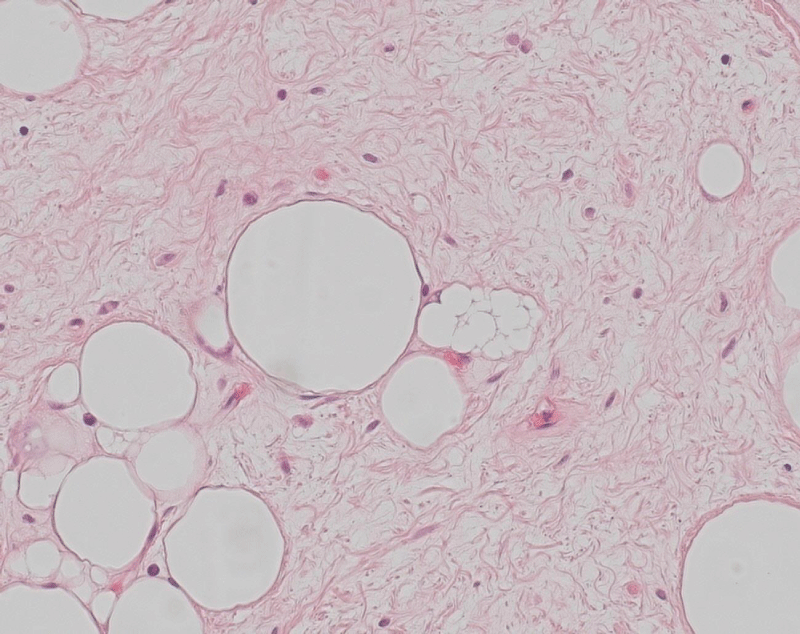 Figure 9: Tumour section (HE 20x): paucicellular collagenous tissue with uniform spindle cells with mild nuclear atypia and scattered bizarre stromal cells and associated with rare multivacuolated lipoblasts and adipocytes.
Figure 9: Tumour section (HE 20x): paucicellular collagenous tissue with uniform spindle cells with mild nuclear atypia and scattered bizarre stromal cells and associated with rare multivacuolated lipoblasts and adipocytes.
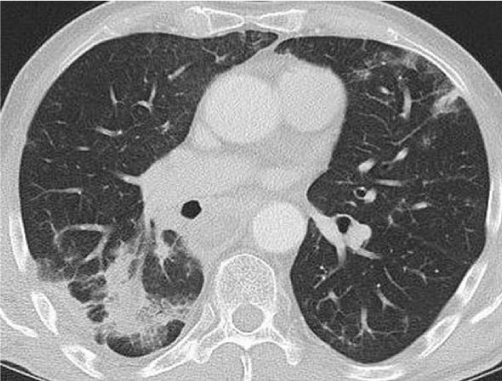 Figure 11: Follow up CT scan one year after tumour debulking and 10 months after radiation therapy: significant decrease in tumour size and pulmonal infiltrates in the right lower lobe and in the lingula, compatible with postradiogenic changes due to fibrosis.
Figure 11: Follow up CT scan one year after tumour debulking and 10 months after radiation therapy: significant decrease in tumour size and pulmonal infiltrates in the right lower lobe and in the lingula, compatible with postradiogenic changes due to fibrosis.
Postoperatively, the patient was once again discussed in the interdisciplinary UCC tumour board. Because of the patient’s preference and the extent of disease, especially given the involvement of the upper aerodigestive tract, complete resection was not favoured. Therefore, the indication for radiotherapy was presented.
The patient was simulated in supine position with arms above the head and the previously acquired contrast-enhanced CT was fused to the planning CT. The Gross Tumour Volume (GTV) was defined as macroscopic tumour apparent in contrast-enhanced CT. Clinical Target Volume (CTV) was defined as the whole esophagus including GTV plus 5 mm margin. Planning Target Volume (PTV) was created by adding a margin of 10 mm to the respective CTV. The volume of the PTV was 957.5 cc. A 15 MV 3D-conformal photon plan with 8 irradiation fields was approved, delivering 2 Gy per fraction up to 50 Gy over 5 weeks. The minimum dose and maximum dose to the PTV were 76% and 108.2% of the prescribed dose. 3, 6 cc were > 107%. The maximum dose was within the PTV. The minimum of 76% was due to air in the PTV and the proximity of the spinal cord to the PTV.
The boost volume was prescribed to the CTV only, due to the maximum tolerated dose of the spinal cord. A 15 MV 3D-conformal photon boost plan with 6 irradiation fields was approved, delivering 2 Gy per fraction up to 10 Gy over 1 week, resulting in a total dose of 60 Gy. The maximum dose to the spinal cord was 44.9 Gy and the mean lung dose was 17.9 Gy (whole lung V20Gy = 35%). Daily image guidance was performed with orthogonal kV-imaging.
After 38 Gy (4 weeks) a contrast-enhanced CT was performed to reevaluate the tumour response. No tumour regression was seen and the treatment was continued up to the approved total dose of 60 Gy.
Because the patient presented with a symptomatic esophagitis after 38 Gy, a supportive therapy with tilidin was initiated. Hereby, sufficient oral nutrition with smooth foods until the end of the radiotherapy was possible. Four days after the end of treatment, the patient presented with a deterioration of the general condition, a grade 3 dysphagia and a suspicion of bipulmonal pneumonia. An in-patient treatment with levofloxacin and dipyrone was initiated, and it was then possible for the patient to be discharged from the hospital after 10 days. A contrast-enhanced CT-scan was performed 6 weeks after the end of radiotherapy, showing radiologic signs comparable with radiation pneumonitis. The patient was in good clinical condition without clinical symptoms, therefore no specific therapy, despite respiratory exercises during rehabilitation, was initiated.
Nine months after the end of treatment the patient was presenting in good performance status (ECOG 0). A partial response was seen in a contrast-enhanced CT-scan and fibrotic changes due to the radiotherapy fields were observed in the lung parenchyma adjacent to the esophagus. An asymptomatic pericardial effusion was diagnosed and controlled by echocardiography.
A follow up one year after tumour debulking and 10 months after the radiation therapy showed a significant decrease of the tumour mass continuing to spread from the cricopharyngeal area down to the carina, but the transversal extent was reduced (from about 70 x 50 x 110 mm to 59 x 56 x 90 mm (width x depth x length ). In addition, new bipulmonal infiltrates compatible with postradiogenic changes due to fibrosis appeared, mostly localized in the right lower lobe (Figure 11). No metastatic lesions were detected. The last follow-up after 4 years displayed a stable disease in CT-scans (data not shown).
A follow up one year after tumour debulking and 10 months after the radiation therapy showed a significant decrease of the tumour mass continuing to spread from the cricopharyngeal area down to the carina, but the transversal extent was reduced (from about 70 x 50 x 110 mm to 59 x 56 x 90 mm (width x depth x length ). In addition, new bipulmonal infiltrates compatible with postradiogenic changes due to fibrosis appeared, mostly localized in the right lower lobe (Figure 11). No metastatic lesions were detected. The last follow-up after 4 years displayed a stable disease in CT-scans (data not shown).
Discussion
Here, we present the second largest esophageal liposarcoma reported to date with involvement of the upper aerodigestive tract and posterior mediastinum. After extensive diagnostics with multiple biopsies from different locations, we indicated an explorative thoracotomy with palliative tumour debulking. Only then was histological confirmation of a well-differentiated liposarcoma possible. From a surgical perspective, the option of a curative intended laryngo-pharyngo-esophagectomy as the procedure of choice was considered, however, it was favored neither by the surgical team nor by the patient due to the expectation of very poor postoperative functional results. The patient underwent radiotherapy with partial response, and a long term control of symptoms and a survival of more than 4 years could be reached.
Since Mansour et al. published the first case of a malignant pedunculated liposarcoma of the esophagus [1], a total number of only 46 cases have been reported [1-46]. The median diameter of the tumour was 12 cm (range 4 to 40 cm). The largest esophageal carcinoma (40x30x15 cm in size) [46].
The treatments performed were dependant on the tumour location, size and type of lesion as well as on the experience of endoscopists and surgeons. In selected patients, flexible or rigid endoscopic polypectomy or transoral surgical polypectomy was performed. In many cases, esophagotomy with polypectomy or wedge resection was accomplished via a cervical approach and only occasionally via a thoracotomy or laparotomy. More extensive procedures included subtotal and total esophagectomy via a one-, two- or three-hole approach. Mandell et al. reported a case of giant liposarcoma involving the upper aerodigestiv tract which was rejected by a laryngo-pharyngo-esophagectomy in 1999 [18]. One patient underwent palliative tumour debulking. Adjuvant radiation [47], neoadjuvant radiotherapy [45] or adjuvant chemotherapy [44] was applied very rarely. Histologically, most of the liposarcomas were surrounded by benign fibrolipomatous of fibrovascular tissue suggesting that sarcomatous transformation may occur within the primary benign submucosal tumour and may finally result in excessive tumour growth and infiltration. Pathological tumour grading was assessed as being low in most patients. Survival of more than 3 years was reported only in patients with “small” and completely resected tumours [42, 48]. A recurrence may develop even after decades [49]. However, few reports provided long-term follow-up information and therefore, comparative data concerning control of symptoms and survival are rare.
Conclusion
Although recurrent deep biopsies are not always sufficient for histological confirmation, malignancy has to be considered in accordance with the endoscopic and radiological findings. Therefore, complete resection of these sarcomas with clear resection margins should be aimed for histological examination. For very advanced tumours, tumour debulking combined with radiation is an ideal option, which results in symptom control and long-term survival.
Compliance with Ethical Guidelines and Conflict of Interest
S. Pistorius, D. Küpper, N. Kiria, J. Pablik, and C. Jentsch declare that they have no competing interests.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, in its most recently amended version. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
The authors state the accompanying manuscript is in compliance with ethical guidelines.
Acknowledgements
We thank Ursula Wehrmann M.D. for performing the flexible esophagoscopy and providing the esophagoscopic figure, Silke Braun M.D. for the bronchoscopy and the bronchoscopic figure and Marc Eisele for taking and providing the intraoperative and specimen photos.
In addition, we thank Marcus Neudert M.D. who carried out the rigid hypopharyngo-, microlaryngo- and esophagoscopies, Bettina Beuthien-Baumann M.D. who performed the PET and Mrs. Weber for editing the manuscript.
References
- Mansour KA, Fritz RC, Jacobs DM, Vellios F. Pedunculated liposarcoma of the esophagus: a first case report. J Thorac Cardiovasc Surg. 1983; 86: 447-450. https://goo.gl/aaDiCg
- Aloraini A, Nahal A, Ferri LE. Transoral endoscopic resection of esophageal liposarcoma. Ann Thorac Surg. 2012; 94: 121-122. https://goo.gl/yhaops
- Arteaga-Gonzalez I, Fernandez EL, Higa K. A minimal invasive approach of esophageal liposarcoma. J Laparoendosc Adv Surg Tech A. 2008; 18: 266-270. https://goo.gl/5eHTr6
- Baca I, Klempa I, Weber JT. Liposarcoma of the esophagus. Eur J Surg Onco. 1991; 17: 313-315. https://goo.gl/6Eem1N
- Bak YT, Kim JH, Kim JG, Lee CH, Lee KN, Choi YH, et al. Liposarcoma arising in a giant lipomatous polyp of the esophagus. Korean J Intern Med. 1989; 4: 86-89. https://goo.gl/Rc7CRQ
- Beaudoin A, Journet C, Watier A, Mongeau CJ, Chagnon M, Beaudry R. Giant liposarcoma of the esophagus. Can J Gastroenterol. 2002; 16: 377-379. https://goo.gl/p56YFb
- Boggi U, Viacava P, Naccarato AG, Giulianotti PC, di Candio G, Battolla L, et al. Giant pedunculated liposarcomas of the esophagus: literature review and case report. Hepatogastroenterology. 1997; 44: 398-407. https://goo.gl/U6BiVP
- Brehant O, Pessaux P, Hennekinne-Mucci S, Barriere E, D'Aubigny N, Aube C, et al. Giant pedunculated liposarcoma of the esophagus. J Am Coll Surg. 2004; 198: 320-321. https://goo.gl/rHvYqF
- Chung JJ, Kim MJ, Kim JH, Lee JT, Yoo HS, Kim KW. Imaging findings of giant liposarcoma of the esophagus. Yonsei Med J. 2003; 44: 715-718. https://goo.gl/62YzRJ
- Cooper GJ, Boucher NR, Smith JH, Thorpe JA. Liposarcoma of the esophagus. Ann Thorac Surg. 1991; 51: 1012-1013. https://goo.gl/3NxUit
- Czekajska-Chehab E, Tomaszewska M, Drop A, Dabrowski A, Skomra D, Orłowski T, et al. Liposarcoma of the esophagus: case report and literature review. Med Sci Monit. 2009; 15: 123-127. https://goo.gl/dEMxKC
- Di Mascio L, Gamble L, Wajed S, Winslet M. Intussuscepting giant liposarcoma of the oesophagus. J Postgrad Med. 2006; 52: 231-232. https://goo.gl/nbyPtV
- Garcia M, Buitrago E, Bejarano PA, Casillas J. Large esophageal liposarcoma: a case report and review of the literature. Arch Pathol Lab Med. 2004; 128: 922-925. https://goo.gl/LXcQ5y
- Ginai AZ, Halfhide BC, Dees J, Zondervan PE, Klooswijk AI, Knegt PP. Giant esophageal polyp: a clinical and radiological entity with variable histology. Eur Radiol. 1998; 8: 264-269. https://goo.gl/sv8quF
- Hasanabadi MS, Amiri M, Tajedini A, Yazdi AK, Heidarali M, Amali A, et al. Huge myxoid liposarcoma of the esophagus: a case report. Acta Med Iran. 2011; 49: 118-121. https://goo.gl/tDxCJ8
- Jakowski JD, Wakely PE, Jr. Rhabdomyomatous well-differentiated liposarcoma arising in giant fibrovascular polyp of the esophagus. Ann Diagn Pathol. 2009; 13: 263-268. https://goo.gl/JXDVQB
- Liakakos TD, Troupis TG, Tzathas C, Spirou K, Nikolaou I, Ladas S, et al. Primary liposarcoma of esophagus: a case report. World J Gastroenterol. 2006; 12: 1149-1152. https://goo.gl/y5zJCM
- Mandell DL, Brandwein MS, Woo P, Som PM, Biller HF, Urken ML. Upper aerodigestive tract liposarcoma: report on four cases and literature review. Laryngoscope. 1999; 109: 1245-1252. https://goo.gl/E595gG
- Mansour KA, Fritz RC, Jacobs DM, Vellios F. Pedunculated liposarcoma of the esophagus: a first case report. J Thorac Cardiovasc Surg. 1983; 86: 447-450. https://goo.gl/CQL1SL
- Maruyama K, Motoyama S, Okuyama M, Sasaki K, Sato Y, Hayashi K, et al. Cervical approach for resection of a pedunculated giant atypical lipomatous tumor of the esophagus. Surg Today. 2007; 37: 173-5. https://goo.gl/ACgSXM
- Masumori K, Yoshioka M, Tanaka Y, Uchida E, Higuchi K, Maeda S, et al. [A case report of esophageal liposarcoma]. Nihon Geka Gakkai Zasshi. 1991; 92: 885-888. https://goo.gl/EyS2ah
- Mica L, Gianom D, Bode B, Jaklin P, Hollinger A. Rare cause of dysphagy: giant polypoid esophageal well-differentiated liposarcoma. Case Rep Gastroenterol. 2007; 1: 7-14. https://goo.gl/qdKVNz
- Moretti R, Cimino F, Vacca U, Rubinato L. Giant low-grade liposarcoma of distal esophagus. Eur J Cardiothorac Surg. 2011; 39: 795. https://goo.gl/TFoN8C
- Myung JK, Jeong JB, Han D, et al. Well-differentiated liposarcoma of the oesophagus: clinicopathological, immunohistochemical and array CGH analysis. Pathol Oncol Res. 2011; 17: 415-420. https://goo.gl/nMzdbX
- Nakazawa T, Kondo T, Niu D, Ma D, Mochizuki K, Kawasaki T, et al. Giant oesophageal liposarcoma mimicking spindle cell liposarcoma and containing eosinophilic cells with rhabdomyoblastic differentiation. J Clin Pathol. 2010; 63: 469. https://goo.gl/dWWmfo
- Ramacciato G, Nigri G, Valabrega S. Clinical Challenges and Images in GI: image 2. A very rare case of dysphagia. Gastroenterology. 2008; 135: 742, 1019. https://goo.gl/YmNePE
- Ruppert-Kohlmayr AJ, Raith J, Friedrich G, Regauer S, Preidler KW, Szolar DH. Giant liposarcoma of the esophagus: radiological findings. J Thorac Imaging. 1999; 14: 316-319. https://goo.gl/ncqFsd
- Sakamaki Y, Miyoshi S, Minami M, Tanaka H, Inada K, Matsuda H. Mediastinal liposarcoma appearing as a tumor arising in the esophageal wall. Jpn J Thorac Cardiovasc Surg. 2001; 49: 679-681. https://goo.gl/F1FPto
- Salis GB, Albertengo JC, Bruno M, Palau G, Gonzalez Villaveiran R, et al. Pedunculated liposarcoma of the esophagus. Dis Esophagus. 1998; 11: 68-71. https://goo.gl/XyaDqc
- Smith MA, Kluck E, Jagannath S, Yang SC. Giant multi-polypoid liposarcoma of the esophagus: an atypical presentation. Ann Thorac Surg. 2010; 89: 610-612. https://goo.gl/FmWNJr
- Sui X, Li Y, Zhao H, Wang J. Giant liposarcoma of the esophagus with Li-Fraumeni-like syndrome. Eur J Cardiothorac Surg. 2011; 40: 1253-1255. https://goo.gl/zgPt1Q
- Torres-Mora J, Moyer A, Topazian M, Alexander J, Wu TT, Seys A, et al. Dedifferentiated liposarcoma arising in an esophageal polyp: a case report. Case Rep Gastrointest Med. 2012; 2012: 141693. https://goo.gl/X81oX6
- Watkin E, Devouassoux-Shisheboran M, Pedeutour F, Lagarde P, Salle M, Ranchère-Vince D, et al. A first reported case of dedifferentiated liposarcoma of the esophagus with molecular diagnosis. J Gastrointest Cancer. 2011; 42: 65-67. https://goo.gl/Rkmz4X
- Will U, Lorenz P, Urban H, Meyer F. Curative endoscopic resection of a huge pedunculated esophageal liposarcoma. Endoscopy. 2007; 39: 15-6. https://goo.gl/y9PKMc
- Xu S, Xu Z, Hou Y, Tan Y. Primary pedunculated giant esophageal liposarcoma: case report. Dysphagia. 2008; 23: 327-30. https://goo.gl/wGbVyP
- Yang B, Shi PZ, Li X, Xu RJ. Well-differentiated liposarcoma of esophagus. Chin Med J (Engl). 2006; 119: 438-440. https://goo.gl/qz3gg7
- Yates SP, Collins MC. Case report: recurrent liposarcoma of the oesophagus. Clin Radiol. 1990; 42: 356-358. https://goo.gl/CT7ymY
- Brett CL, Miller DH, Jiang L, Wolfsen HC, Attia S, Hintenlang L, et al. Dedifferentiated Liposarcoma of the Esophagus: A Case Report and Selected Review of the Literature. Rare Tumors. 2016; 8: 6791. https://goo.gl/8g96nf
- Takiguchi G, Nakamura T, Otowa Y, Tomono A, Kanaji S, Oshikiri T, et al. Successful resection of giant esophageal liposarcoma by endoscopic submucosal dissection combined with surgical retrieval: a case report and literature review. Surg Case Rep. 2016; 2: 90. https://goo.gl/qTkjbp
- Lin ZC, Chang XZ, Huang XF, Zhang CL, Yu GS, Wu SY, et al. Giant liposarcoma of the esophagus: A case report. World J Gastroenterol. 2015; 21: 9827-9832. https://goo.gl/VLnLZP
- Riva G, Sensini M, Corvino A, Vittone F, Garzaro M, Pecorari G. Rare Giant Pedunculated Liposarcoma of the Hypopharynx: Case Report and Review of Literature. J Gastrointest Cancer. 2016; 47: 449-453. https://goo.gl/Fvtofr
- Valiuddin HM, Barbetta A, Mungo B, Montgomery EA, Molena D. Esophageal liposarcoma: Well-differentiated rhabdomyomatous type. World J Gastrointest Oncol. 2016; 8: 835-839. https://goo.gl/Fj9o3Z
- Yo I, Chung JW, Jeong MH, Lee JJ, An J, Kwon KA, et al. Huge liposarcoma of esophagus resected by endoscopic submucosal dissection: case report with video. Clin Endosc. 2013; 46: 297-300. https://goo.gl/gBLFHP
- Marolla A, Pardolesi A, Camplese P, Politi R, Sacco R. Giant posterior mediastinal liposarcoma invading the esophagus: a case report. Rays. 2006; 31: 17-9. https://goo.gl/FBrDaT
- Gethin-Jones TL, Evans NR, 3rd, Morse CR. Surgical management of mediastinal liposarcoma extending from hypopharynx to carina: case report. World J Surg Oncol. 2010; 8: 13. https://goo.gl/VSxuKj
- Taki K, Watanabe M, Iwagami S, Nagai Y, Iwatsuki M, Ishimoto T, et al. Giant liposarcoma of the posterior mediastinum and retroperitoneum. BMJ Case Rep. 2011; 2011. https://goo.gl/woxkKo
- Czekajska-Chehab E, Tomaszewska M, Drop A, Dabrowski A, Skomra D, Orłowski T, et al. Liposarcoma of the esophagus: case report and literature review. Med Sci Monit. 2009; 15: 123-127. https://goo.gl/eeQ7Vd
- Di Mascio L, Gamble L, Wajed S, Winslet M. Intussuscepting giant liposarcoma of the oesophagus. J Postgrad Med 2006; 52: 231-232. https://goo.gl/TqBnV8
- Beaudoin A, Journet C, Watier A, Mongeau CJ, Chagnon M, Beaudry R. Giant liposarcoma of the esophagus. Can J Gastroenterol. 2002; 16: 377-379. https://goo.gl/ztCPRG
Authors submit all Proposals and manuscripts via Electronic Form!