Anne M. Seidler1, Daniel I. Wasserman1, Yana Turkowski2*, Aldo Gonzalez Serva2 and Nellie Konnikov2
1Department of Dermatology at Tufts University School of Medicine, Boston, Massachusetts
2Department of Veterans Affairs Medical Center, Boston, Massachusetts
*Address for Correspondence:Yana Turkowski, Department of Veterans Affairs Medical Center, Boston, Massachusetts, E- mail: lyyavi@yahoo.com
Dates: Submitted: 01 June 2017; Approved: 07 June 2017; Published: 10 June 2017
Citation this article: Seidler AM, Wasserman DI, Turkowski Y, Serva AG, Konnikov N. Unusual Presentation of Amyopathic Dermatomyositis. Sci J Clin Res Dermatol. 2017;2(1): 014-017
Copyright: © 2017 Seidler AM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Our case describes an unusual presentation of dermatomyositis in a patient with ovarian carcinoma. The eruption appeared as a venous stasis like dermatitis. The temporal sequence of onset after chemotherapy administration suggested a possible drug-induced process. However, in the context of underlying ovarian carcinoma, a paraneoplastic process offered an alternative explanation for dermatomyositis.
Dermatomyositis (DM) is a rare autoimmune disorder that primarily affects skin and skeletal muscle with a prevalence of 0.6-1.0 per 100.000 people. Women are affected approximately three times as much as men [1]. Hallmark cutaneous features include heliotrope rash-a lilac discoloration of the upper eyelids, Gottron’s papules- erythematous or violaceous papules and plaques on the dorsal surfaces of the finger joints and knuckles of the hands, and macular erythemas. Cutaneous manifestations classically distributed on the face, neck, anterior chest (V sign), shoulders (Shawl sign), elbows, the knees, and malleoli [2]. Unusual findings have included acquired icthyosis [3], bleomycin-like flagellate erythema [4], papular mucinosis [5], and follicular hyperkeratosis [6], and vesiculobullous lesions [7].
Amyopathic Dermatomyositis (ADM) affects 21% of patients with dermatomyositis [8]. ADM has the same skin lesions as classic dermatomyositis for 6 months or longer, but without evidence of proximal muscle weakness or serum muscle enzyme abnormalities. In order to establish the diagnosis there should be a biopsy confirmation.
Udkoff, et al. [9] by reviewing the literature, identified 88 cases of ADM associated with malignancy. The diagnosis of cancer prior to ADM was found in 68% of patients, was coincident in 16% of patients, and followed in 16% of patients.
Classic and amyopathic dermatomyositis diagnosed patients have a 14-28% probability of developing cancer, with lung cancer being the most common in cases of classic DM and breast cancer being the most common in cases of ADM [9]. Moreover, ovarian cancer and nasopharyngeal cancer (particularly within Asian population) are the second and third most common tumors in both amyopathic and classic dermatomyositis [9]. In addition, DM-like eruptions have been associated with etoposide and cyclophosphamide chemotherapies in case reports [10]. A long-term treatment with hydroxyurea may also induce skin eruptions imitating those of DM [11].
We present a case of clinically ADM in a patient with ovarian carcinoma. Although DM in this clinical setting is well recognized, the case we present is unique and characterized by unusual distribution of cutaneous findings. The temporal sequence of the eruption which appeared after carboplatin/paclitaxel administration raised the possibility of a drug-associated DM.
Case Report
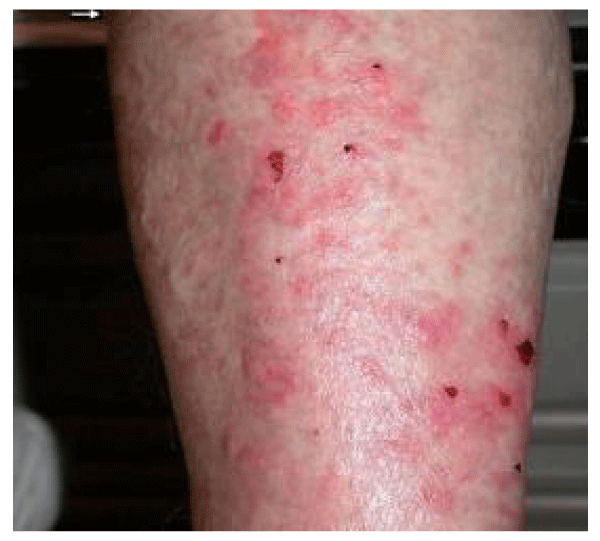
An 81-year-old Caucasian woman with underlying ovarian carcinoma, undergoing carboplatin/paclitaxel chemotherapy, presented in July 2004 with 1-month-old, bilateral, symmetric, pruritic papules, and confluent plaques confined to the gaiter area, extending bilaterally from just above the malleoli to below the knees (Figure 1). The patient had no other physical findings on the face, chest, back, hands, or feet. She stated that the skin eruption had appeared 3 days after her second treatment with carboplatin/paclitaxel. A similar rash had appeared, but spontaneously resolved within 10 days after her first chemotherapeutic treatment.
The patient had been given the diagnosis of stage IIIB ovarian carcinoma in May 2004. She was receiving intravenous infusion of paclitaxel (260 mg) and carboplatin (300 mg) every 3 to 4 weeks. When the patient reported to oncology for her third cycle of chemotherapy in July 2004, she voiced concern that the chemotherapy might exacerbate the eruption on her legs, which had persisted without improvement since her previous treatment. As a result, she was referred to dermatology for immediate consultation.
The clinical differential diagnosis included venous stasis dermatitis, contact dermatitis, or a drug eruption. A punch biopsy was performed on the left leg, and topical triamcinolone was prescribed for the presumptive diagnosis of venous stasis dermatitis. The histopathologic diagnosis was consistent with DM. Given the fact that the patient had underlying ovarian cancer, it was concluded that DM might represent a paraneoplastic syndrome. Because it was expected that the rash would likely improve with further treatment for the ovarian carcinoma, the patient received a third cycle of chemotherapy in July 2004.
Overall, the patient’s ovarian carcinoma responded to the chemotherapy treatment as evidenced by a significant reduction of the carbohydrate antigen-125 level (CA-125) and reduction of as cites and omental disease. However, the skin presentations of DM persisted without change. It was recommended that the patient receive a fourth cycle of chemotherapy, but the patient declined because of side effects of the treatment. Without any further treatment the course of the disease deteriorated. Her care was transferred to a hospice, and she passed away 3 months after her final chemotherapy treatment.
Histopathology
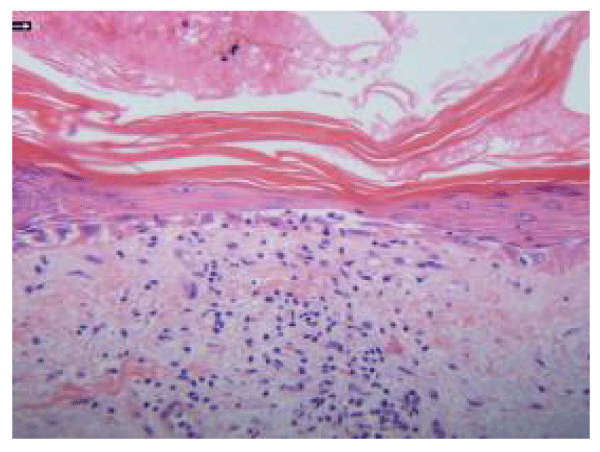
Microscopic examination of the biopsy specimen revealed confluent and early bullous interface dermatitis with vacuolar alteration of the basal cell layer (Figure 2). Epidermal atrophy, orthokeratosis, and scant perivascular and interstitial lymphocytic infiltrate in the dermis were present (Figure 2). Necrotic basal keratinocytes and squamatization were present. Venules were prominent but not increased in number or particularly dilated. Alcian blue stain produced negative results for dermal mucin deposition.
Discussion
Although the eruption was limited to the legs, histopathologic findings were consistent with DM. In addition, early bullous changes were present in the basal layer, which have been described in gynecologic malignancies [7] and may represent acute, severe disease [12]. The main histopathologic differential diagnosis of DM is early, acute, or subacute lupus erythematosus. The presence of massive interface changes (with prominent vacuolization and apoptosis of keratinocytes), the lack of deep lymphocytic infiltrate, and the absence of mucin in our patient would be unusual for lupus erythematosus. Although the presence of dermal mucin would have helped support the diagnosis of DM, its absence did not militate against it, particularly because mucin is a more constant finding in lupus.
The temporal sequence of the eruption after each of two administrations of carboplatin/paclitaxel and the spontaneous resolution of the first eruption within 10 days of a drug-free interval suggested a possible association with drug therapy. Carboplatin and paclitaxel have rarely been associated with mild cutaneous hypersensitivity reactions, but have not been associated with DM to our knowledge. However, cases of DM or DM-like syndromes have been associated with a wide range of drugs [13], including other chemotherapies, specifically hydroxyurea [11], cyclophosphamide [10] and etoposide [10]. As in our case, these cases had histopathologic findings consistent with DM, they lacked muscular weakness, and DM spontaneously resolved during a drug-free interval [10,14]. (Table 1) displays medications that have been associated with drug-induced DM by previous authors.
| Table 1: Drugs related to DM onset, described by previous authors. | ||
| Drug category | Drug name | References |
| Cytotoxic/antitumor antibiotic | Hydroxyurea | 11,14-22 |
| HMG Co-A reductase inhibitor | Atorvastatin | 23 |
| Lovastatin | 24 | |
| Pravastatin | 25 | |
| Simvastatin | 26-28 | |
| Chelator | Penicillamine | 9-34 |
| Local anesthetic (Amide) | Carticaine | 35 |
| Nonsteroidal anti-inflammatory | Niflumic acid | 36 |
| Phenylbutazone | 37 | |
| Alkylating agent | Cyclophosphamide | 10 |
| Topoisomerase inhibitor (Podophyllum) | Etoposide | 10 |
| Kinase inhibitor | Imatinib mesylate | 38 |
| Interferon | Interferon alfa-2b | 39 |
| Antidote | Ipecac | 40 |
| Proton pump inhibitor | Omeprazole | 41 |
| Anticonvulsant | Phenytoin | 42 |
| Sulfa antimicrobial | Sulphacetamide sodium 10% eye drops | 43 |
| Antimetabolite (5-fluorouracil) | Tegafur | 44 |
| Alpha-blocker | Alfuzosin | 45 |
| Tumor necrosis factor alpha inhibitor | Etanercept | 46 |
| Fibrate | Gemfibrozil | 47 |
| Vaccine | BCG vaccine | 48 |
| HMG Co-A: Hydroxymethylglutaryl-Coenzyme A. | ||
Further efforts are needed to differentiate DM in the context of malignancy from DM-like eruptions in which certain drugs may have a role.
According to Fiorentino et al. [49] anti-NXP-2 and a novel antibody, anti-p155/140, which was later identified as autoantibody to human transcriptional intermediary factor 1-gamma (TIF1-ϒ) are found in 55% of patients with cancer-associated DM. Carcinoembrionic Antigen (CEA), carbohydrate antigen-125, carbohydrate antigen 19-9, and carbohydrate antigen 15-3 could be used for the detection of solid tumors in DM [50]. Autoantibodies have a very promising role in the evaluation of risk of malignancy in patients with DM.
The cancer screening recommendations, recognition of new antibodies associated with malignancy can be offered to patients with ADM in order to make accurate diagnosis and conduct better treatment for the patients.
We emphasize the importance of clinicopathological correlation, significance of the biopsy as a most specific diagnostic tool for any dermatologic disorder of unknown cause. In our case, histopathology detected the disease which was not considered clinically. Performing a biopsy is a highly accurate and a cost-effective modality to diagnose the patients with ADM. Further research needs to be performed in order to define guidelines for cancer screening in DM.
References
- Hong MK, Lee MH, Ding DC, Chu SC, Chu TY. High grade serous ovarian carcinoma with serous tubal intraepithelial carcinoma in a case presented with atypical glandular cell favor neoplasm cervical cytology and dermatomyositis. Taiwanese Journal of Obstetrics and Gynecology. 2015; 54: 183-186. https://goo.gl/ojSqnB
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975; 292: 344-347. https://goo.gl/w6uMeC
- Inuzuka M, Tomita K, Tokura Y, Takigawa M. Acquired ichthyosis associated with dermatomyositis in a patient with hepatocellular carcinoma. Br J Dermatol. 2001; 144: 416-417. https://goo.gl/RA2gxi
- Nousari HC, Ha VT, Laman SD, Provost TT, Tausk FA. “Centripetal flagellate erythema”: a cutaneous manifestation associated with dermatomyositis. J Rheumatol. 1999; 26: 692-695. https://goo.gl/SwEnCl
- Del Pozo J, Almagro M, Martinez W, Yebra Pimentel MT, Garcia Silva J, et al. Dermatomyositis and mucinosis. Int J Dermatol. 2001; 40: 120-124. https://goo.gl/uK0DF5
- Lupton JR, Figueroa P, Berberian BJ, Sulica VI. An unusual presentation of dermatomyositis: the Wong type variant revisited. J Am Acad Dermatol. 2000; 43: 908-912. https://goo.gl/LMR6PO
- Kubo M, Sato S, Kitahara H, Tsuchida T, Tamaki K. Vesicle formation in dermatomyositis associated with gynecologic malignancies. J Am Acad Dermatol. 1996; 34: 391-394. https://goo.gl/0XYuiZ
- Bendewald MJ, Wetter DA, Li X, Davis MD. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010; 146: 26-30. https://goo.gl/GEZeHl
- Jeremy Udkoff, Cohen PR. Amyopathic Dermatomyositis: A Concise Review of Clinical Manifestations and Associated Malignancies. Am J Clin Dermatol. 2016; 17: 509-518. https://goo.gl/zxxIeE
- Ruiz Genao DP, Sanz Sánchez T, Bartolomé González B, Fernández Herrera J, García Díez A. Dermatomyositis-like reaction induced by chemotherapeutical agents. Int J Dermatol. 2002; 41: 885-887. https://goo.gl/fo2SxH
- Dacey MJ, Callen JP. Hydroxyurea-induced dermatomyositis-like eruption. J Am Acad Dermatol. 2003; 48: 439-441. https://goo.gl/JNrZqQ
- McCollough ML, Cockerell CJ. Vesiculo-bullous dermatomyositis. Am J Dermatopathol. 1998; 20:170-174. https://goo.gl/Q0phYd
- Dourmishev AL, Dourmishev LA. Dermatomyositis and drugs. Adv Exp Med Biol. 1999; 455:187-191. https://goo.gl/lTUpAv
- Senet P, Aractingi S, Porneuf M, Perrin P, Duterque M. Hydroxyurea-induced dermatomyositis-like eruption. Br J Dermatol. 1995; 133: 455-459. https://goo.gl/vJShgz
- Vélez A, López-Rubio F, Moreno JC. Chronic hydroxyurea-induced dermatomyositis-like eruption with severe dermal elastosis. Clin Exp Dermatol. 1998; 23: 94-95. https://goo.gl/ulX73Z
- Oskay T, Kutluay L, Ozyilkan O. Dermatomyositis-like eruption after long-term hydroxyurea therapy for polycythemia vera. Eur J Dermatol. 2002; 12: 586-588. https://goo.gl/s66HyJ
- Varma S, Lanigan SW. Dermatomyositis-like eruption and leg ulceration caused by hydroxyurea in a patient with psoriasis. Clin Exp Dermatol. 1999; 24: 164-166. https://goo.gl/Zu8nOC
- Rocamora V, Puig L, Alomar A. Dermatomyositis-like eruption following hydroxyurea therapy. J Eur Acad Dermatol Venereol. 2000; 14: 227-228. https://goo.gl/Dz0o6K
- Daoud MS, Gibson LE, Pittelkow MR. Hydroxyurea dermopathy: a unique lichenoid eruption complicating long term therapy with hydroxyurea. J Am Acad Dermatol. 1997; 36: 178-182. https://goo.gl/qgRd9E
- Oh ST, Lee DW, Lee JY, Cho BK. Hydroxyurea-induced melanonychia concomitant with a dermatomyositis-like eruption J Am Acad Dermatol. 2003; 49: 339-341. https://goo.gl/REIdKe
- Marie I, Joly P, Levesque H, Heron F, Courville P, Cailleux N, et al. Pseudo-dermatomyositis as a complication of hydroxyurea therapy. Clin Exp Rheumatol. 2000; 18 536-537. https://goo.gl/rNwXIR
- Bahadoran P, Castanet J, Lacour JP, PerrinC, Del Giudice P, Mannocci N, et al. Pseudo-dermatomyositis induced by long-term hydroxyurea therapy: report of two cases. Br J Dermatol. 1996; 134: 1161-1163. https://goo.gl/1pmjSb
- Noel B, Cerottini JP, Panizzon RG. Atorvastatin-induced dermatomyositis. Am J Med. 2001; 110: 670-671. https://goo.gl/C03kEH
- Rodriguez Garcia JL, Serrano Commino M. Lovastatin-associated dermatomyositis. Postgrad Med J. 1996; 72- 694. https://goo.gl/vRqwE7
- Schalke BB, Schmid B, Toyk K, Hartung HP. Pravastatin-associated inflammatory myopathy. N Engl J Med. 1992; 327: 649-650. https://goo.gl/OIhbla
- Khattak FH, Morris IM, Branford WA. Simvastatin-associated dermatomyositis. Br J Rheumatol. 1994; 33: 199. https://goo.gl/HUdpZ8
- Hill C, Zeitz C, Kirkham B. Dermatomyositis with lung involvement in a patient treated with simvastatin. Aust N Z J Med. 1995; 25: 745-746
- Vasconcelos OM, Campbell WW. Dermatomyositis-like syndrome and HMG-CoA reductase inhibitor (statin) intake. Muscle Nerve. 2004; 30: 803-807. https://goo.gl/Xa0jTZ
- Fernandes L, Swinson DR, and Hamilton EB. Dermatomyositis complicating penicillamine treatment. Ann Rheum Dis. 1977; 36: 94-95. https://goo.gl/60xa81
- Simpson NB, Golding JR. Dermatomyositis induced by penicillamine. Acta Derm Venereol. 1979; 59: 543-544. https://goo.gl/lXzHGb
- Wojnarowska F. Dermatomyositis induced by penicillamine. J R Soc Med. 1980; 73: 884-886. https://goo.gl/XdKwkE
- Carroll GJ, Will RK, Peter JB, Garlepp MJ, Dawkins RL. Penicillamine induced polymyositis and dermatomyositis. J Rheumatol. 1987; 14: 995-1001. https://goo.gl/Fc1sng
- Lund HI, Nielsen M. Penicillamine-induced dermatomyositis: a case history. Scand J Rheumatol. 1983; 12: 350-352. https://goo.gl/9Uk555
- Petersen J, Halberg P, Hojgaard K, Lyon BB, Ullman S. Penicillamine-induced polymyositis-dermatomyositis. Scand J Rheumatol. 1978; 7: 113-117. https://goo.gl/zslj86
- Rose T, Nothjunge J, Schlote W. Familial occurrence of dermatomyositis and progressive scleroderma after injection of a local anesthetic for dental treatment. Eur J Pediatr. 1985; 143: 225-228. https://goo.gl/VT0M2u
- Grob JJ, Collet AM, Bonerandi JJ. Dermatomyositis-like syndrome induced by nonsteroidal anti-inflammatory agents. Dermatologica. 1989. 178: 58-59. https://goo.gl/lFVxT2
- Curran JJ, Jamieson TW. Dermatomyositis-like syndrome associated with phenylbutazone therapy. J Rheumatol. 1987; 14: 397-398
- Kuwano A, Asahina R, Watanabe M, Fujimoto H, Ihn K. Tamaki. Heliotrope-like eruption mimicking dermatomyositis in a patient treated with imatinib mesylate for chronic myeloid leukemia. Int J Dermatol. 2006; 45: 1249-1251. https://goo.gl/kWpK6C
- Dietrich LL, Bridges AJ, Albertini MR. Dermatomyositis after interferon alpha treatment. Med Oncol. 2000; 17: 64-69. https://goo.gl/eodff5
- Bennett HS, Spiro AJ, Pollack MA, Zucker P. Ipecac-induced myopathy simulating dermatomyositis. Neurology. 1982; 32: 91-94. https://goo.gl/KwFCIL
- Pan Y, Chong AH, Williams RA, Green J, Sinclair R. Omeprazole-induced dermatomyositis. Br J Dermatol. 2006; 154: 557-558. https://goo.gl/kqZSLz
- Dimachkie MM, Vriesendorp FJ, Heck KA. Phenytoin-induced dermatomyositis: case report and literature review. J Child Neurol. 1998; 13: 577-580. https://goo.gl/HOiWAU
- Mackie BS, Mackie LE. Systemic lupus erythematosus-dermatomyositis induced by sulphacetamide eye drops. Australas J Dermatol. 1979; 20: 49-50. https://goo.gl/ZAfQ3Q
- Akiyama C, Osada A, Sou K, Yasaka N, Ohtake N, Furue M, Tamaki K. A case of dermatomyositis triggered by tegafur. Acta Derm Venereol. 1997; 77: 490. https://goo.gl/x1skPp
- Vela Casasempere P, Borras Blasco J, Navarro Ruiz A. Alfuzosin-associated dermatomyositis. Br J Rheumatol 1998; 37: 1135-1136. https://goo.gl/kny1fR
- Hall HA, Zimmermann B. Evolution of dermatomyositis during therapy with a tumor necrosis factor alpha inhibitor. Arthritis Rheum. 2006; 55: 982-984. https://goo.gl/8iNvHv
- Flores Suarez LF, Morales H, Angeles A, Kraus A. Drug-induced amyopathic dermatomyositis. J Clin Rheumatol. 2002; 8: 50-54. https://goo.gl/S32Ln3
- Kåss E, Straume S, Mellbye OJ, Munthe E, Solheim BG. Dermatomyositis associated with BCG vaccination. Scand J Rheumatol. 1979; 8: 187-191. https://goo.gl/CDZlHw
- Fiorentino DF, Chung LS, Christopher Stine L, Zaba L, Li S, Mammen AL, et al. Most Patients With Cancer-Associated Dermatomyositis Have Antibodies to Nuclear Matrix Protein NXP-2 or Transcription Intermediary Factor 1γ. Arthritis and Rheumatism. 2013; 65: 2954-2962. https://goo.gl/aWCtvq
- Tiniakou E, Mammen AL. Idiopathic Inflammatory Myopathies and Malignancy: a Comprehensive Review. Clinic Rev Allerg Immunol. 2017; 52: 20-33. https://goo.gl/t8CCiS
Authors submit all Proposals and manuscripts via Electronic Form!