Short Communication
Dietary Supplements: knowledge and Adverse Event Reporting among Emergency Medicine Physicians
Jonathan M. Scott1*, Alexandra S. Ortiz2, Selasi Attipoe MA3 and Patricia A. Deuster1
1School of Medicine, Uniformed Services University, Bethesda, Maryland
2San Antonio Military Medical Center, Joint Base San Antonio, San Antonio, Texas
3College of Public Health, The Ohio State University, Columbus, Ohio
*Address for Correspondence: Jonathan M. Scott, Department of Military and Emergency Medicine, School of Medicine, Uniformed Services University, 4301 Jones Bridge Rd, Bethesda, MD 20814, Tel: +301-295-5428; Fax: +301-295-5914; E-mail: jonathan.scott@usuhs.edu
Dates: 03 April 2018; Approved: 11 April 2018; Published: 15 April 2018
Citation this article: Scott JM, Ortiz AS, Selasi Attipoe MA, Deuster PA. Dietary Supplements: knowledge and Adverse Event Reporting among Emergency Medicine Physicians. American J Emerg Crit Care Med. 2018;1(1): 001-004.
Copyright: © Scott JM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Dietary supplement; Adverse events; Public health; Physicians
Abstract
Background: The Food and Drug Administration relies on adverse event reports linked with health risks to remove potentially harmful dietary supplements from the market. Many emergency medicine physicians encounter suspected adverse events related to dietary supplement use but we do not know what proportion of those adverse events are reported to the Food and Drug Administration. The objective of the study was to determine emergency medicine physicians’ practices regarding adverse event reporting and knowledge of dietary supplements.
Methods: A prospective, cross-sectional study was conducted across five medical centers around the U.S: three military and two civilian. A web-based survey was distributed to emergency medicine attending physicians and emergency medicine residents. The questionnaire was created and administered using Lime Survey software. An administrator at each site communicated study details to emergency medicine physicians and residents via email. The survey was kept open for five months. To preserve participant anonymity, neither email domains, email addresses, Internet Protocol addresses, nor any other personally identifiable or demographic information were collected.
Results: A total of 90 (54 attending physicians and 36 residents) individuals completed the survey. The majority, 73 (81%), of respondents had encountered an adverse event associated with dietary supplement use. A mere, 16 (18%) reported the adverse event to any source, including only one physician reporting the adverse event to the Food and Drug Administration. The majority of respondents did not know where 76 (84%) or how 76 (84%) to report adverse events.
Conclusion: Emergency medicine physicians are likely to encounter patients with suspected adverse events associated with dietary supplements. There is a critical need to ensure physicians known how and where to report suspected adverse events. Addressing these needs could impact both patient care and public safety.
Abbreviations
AE: Adverse Events; DoD: Department of Defense; DS: Dietary Supplements; EM: Emergency Medicine; FDA: Food and Drug Administration; OPSS: Operation Supplement Safety
Introduction
Dietary Supplements (DS) marketed for weight-loss, bodybuilding, energy-boosting, and other products may pose a public health risk [1-4]. Currently, the Food and Drug Administration (FDA) requires a sufficient number of Adverse Events (AE) linked with negative health effects to remove DS from the market. Importantly, how many AE are being reported to the FDA and are they sufficient to generate a signal to remove products?
We previously reported that over 60% of military and sports medicine physicians have encountered AE from dietary supplements, [5,6] yet less than 32% know where or how to report them. Importantly, less than 1% [5] and 4% [6] of those who do report, report them to the FDA. This raises the question “how can signals be detected if the FDA does not receive the requisite reports?”
In 2015 Geller et al. [7] reported that DS were responsible for more than 23,000 visits to emergency departments each year in the U.S. This suggests that Emergency Medicine (EM) physicians regularly encounter patients with AE associated with DS [7]. One important question is “what do EM physicians know about DS and AE reporting?” The purpose of this study was to examine EM physicians’ knowledge of DS and practices regarding AE reporting. A survey similar to that used by Cellini [5] and Pascale [6] was implemented with minor modifications to improve readability.
Methods
This cross-sectional study was approved by the Uniformed Services University Institutional Review Board. Prospective EM departments were contacted regarding their interest in participation. The web-based survey was distributed to EM physicians and residents at five medical centers around the U.S: three military and two civilian. Although not nationally representative, the sample demonstrates geographic and demographic (military v. civilian) diversity. All EM physicians and residents at those centers received an e-mail with a link to participate in the survey. The questionnaire was created and administered using LimeSurvey (LimeService, Hamburg, Germany) software.
An administrator at each site communicated study details to EM physicians and residents via email. The initial email introduced the study, stated its purpose, invited the recipient to participate, and provided a link to the survey, which was kept open for five months. Over the course of five months, up to two reminders were sent. To preserve participant anonymity, neither email domains, email addresses, Internet Protocol addresses, nor any other personally identifiable or demographic information were collected.
The primary outcome of this study was AE reporting behaviors among EM physicians and residents. Other outcomes include self-reported knowledge about DS and physician-patient communication about DS.
Frequency distributions for group characterization and chi-square analyses were used to assess associations between professional status (physician or resident) and outcome variables. Statistical analyses were performed using IBM SPSS 20.0 for Windows (SPSS Inc., Chicago, IL., USA).
Results
The study questionnaire and responses can be found in the eMethods. 90 complete responses (90/354, 25%) were received from the five EM departments surveyed. Overall, 36 (40%) of respondents were residents and 54 (60%) attending physicians.
Figure 1 provides the frequency of responses to questions about DS: whether they a) routinely asked patients about DS, b) had a reliable source for information for DS, c) had observed a suspected AE from a DS, and d) had observed and reported a suspected AE from a DS for attending physicians and residents. Performance-enhancing and weight-loss were the most frequently encountered DS.
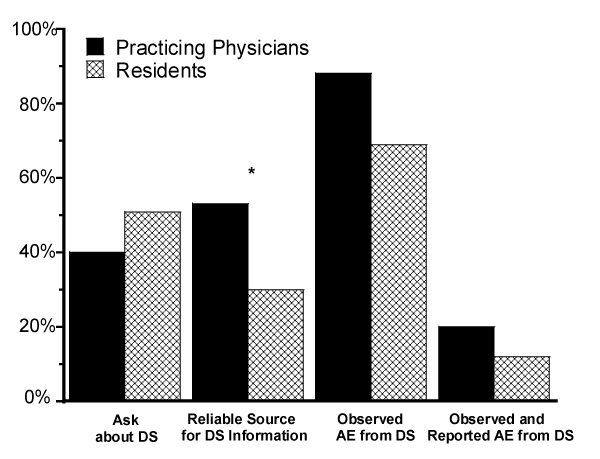 Figure 1: Dietary supplement adverse event reporting practices of EM residents vs. practicing EM physicians.
Dietary supplement Adverse Event (AE) reporting practices of EM residents (n = 36) vs. practicing EM physicians (n = 54) (*P < 0.05).
Figure 1: Dietary supplement adverse event reporting practices of EM residents vs. practicing EM physicians.
Dietary supplement Adverse Event (AE) reporting practices of EM residents (n = 36) vs. practicing EM physicians (n = 54) (*P < 0.05).
The majority, 73 (81%), of respondents had encountered an AE associated with DS use, while only 16 (18%) reported the AE to any source. AE were reported to various sources: poison control 8 (62%), hospital databases 7 (54%), or another specialist 4 (31%); only one physician reported the AE to the FDA. The majority of respondents did not know where 76 (84%) or how 76 (84%) to report AE. Only 7 (8%) reported they had some training in reporting AE.
Approximately 39 (43%) of respondents reported receiving training on DS and a similar proportion 37 (41%) routinely ask patients about DS use. However, only 18 (20%) received training on how to interview patients about DS use and 12 (13%) on how to counsel patients on appropriate selection and use of DS. Most, 47 (52%), reported not feeling confident answering patients’ questions about DS. Only 40 (44%) reported having a reliable source of information about DS.
Chi-square analysis revealed no significant associations between professional status and encounters with or reporting of AE, nor with asking patients about their use of DS. However, significantly more (P < 0.05) attending physicians than residents indicated they had a reliable source for DS information (Figure 1).
Discussion
EM physicians are likely to encounter patients who experience AE associated with DS use. The present study suggests that EM physicians could benefit from education about DS and training on how and where to report suspected AE from DS.
Previous studies have established that healthcare providers have significant knowledge gaps about the risks, benefits, and regulation of DS [5,6,8]. Because the FDA relies on AE reports to guide investigations, detect signals, and remove harmful DS from the market, the low rate of AE reporting to the FDA is alarming. Interestingly, between 2008 and 2011, only 29% of the 6,307 AE related to DS reported to the FDA came from sources other than manufacturers [9]. The low AE reporting rates by healthcare professionals highlight a deficiency in the signal detection system.
Perhaps the most important factors in improving this knowledge gap and the attendant under-reporting trend are provider training and a simple workflow solution to reporting. Previous research has shown that physicians who have confidence and knowledge about DS are more likely to discuss the topic with patients [10,11]. Many of the EM physicians reported not having received any training on many important issues related to DS. Even physicians who reported training in DS lacked education around the importance of and specific procedures for reporting AE to the FDA. Nearly all respondents stated they would benefit from additional training on DS, including AE reporting procedures, which is consistent with previous findings [11].
Several organizations, including FDA and the Department of Defense (DoD), have begun developing potential solutions to the knowledge and training gaps, but these solutions are far from complete. FDA’s Safety Reporting Portal provides a centralized location for anonymously reporting AE associated with DS use, but any process beyond the normal work flow is tedious for busy providers and the reported data must be shared [11]. The DoD provides resources for health care providers, the military community, leaders, and DoD civilians through Operation Supplement Safety (OPSS), the “go-to” website for DS. Other websites, such as NIH’s Office of Dietary Supplements and Natural Medicines, provide information about individual DS, but some require a subscription and are not well known. Reliable sources of information on DS are needed for all providers.
In summary, EM physicians are likely to encounter patients with suspected AE associated with DS. Further, EM and other physicians would benefit from additional training about how and where to report suspected AE to the FDA. These findings highlight the importance of formal training about DS and AE reporting procedures. Addressing these needs could change EM practices and increase AE reporting to FDA, which impact both patient care and public safety.
Acknowledgements
Author Contributions
JM Scott and S Attipoe had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: P.A. Deuster. Acquisition of data: A. Shams and S. Attipoe. Analysis and interpretation of data: J.M. Scott and S. Attipoe. Drafting of the manuscript: J.M. Scott, A. Shams, S. Attipoe, and P.A. Deuster. Critical revision of the manuscript for important intellectual content: J.M. Scott, A. Shams, S. Attipoe, and P.A. Deuster. Statistical analysis: J.M. Scott and S. Attipoe. Obtained funding: P.A. Deuster. Administrative, technical or material support: A. Shams and S. Attipoe. Study supervision: P.A. Deuster.
Funding/Support
This work was supported by the Center Alliance for Nutrition and Dietary Supplements Research, NB91FD.
Role of the Sponsors
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer
The views expressed are those of the authors and do not reflect the official position of the Uniformed Services University, The United State Army, or the United States Department of Defense.
References
- Damin-Pernik M, Espana B, Lefebvre S, Fourel I, Caruel H, Benoit E, et al. Management of rodent populations by anticoagulant rodenticides: toward third-generation anticoagulant rodenticides. Drug Metab Dispos. 2017; 45: 160-165. https://goo.gl/3VJ4kW
- King N, Tran MH. Long-acting anticoagulant rodenticide (superwarfarin) poisoning: a review of its historical development, epidemiology, and clinical management. Transfus Med Rev. 2015; 29: 250-258. https://goo.gl/XdKKd1
- Tie JK, Stafford DW. Structural and functional insights into enzymes of the vitamin k cycle. J Thromb Haemost. 2016; 14: 236-247. https://goo.gl/BQSXq9
- Spahr JE, Maul JS, Rodgers GM. Superwarfarin poisoning: a report of two cases and review of the literature. Am J Hematol. 2007; 82: 656-660. https://goo.gl/6GiZcN
- Raymond DH, Marie MB, Giffe TJ. Hamilton & Hardy’s industrial toxicology, sixth edition [M/OL]. John Wiley & Sons, Inc. 2015: 921-922. https://goo.gl/ftJU6P
- Feinstein DL, Akpa BS, Ayee MA, Boullerne AI, Braun D, Brodsky SV, et al. The emerging threat of superwarfarins: history, detection, mechanisms, and countermeasures. Ann NY Acad Sci. 2016; 1374: 111-122. https://goo.gl/xuA8wY
- Imran M, Shafi H, Wattoo SA, Chaudhary MT, Usman HF. Analytical methods for determination of anticoagulant rodenticides in biological samples. Forensic Sci Int. 2015; 253: 94-102. https://goo.gl/u1KhF1
Authors submit all Proposals and manuscripts via Electronic Form!




























