Research Article
Heat Shock Transcription Factor (HSF) is Down-Regulated in a Drosophila Model of Parkinson's Disease
Pokrzywa M1, Pawelek K1 and Pernilla Wittung-Stafshede2*#
1Airoptic Sp. z o.o., Poznan, Poland.
2Department of Biology and Biological Engineering, Chalmers University of Technology, Gothenburg, Sweden
#Authors contributed equally
*Address for Correspondence: Pernilla Wittung-Stafshede, Department of Biology and Biological Engineering, Chalmers University of Technology, Gothenburg, Sweden, Tel: +467-660-722-83; ORCID ID: 0000-0003-1058-1964; Email: pernilla.wittung@chalmers.se
Dates: Submitted: 21 March 2018; Approved: 13 April 2018; Published: 17 April 2018
Citation this article: Pokrzywa M, Pawelek K, Wittung-Stafshede P. Heat Shock Transcription Factor (HSF) is Down-Regulated in a Drosophila Model of Parkinson's Disease. Alzheimers Parkinsons Dis Open Access. 2018;4(1): 001-005.
Copyright: © 2017 Pokrzywa M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Drosophila; Synuclein; Heat shock transcription factor; Parkinson's disease
Abstract
Alpha-Synuclein (aS) aggregation and deposition into Lewy bodies is involved in Parkinson's disease (PD) progression, but the underlying mechanisms are unclear. Therefore, animal models such as Drosophila with high genetic power are of interest. Here we show that protein levels of the fly heat shock transcription factor HSF (corresponding to HSF-1 in humans) are reduced as a function of increased human aS levels in a Drosophila PD model. This result implies that PD like Huntington's disease involves HSF-1 degradation and, moreover, that fruit flies can act as a model system for further studies dissecting the pathways connecting HSF, aS and PD.
Introduction
Neurodegenerative diseases, such as Parkinson's Disease (PD), are associated with accumulation of misfolded and aggregated proteins, resulting in neuronal dysfunction and cell death [1]. The assembly of the synaptic protein a-Synuclein (aS) to amyloid fibrils has been linked to the molecular basis of PD. aS is the major constituent protein in the amyloid aggregates found in Lewy body inclusions, which are pathological hallmarks of PD, and duplications, triplications and point-mutations in the aS gene are related to familial PD cases [2-5]. Chaperone proteins are considered the first line of defense against misfolded and aberrantly aggregated proteins. Chaperone expression in humans is primarily determined by the activation of Heat Shock Transcription Factor 1 (HSF-1), a master stress-protective transcription factor found in most organisms [6]. Several reports have linked intracellular HSF-1 loss to neurodegeneration, with Huntington's disease as the best characterized example [7,8] for which there is direct evidence of huntingtin-mediated HSF-1 degradation [9]. It appears that HSF-1 degradation is also a key part of the deleterious cascade in PD, as a recent study elegantly demonstrated HSF-1 degradation caused by aggregated aS in neuroblastoma and HEK293 cell lines [10,11]. Enhancing protein folding capacity of cells, via elevated expression of chaperone proteins, may have therapeutic potential against neurodegeneration [12].
We recently extended our biophysical work on aS amyloid formation [13-17] to mice and Drosophila models [18,19]. In comparison to mice, fly models are attractive as they have a short life cycle, very low comparative costs and allow for powerful genetic manipulations [20]. Several fly models recapitulate essential features of PD [20] upon aS over-expression [21,22] including selective and progressive loss of dopaminergic neurons [23,24]. HSF is encoded by a single-copy gene in Drosophila, and is similar to human HSF-1: it is induced by heat stress and activation involves stress-induced oligomerization that promotes DNA binding [25]. Sequence and functional features of all metazoan HSF proteins (including the Drosophila HSF and human HSF-1) are similar and include an N-terminal DNA-binding domain, followed by a long hydrophobic repeat sequence that contains the oligomerization domain, and then, in the C-terminus, a transactivation domain [26]. Notably, the C-terminal region of human HSF-1 could functionally substitute for the corresponding region of Drosophila HSF [27].
Here we investigated a putative link between HSF and human aS in a Drosophila PD model that expresses human aS. We previously used these aS-expressing flies as an in vivo PD model to test the effects of small molecule compounds known to modulate aS amyloid formation in vitro [18]. To analyze motor functions of aS-expressing flies quantitatively, and as a function of small molecule drug leads, we developed an optical automated analyzer of walking and climbing locomotor behavior of fruit-flies [28]; see also http://www.airoptic.pl/en/about-us/research-programs. From our current experiments, we find an inverse correlation between the amounts of levels of HSF and aS proteins in the fruit flies, suggesting that HSF (HSF-1 in humans) down-regulation is linked to PD development. Therefore, underlying molecular mechanisms and pathways can be investigated (and eventually screened for modulators) in this attractive and genetically powerful model organism.
Materials and Methods
Expression of WT aS (stock #8146; w[*]; P{w[+ mC] = UAS-Hsap\SNCA.F} 5B, Bloomington Drosophila Stock Center BDSC, Indiana University) and mutant A30P aS (BDSC #8147; w[*]; P{w[ + mC] = UAS-Hsap \ SNCA.A30P}40.1) was achieved with a pan-neuronal nSyb-Gal4#2-1 driver line used previously [29]. For controls, we used progeny of w1118w (BDSC #6326) or wild-type Oregon-R strain (BDSC #6361) crossed with the nSyb-GAL4. Flies were kept at 60% humidity (20°C; 12:12 h light:dark cycle, standard food) until eclosion, and at 29°C (low-melt fly food [18]) post eclosion
Protein extraction followed a modified protocol from [30]. For each analysis sample, twenty fly heads were homogenized in extraction buffer (20 mM Tris pH 7.6, 50 mM NaCl, 1% Triton X-100, protease inhibitor cocktail), vortexed and incubated on ice (30 min). After centrifugation (60 min, 15 x 1000g, 4°C), supernatants were mixed with 4x LDS Sample Buffer and DTT containing 10x Sample Reducing Agent. Pellets were re-suspended in SDS buffer (50 mM Tris pH 7.6, 5 mM EDTA, 4% SDS), vortexed and boiled (10 min). Supernatants after centrifugation (10 min, 15 x 1000g) were mixed with 4x LDS Sample Buffer and DTT, as above, boiled and frozen until use. Three different sets of 20-day old fly samples (wild-type aS-, A30P aS-expressing and control) were analyzed and data shown represent mean values ± SD. Additional samples from 10-day old flies (wild-type aS-expressing and control) were also taken.
For Western blot analyses, all protein samples were acetone-precipitated (ThermoFisher Scientific, TR0049.1) and re-suspended in SDS buffer. Protein concentration was estimated with Pierce Microplate BCA-RAC Protein Assay Kit. After 20 min boiling, proteins (4,5 µg/lane) were resolved on NuPAGE® Novex® 4-12% Bis-Tris Protein Gels in MES-SDS running buffer and blotted onto nitrocellulose membrane using iBlot2 gel transfer device. Primary antibodies used were mouse monoclonal against a-tubulin (1:5000, clone B-5-1-2, Life Technologies), rabbit polyclonal against human aS 1:1000 (AlexoTech AB, Sweden) and rabbit polyclonal against HSF 1:1000 (Dr C. Wu) [31]. Detection was performed with Western Breeze Chromogenic kit anti-mouse or anti-rabbit, respectively. HSF and aS levels were quantified using Gel-Doc XR+ Imager and Image Lab 5.2 software (Bio-Rad). Recombinant, human wild-type aS standards (AlexoTech AB) were used.
Graphs and statistical data analysis were generated with IBM SPSS 20 Statistics (IBM Corporation, Armonk, NY). Statistical significance was determined by General Linear Model multivariate analysis of variance (Multivariate GLM, also known as MANOVA), followed by Fisher's post hoc. The mean difference was considered to be statistically significant at the 95% confidence level. Final figures were assembled with Adobe Photoshop and Illustrator CC 2015.5 (Adobe Systems, San Jose, CA).
Results and Discussion
To ensure robust expression of human aS, we used a Neural Synaptobrevin Promoter (nSyb-GAL4), a type that was previously shown to yield about 60% increased aS levels compared to the broadly used elav-GAL4 neuronal promoter [18,21]. As previously reported, pan-neuronal expression of aS accelerates climbing deficits normally seen later in life in control flies. This premature locomotor decline has been associated with intracellular accumulation of aS and the specific loss of dopaminergic neurons [20,32]. Longevity, on the other hand, was shown to be insensitive to aS expression in flies and, for flies raised at our conditions, the median life time is about 27 days [20,32].
Next, we took advantage of these aS-expressing fruit flies to assess for a putative link between HSF and aS protein levels. Using Western blot analysis, we first confirmed that we can detect HSF protein in normal flies using a polyclonal antibody that was a kind gift from Dr. Wu [33].The electrophoresis band pattern for HSF (detected with this antibody) was similar to previously reported [33] and, for quantitative analysis, we normalized HSF bands to tubulin. Next, we selected the time point of 20-day old flies for comparison of protein levels, as it is close to the median life span of the flies. When we analyzed levels of HSF (Figure 1A) and aS (Figure 1B) in protein extracts from fly heads of 20-day old flies over-expressing either wild-type or the disease-causing mutant A30P aS, a strong reduction of HSF amount paralleled increased levels of aS compared to control aS non-expressing flies (Figure 1C).
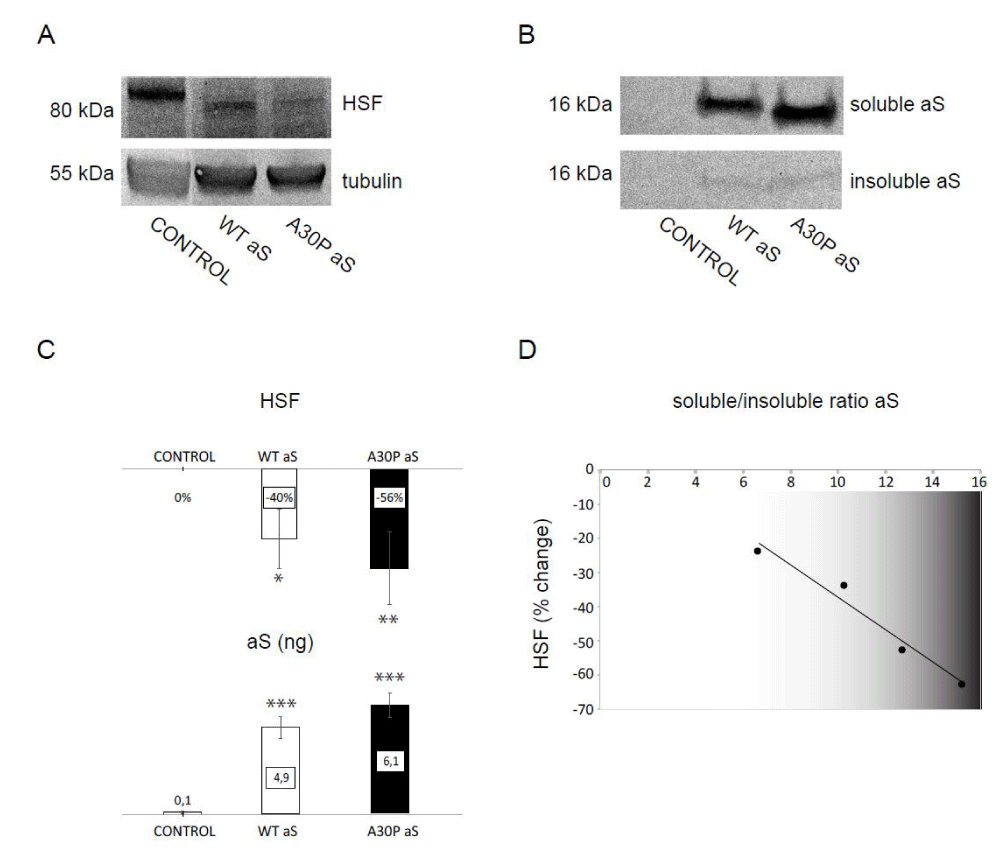 Figure 1:Reduction of HSF levels in a fly PD model
A: Immunoblot assay showing the expression levels of HSF versus tubulin levels in head protein extracts from control, wild-type aS- (WT aS) and A30P aS-expressing (A30P aS) flies
B: Western blot of aS detected in soluble and insoluble fractions of fly head protein extracts from control, WT aS- and A30P aS-expressing flies
C: Changes in HSF levels relative to control in aS-expressing either WT or A30P aS flies at 20 days (top) with corresponding changes in total aS levels (quantified in ng). Quantification of the western blot images was done by densitometry. Recombinant human aS standards (at two concentrations 1,25 and 2,5 ng per lane) were used to quantify aS levels in both fractions of fly protein extracts. Bars represent mean values ±SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001
D: Change in HSF level in WT and A30P aS-expressing flies relative to control, aS non-expressing flies (y axis) versus soluble/insoluble aS ratio (x-axis) detected in 20 day old flies analyzed with linear regression. A strong, negative correlation was found (R2 = 0.96) between levels of HSF and aS solubility.
Figure 1:Reduction of HSF levels in a fly PD model
A: Immunoblot assay showing the expression levels of HSF versus tubulin levels in head protein extracts from control, wild-type aS- (WT aS) and A30P aS-expressing (A30P aS) flies
B: Western blot of aS detected in soluble and insoluble fractions of fly head protein extracts from control, WT aS- and A30P aS-expressing flies
C: Changes in HSF levels relative to control in aS-expressing either WT or A30P aS flies at 20 days (top) with corresponding changes in total aS levels (quantified in ng). Quantification of the western blot images was done by densitometry. Recombinant human aS standards (at two concentrations 1,25 and 2,5 ng per lane) were used to quantify aS levels in both fractions of fly protein extracts. Bars represent mean values ±SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001
D: Change in HSF level in WT and A30P aS-expressing flies relative to control, aS non-expressing flies (y axis) versus soluble/insoluble aS ratio (x-axis) detected in 20 day old flies analyzed with linear regression. A strong, negative correlation was found (R2 = 0.96) between levels of HSF and aS solubility.
The total aS amount is the sum of soluble and insoluble fractions (using Triton to solubilize proteins), with the majority (around 90%) of aS appearing in the soluble fraction (Figure 1B). Although total aS roughly correlates inversely with the level of HSF (i.e., high aS means low HSF; Figure 1C), a negative linear correlation (R2 coefficient of 0.96) is observed when change in HSF level is plotted against aS soluble/insoluble ratio for individual samples (Figure 1D). This observation implies that the higher the soluble aS fraction is detected in flies, the more extensive loss of HSF protein is observed. Thus, monomeric or oligomeric fractions of aS (not insoluble amyloids) promote the reactions that reduce HSF levels. This is of importance as aS oligomers are thought to be the most toxic species in PD [34-37]. Thus, one path to cell death in PD in human neurons may be aS-oligomer mediated reduction of HSF-1, resulting in increased sensitivity to various cellular stresses and perturbations. We speculate that this may be a common mechanism that links HSF-1 to both Huntington's disease and PD, and possible to other neurodegenerative disorders as well.
To conclude, the experimental tractability and similarity of its biological pathways to those of humans have placed fruit flies at the forefront of research on human neurodegenerative diseases [23,24]. Here we discovered that, in similarity with what was found in human cells and in mice [9,10], HSF levels are strongly reduced upon accumulation of aS in Drosophila brains. This result opens up for exploitation of the extensive genetic tool-kit offered by fruit flies to study gene products and pathways involved in HSF inactivation in PD.
Acknowledgement
Funding is acknowledged from Knut and Alice Wallenberg Foundation and Swedish Research Council. We thank Carl Wu (National Cancer Institute) for the kind gift of the HSF antibody and Dennis Thiele (Duke University) for helpful discussions.
References
- Peewee W, Klaus Sappi, Caroline M. Tanner, Glenda M. Halliday, Patrik Brundin, Jens Volkmann, et al. Parkinson disease. Nature reviews. Disease primers. 2017; 3: 17013. https://goo.gl/2pDQns
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997; 388: 839-840. https://goo.gl/LTE3U6
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson´ s disease. Science. 1997; 276: 2045-2047. https://goo.gl/oAPiBp
- Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson´ s disease: a systematic review and meta-analysis. Mov Disord. 2014; 29: 1583-1590. https://goo.gl/1V3fc2
- Breydo L, Wu JW, Uversky VN. Alpha-synuclein misfolding and Parkinson´ s disease. Biochim Biophys Acta. 2012; 1822: 261-285. https://goo.gl/rUupDo
- Cotto JJ, Morimoto RI. Stress-induced activation of the heat-shock response: cell and molecular biology of heat-shock factors. Biochem Soc Symp. 1999; 64: 105-118. https://goo.gl/CQMfdX
- Neef DW, Jaeger AM, Thiele DJ. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat Rev Drug Discov. 2011; 10: 930-944. https://goo.gl/g4BxV2
- Batista-Nascimento L, Neef DW, Liu PC, Rodrigues-Pousada C, Thiele DJ. Deciphering human heat shock transcription factor 1 regulation via post-translational modification in yeast. PloS one. 2011; 6: e15976. https://goo.gl/nttZff
- Gomez-Pastor R, Burchfiel ET, Neef DW, Jaeger AM, Cabiscol E, McKinstry SU, et al. Abnormal degradation of the neuronal stress-protective transcription factor HSF1 in Huntington´ s disease. Nature communications. 2017; 8: 14405. https://goo.gl/WPLpZR
- Kim E, Wang B, Sastry N, Masliah E, Nelson PT, Cai H, et al. NEDD4-mediated HSF1 degradation underlies alpha-synucleinopathy. Hum Mol Genet. 2016; 25: 211-222. https://goo.gl/e4CzJ2
- Gomez-Pastor R, Burchfiel ET, Thiele DJ. Regulation of heat shock transcription factors and their roles in physiology and disease. Nature reviews. Molecular cell biology. 2018; 19: 4-19. https://goo.gl/c7E316
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005; 280: 33097-33100. https://goo.gl/4EtKav
- Horvath I, Wittung-Stafshede P. Cross-talk between amyloidogenic proteins in type-2 diabetes and Parkinson´ s disease. Proc Natl Acad Sci U S A. 2016; 113: 12473-12477. https://goo.gl/fBNS7e
- Horvath I, Christoph F. Weise, Emma K. Andersson, Erik Chorell, Magnus Sellstedt, Christoffer Bengtsson, et al. Mechanisms of protein oligomerization: inhibitor of functional amyloids templates alpha-synuclein fibrillation. J. Am. Chem. Soc. 2012; 134: 3439-3444. https://goo.gl/1tCBn6
- Kiskis J, Horvath I, Wittung-Stafshede P, Rocha S. Unraveling amyloid formation paths of Parkinson´ s disease protein a-synuclein triggered by anionic vesicles. Quarterly reviews of biophysics. 2017; 50. https://goo.gl/mxpcJn
- Sharma SK, Chorell E, Steneberg P, Vernersson-Lindahl E, Edlund H, Wittung-Stafshede P. Insulin-degrading enzyme prevents alpha-synuclein fibril formation in a nonproteolytical manner. Sci Rep. 2015; 5: 12531. https://goo.gl/9eF75H
- Erik C, Emma A, Margery LE, Neha J, Anna G, Jorgen A, et al. Bacterial chaperones CsgE and CsgC differentially modulate human alpha-synuclein amyloid formation via transient contacts. PloS one. 2015; 10: https://goo.gl/LxXLj3
- Chermenina M, Chorell E, Pokrzywa M, Antti H, Almqvist F, Stromberg I, et al. Single injection of small-molecule amyloid accelerator results in cell death of nigral dopamine neurons in mice. NPJ Parkinsons Dis. 2015; 1: 15024. https://goo.gl/14MJTz
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016; 167: 1469-1480. https://goo.gl/AthQev
- Feany MB, Bender WW. A Drosophila model of Parkinson´ s disease. Nature. 2000; 404: 394-398. https://goo.gl/oFWcXu
- Chen AY, Wilburn P, Hao X, Tully T. Walking deficits and centrophobism in an alpha-synuclein fly model of Parkinson´ s disease. Genes Brain Behav. 2014; 13: 812-820. https://goo.gl/TYtyLg
- Riemensperger T, Issa AR, Pech U, Coulom H, Nguyen MV, Cassar M, et al. A single dopamine pathway underlies progressive locomotor deficits in a Drosophila model of Parkinson disease. Cell Rep. 2013; 5: 952-960. https://goo.gl/6w5pWq
- McGurk L, Berson A, Bonini NM. Drosophila as an In Vivo model for human neurodegenerative disease. Genetics. 2015; 201: 377-402. https://goo.gl/oFQTtQ
- Perrimon N, Bonini NM, Dhillon P. Fruit flies on the front line: the translational impact of Drosophila. Dis Model Mech. 2016; 9: 229-231. https://goo.gl/o55vnH
- Zhong M, Orosz A, Wu C. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Molecular cell. 1998; 2: 101-108. https://goo.gl/8vjSSa
- Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004; 9: 122-133. https://goo.gl/ZVuWBC
- Wisniewski J, Orosz A, Allada R, Wu C. The C-terminal region of Drosophila Heat Shock Factor (HSF) contains a constitutively functional transactivation domain. Nucleic acids research. 1996; 24: 367-374. https://goo.gl/ZeUxGf
- Pokrzywa M, Katarzyna P, Weronika EK, Szymon S, Erik C, Fredrik A, et al. Effects of small-molecule amyloid modulators on a Drosophila model of Parkinson´ s disease. PloS one. 2017; 12: e0184117. https://goo.gl/zqRHCr
- Iakovleva I, Afshan B, Malgorzata P, Malin W, A. Elisabeth Sauer-Eriksson, Anders O. The flavonoid luteolin, but not luteolin-7-O-glucoside, prevents a transthyretin mediated toxic response. PloS one. 2015; 10: e0128222. https://goo.gl/k8tJHW
- Auluck PK, Meulener MC, Bonini NM. Mechanisms of suppression of {alpha}-synuclein neurotoxicity by geldanamycin in drosophila. J Biol Chem. 2005; 280: 2873-2878. https://goo.gl/2eGjEH
- Zimarino V, Wilson S, Wu C. Antibody-mediated activation of drosophila heat shock factor in vitro. Science. 1990; 249: 546-549. https://goo.gl/V4WD8v
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson´ s disease. Science. 2002; 295: 865-868. https://goo.gl/AscGLT
- Westwood JT, Wu C. Activation of Drosophila heat shock factor: conformational change associated with a monomer-to-trimer transition. Mol Cell Biol. 1993; 3: 3481-3486. https://goo.gl/FvJmrG
- Deas E, Cremades N, Angelova PR, Ludtmann MH, Yao Z, Chen S, et al. Alpha-synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in Parkinson´ s disease. Antioxid Redox Signal. 2016; 1: 376-391. https://goo.gl/rPVePS
- Gajula Balija MB, Griesinger C, Herzig A, Zweckstetter M, Jackle H. Pre-fibrillar alpha-synuclein mutants cause Parkinson´ s disease-like non-motor symptoms in Drosophila. PloS one. 2011; 6: e24701. https://goo.gl/HDqYEv
- Nors PM, Vito F, Istvan H, Andreas van M, Katrine NT, Christoph Weise, et al. Direct correlation between ligand-induced alpha-synuclein oligomers and amyloid-like fibril growth. Scientific reports. 2015; 5: 10422. https://goo.gl/MC3tQ1
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, et al. In vivo demonstration that a-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011; 108: 4194-4199. https://goo.gl/Dbfe9K
Authors submit all Proposals and manuscripts via Electronic Form!




























