An Update on the Progress towards COVID-19 Medication
Satya P Gupta*
Department of Pharmaceutical Technology, Meerut Institute of Engineering Technology, Meerut-250005, India
*Address for Correspondence: Satya P Gupta, Department of Pharmaceutical Technology, Meerut Institute of Engineering Technology, Meerut-250005, India, ORCID: https://orcid.org/0000-0003-1063-443X; E-mail: spgbits@gmail.com
Submitted: 05 September 2020; Approved: 09 September 2020; Published: 17 September 2020
Citation this article: Gupta SP. An Update on the Progress towards COVID-19 Medication. American J Clin Anat Physiol. 2020;2(1): 004-0012.
Copyright: © 2020 Gupta SP. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: SARS-CoV-2; COVID-19; Drug repurposing; COVID-19 vaccine; Convalescent plasma; Immune modulators; Immune modulators; Stem cell therapy
Download Fulltext PDF
The coronavirus disease 2019 (COVID-19) is now a pandemic and has created a panic in the entire world, but despite the war-footing efforts of our scientists, there has been so far no full-proof medication for it. This article presents where we actually stand in this respect and how far we have still to go. A comprehensive coverage of the progress made in all directions as to the development of therapeutics, vaccine, antibody testing, convalescent plasma, immune modulators, stem cell therapy, etc., under one roof may be useful to speed up the finding of the solution of this disease.
Introduction
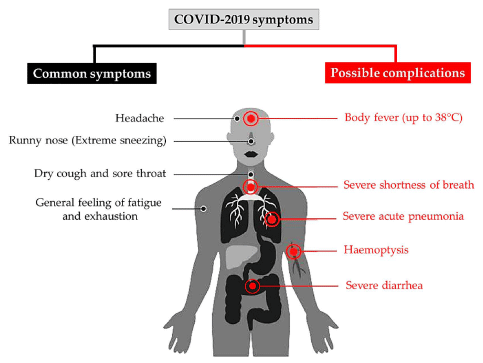
The coronavirus disease 2019 (COVID-19) is caused by a new member of the family of coronaviruses (CoVs), named as 2019-CoV-2, due to its emergence in the year 2019. Since this new virus was found to have its RNA genome about 82% similar to that of Severe Acute Respiratory Syndrome (SARS) coronavirus, it was renamed as severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2). The most common symptoms of this virus, as reported by World Health Organization (WHO) based on more than 70,000 cases in China could be beautifully represented by a schematic diagram (Fig.ure 1) [1]. In fact, the list of lingering maladies from COVID-19 is longer and more varied than what is shown in figure 1 or what most doctors could have imagined. The list also includes a racing heartbeat, achy joints, foggy thinking, a persistent loss of sense of smell, and damage to the heart, lungs, kidneys, and brain. COVID-19 may also lead to Acute Respiratory Distress (ARD)-like syndrome which is associated with severe lung condition, where fluid fills up the air sacs in your lungs, lowering the amount of oxygen or increasing the amount of carbon dioxide in your bloodstream.
However, ever since the outbreak of this virus was declared as pandemic in March 2020 by World Health Organization (WHO), the whole world is at race to win over it. The governments and pharmaceutical companies all over the world are struggling for it with the hope that they will have some solution soon. We are now only at mid-way, but let us see where we now really stand and how far we still have to go. A comprehension of movement in all directions with respect to medication of COVID-19, such as discovery of antivirals, vaccines, antibodies, etc., may shorten our path to the victory, hence this note. This article is not meant to go into the detail of structure of SARS-CoV-2, as number of publications are available on it, but its crucial structural features that constitute the important targets for the development of its medication can be mentioned [2].
In general, all coronaviruses including SARS-CoV-2 are spherical positive single-stranded RNA viruses and contain spike (S) proteins projecting from the virion surface. These spike proteins are meant to mediate the entry of CoVs into the host cells, and for SARS-CoV the receptor in the host cells has been found to be Angiotensin-Converting Enzyme 2 (ACE2). The S protein has two domains, S1 and S2, where the former has a Receptor-Binding Domain (RBD) that mediates the binding to host cell receptor. Also, there are two proteases in COVs, coronavirus main protease (3CLpro) and papain-like protease (PLpro) that mediate proteolytic cleavage of~800 kDa polypeptide, produced by coronaviruses, to generate various proteins. This processing of co-terminal polyproteins leads to components for packing new virions, after which there occurs the replication of viral genome through a replicase, RNA-dependent RNA polymerase (RdRp), encoded in the virus. Thus, the replication of CoVs involves S protein, coronavirus main protease (3CLpro), a papain-like protease (PLpro), and a replicase, RNA-dependent RNA polymerase (RdRp) [3], consequently all these four components constitute good targets for the development of therapeutics for COVID-19.
Antiviral Medication
Mutation can alter biochemical pathway of drugs metabolism because a lot of gene products of CYP450 related to drugs metabolism which involve pharmacokinetic and pharmacodynamics genes function and can lead to adverse drug reaction in human. Similar phenomena was founded on genes mutation that involved drug receptor interaction that influenced drug transport into cell target, it can rapid or slow, hence consideration about genetic factors that influenced drug metabolism is necessary.
So far antiviral medication is concerned, no new drug could be designed so far, but only drug repurposing strategies have been applied. In such trials, remdesivir, an antiviral, has drawn the greatest attention. This is a brand new drug to be given by intravenous infusion and which has shown some effect against SARS, MERS (Middle East respiratory syndrome) and Ebola in cell and animal models, and in a recent in vitro study it has been found to prevent human cells from being infected with SARS-CoV-2. Remdesivir was first developed by Gilead Sciences to only target Ebola. It inhibits viral replication through premature termination of RNA transcription. Because of its positive effect on SARS-CoV-2, FDA issued an emergency use authorization (EUA) for it on May 1, 2020, in United States, but this EUA does not mean that remdesivir is full proof medication for COVID-19, rather it simply meant that doctors may take it for hospitalized patients with severe COVID-19 symptoms. Recently a group of authors [4] conducted a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in adults hospitalized with COVID-19 with evidence of lower respiratory tract involvement to find that this drug was superior to placebo in shortening the time to recovery. However, their overall observation was that remdesivir can be used for patients who are hospitalized with COVID-19 and require supplemental oxygen therapy and that treatment with an antiviral drug alone is not likely to be sufficient. Antiviral drugs may require the combination with other medication.
The other two drugs that drew attention for the treatment of COVID-19 are hydroxychloroquine (HCQ) and chloroquine. These two medications have been used for many decades to treat malaria and autoimmune conditions like rheumatoid arthritis and lupus. Both the drugs have been found to have in vitro activity against SARS-CoV and SARS-CoV-2, with hydroxychloroquine having relatively higher potency against SARS-CoV-2. They are currently undergoing clinical studies to test their efficacy and safety in the treatment of COVID-19 in China and some promising results have been achieved thus far [5]. They have been approved by the FDA to be tested against COVID-19 [1,5]. However, on June 15, 2020, FDA has revoked the EUA to use HCQ and chloroquine to treat COVID-19 in certain hospitalized patients when no clinical trial is available or participation is not feasible. FDA took this decision on the basis that these medicines did not show any benefit for decreasing the likelihood of death or speedy recovery when subjected to a randomized clinical trial in hospitalized patients. Both the drugs are also found to have several side effects, such as worsening vision, nausea, and digestive disorders, as well as more severe cases can lead to heart failure. In a recent study [6], Mehra et al. reported that in a large multinational real-world analysis they could not find any benefit of HCQ or chloroquine (when used alone or in combination with a macrolide) on in-hospital outcomes, when initiated early after diagnosis of COVID-19, rather it was observed that there was an increased hazard for clinically significant occurrence of ventricular arrhythmias and increased risk of in-hospital death with this desease.
Next on the trial were two anti-retroviral drugs, lopinavir/ritonavir, which were found to be active against SARS-CoV [7], though designed by AbbVie to treat HIV (AIDS) under the name Kaletra. However, no benefit was observed with this drug for SARS-CoV-2 compared to standard care when tried for 99 patients with positive infections [8,9]. Recently on June 30, 2020, a RECOVERY (The Randomised Evaluation of COVID-19 Therapy) clinical trial conducted in the UK demonstrated no benefit with lopinavir-ritonavir. Although, initially WHO perceived that there may be benefits to using lopinavir/ritonavir with other drugs such as interferon-β, oseltamivir or ribavirin [10], but recently on July 4, 2020, WHO accepted the recommendation from the Solidarity Trial’s International Steering Committee to discontinue the trial’s hydroxychloroquine and lopinavir/ritonavir arms. The Solidarity Trial was established by WHO to find an effective COVID-19 treatment for hospitalized patients.
A common corticosteroid drug, dexamethasone, is also being tried for COVID-19. Dexamethasone usually has anti-inflammatory effects, but according to a recent report (June 25, 2020), The National Institutes of Health COVID-19 Treatment Guidelines Panel provides recommendations for dexamethasone in patients with COVID-19 [11]. According to an unpublished analysis, an open-label trial in United Kingdom (UK) for hospitalized patients, randomized to receive dexamethasone, had exhibited that such patients had reduced rate of mortality as compared to those who received standard of care [12]. This benefit was however observed only in patients seriously ill with COVID-19 but not in patients with milder disease. WHO welcomed these initial clinical trial results from UK, which showed that dexamethasone can be lifesaving for patients who are critically ill with COVID-19 [13].
Macrolides such as azithromycin have long been hypothesized to have therapeutic benefits on viral infections, either via their anti-inflammatory or off-target effects on viral replication. A group of researchers at University of California at San Francisco in US has started a clinical trial of a macrolide, azithromycin, to treat COVID-19. In March this year, Pfizer suggested the use of azithromycin in combination with hydroxychloroquine after a positive response in a COVID-19 trial performed in France, showing that the rate of virologic cure was highest in people who received this combination.
In addition to the above drugs discussed, there are several other drugs under trial, but currently there are no FDA-approved treatments for coronavirus. According to El-Aziz et al. [1], a drug tocilizumab, marketed as actemra to treat patients with moderate to severe rheumatoid arthritis to lower inflammation, has been approved in China for the treatment of severe complications related to SARS-CoV-2. FDA also has officially approved a phase 3 trial of actemra in severe COVID-19 patients. Darunavir, an anti-retroviral HIV-1 protease inhibitor, has also drawn the attention of Chinese researchers [5] to treat SARS-CoV-2 infection, as well as an anti-parasitic drug called ivermectin has also been observed in an in vitro study by researchers at Monash University in Melbourne, Australia, to be effective against this virus [14]. However, even though currently there is no specific anti-SARS-CoV-2 medication, a recent work of Gao et al, [15] has emphasized that all potential anti-SARS-CoV drugs may also be effective against SARS-CoV-2 as well, since 3CL proteases of both SARS-CoV-2 and SARS-CoV have very high degree of three-dimensional structural similarity [16]. The 3CL protease (3CLpro) is one of the four agents: Spike Protein (SP), coronavirus main protease (3CLpro), papain-like protease (PLpro), and RNA-dependent RNA polymerase (RdRp), that play important roles in the replication of SARS-CoV as well as SARS-CoV-2 and thus constitute good targets for the development of potent inhibitors of these viruses. Therefore, Gao et al. [15] developed a validated machine learning model, curating the largest available experimental data set for SARS-CoV-2 or SARS-CoV 3CLpro inhibitors, to find that many existing drugs might be potentially potent to SARS-CoV-2. Through this study, these authors highlighted 20 drugs from a large FDA-approved drugs as well as 20 drugs from a huge investigational or off-market drugs as highly potent medications against SARS-CoV-2 as shown in tables 1 and 2, respectively. This work of Gao et al. may be useful to develop successful therapeutics for the treatment of COVID-19.
However, some authors found that binding affinity of SARS-CoV-2 toward its receptor ACE2 is much more enhanced as compared to that of SARS-CoV and attributed this to be the hallmark for its enhanced infectivity [17]. Thus, attention should be focused to investigate highly potent inhibitors of ACE2.
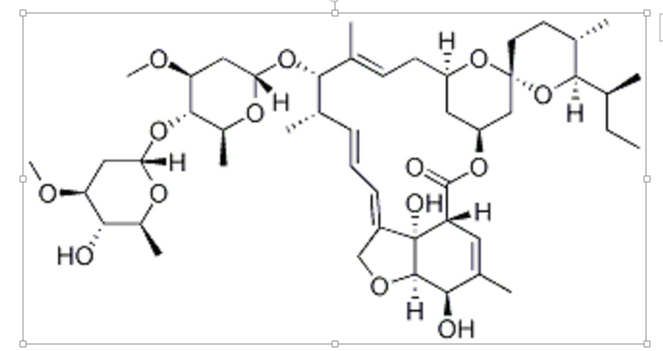
Of the above drugs discussed, ivermectin, whose structure is given below (I), has drawn special attention. In addition to Caly et al.’s report that ivermectin is a potent inhibitor of SARS-CoV-2, some other authors also have expressed their views in its favor, e.g., Sharun et al. [18] have pointed out that since ivermectin is an FDA-approved anti-parasitic drug, there is no problem in repurposing it for anti-SARS-CoV-2 therapy, but some authors as Schmith et al. [19] cautioned that its dose needs to be adjusted vis-à-vis the one used in in vitro studies by Caly et al. [14]. In fact, Schmith et al. [19] have expressed the hope that if interdisciplinary collaboration is adopted, ivermectin can achieve success, if not alone, then in combination with some other drug such as hydroxychloroquine in particular. This can lead to synergistic inhibitory effect on SARS-CoV-2, where hydroxychloroquine may inhibit the entry of the virus into the host cells and ivermectin may inhibit viral replication [20].
I, Ivermectin
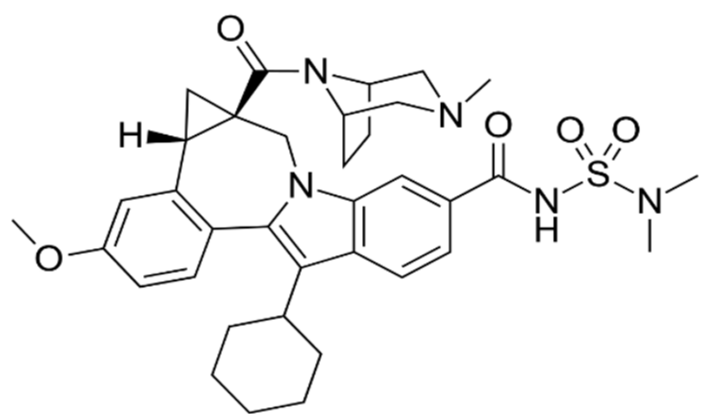
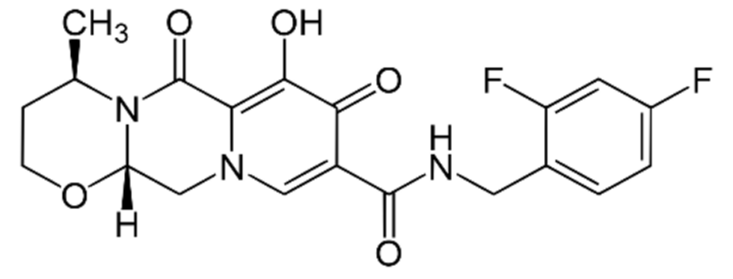
In fact, in addition to the four structural proteins mentioned previously, i.e., S protein, coronavirus main protease (3CLpro), a papain-like protease (PLpro), and a replicase, RNA-dependent RNA polymerase (RdRp), coronaviruses also have some Nonstructural Proteins (NSPs), the proteins that are encoded by a virus but do not form the part of viral particle, but play specific roles in their replication. There are 16 such NSPs, some of which are better characterized than others [21,22], such that NSP5, NSP7-10, NSP12, and NSP15-16 of SARS-CoV-2 have their structures deposited in the Protein Data Bank (PDB) with PDB IDs as 6LU7, 6M71, 7BV1, 6W4B, 6ZET, 7BV2, 6W01, and 6W4H, respectively, along with that of S protein as 6VYB [23]. In order to find the effective drugs against SARS-CoV-2 the earliest possible, Xu et al. [23] performed virtual screening using drugs from the Drug Bank, targeting some of the viral proteins and human ACE2 receptors. In their study, these authors found three drugs to be quite promising to act against SARS-CoV-2. These drugs are beclabuvir (II), bictegravir (III) and dolutegravir (IV). All these three drugs are in the category of protease inhibitors and are reported now to have strong interactions with NSP5. Out of these three, beclabuvir is antiviral drug to treat HCV infection, while the other two are meant for the treatment of HIV infection and are used in combination with other drugs. Both bictegravir and dolutegravir produce their anti-HIV effect through integrase inhibition.
II, Beclabuvir
III, Bictegravir
IV, Dolutegravir
Vaccine Development
So far the vaccine about COVID-19 is concerned, there are multiple efforts in progress to develop such a vaccine, but till today not a single vaccine has completed its clinical trials. The Coalition for Epidemic Preparedness Innovations (CEPI), which is organizing funds for the development of vaccine world-wide has indicated that a vaccine may be available under emergency use protocols in less than 12 months or by early 2021. As of July 2020, 205 vaccine candidates were in development, with 19 in human testing: one in Phase II-III interventional trial using thousands of participants, two in Phase II efficacy and dose-testing studies, four in Phase I–II safety and efficacy trials, and twelve in Phase I trials [24-26].
Because of SARS-CoV-2 pandemic, there have been some unprecedented public/private partnerships, such as Operation Warp Speed (OWS) within which US National Institutes of Health (NIH) has partnered with more than 18 biopharmaceutical companies, The COVID-19 Prevention Trials Network (COVPN) that combines clinical trial networks funded by the National Institute of Allergy and Infectious Diseases (NIAID), such as HIV Vaccine Trials Network (HVTN), HIV Prevention Trials Network (HPTN), Infectious Diseases Clinical Research Consortium (IDCRC), and the AIDS Clinical Trials Group. Under OWS, the US government has chosen three vaccine candidates to fund for Phase 3 trials: Moderna’s mRNA-1273 in July, The University of Oxford and AstraZeneca’s AZD1222 (formerly known as ChAdOx1 nCoV-19) in August, and Pfizer and BioNTech’s BNT162 in September. A COVID-19 vaccine tracker has presently (Sept 3, 2020) listed 3 more vaccine candidates currently in Phase 3 trials [27]. These vaccines are inactivated vaccine of China National Pharmaceutical Group (Sinopharm), Coronavac of Sinovac Biotech Ltd (China), and Ad5-nCoV of CanSino Biologics. In addition to these vaccines, Indian company Bharat Biotech designed a vaccine called Covaxin. It has been in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV) and has demonstrated an encouraging safety profile in a Phase I clinical trial conducted in the country. Covaxin is an inactivated vaccine candidate. It has been granted Phase 2 trials also by India’s regulatory Agency.
Strategies are being planned to bring licensing a vaccine on fast-track so that the usually lengthy duration of Phase II–III trials (typically many years) can be shortened [28-30]. Such strategies may enable to bypass typical Phase III research, provided preliminary proof of safety and efficacy of a candidate vaccine in laboratory animals and healthy humans has been established. This will accelerate the path to license a COVID-19 vaccine [28,29,31]. However, it is suspected that the rapid development and urgency of producing a vaccine for the COVID‑19 pandemic may increase the risks and failure rate of delivering a safe, effective vaccine [32,33]. A recent report from a group of scientists [34] is that they did a phase I/II single-blind, randomized controlled trial in five trial sites in the UK of a chimpanzee adenovirus-vectored vaccine (ChAdOx1 nCoV-19, now AZD1222) expressing the SARS-CoV-2 spike protein compared with a meningococcal conjugate vaccine (MenACWY) as control and found that this vaccine showed an acceptable safety profile and homologous boosting increased antibody responses. This finding encouraged the authors to go for large-scale evaluation of this candidate vaccine in an ongoing phase 3 program. The study is going on with International Standard Randomised Controlled Trial Number (ISRCTN) 15281137 and ClinicalTrials.gov, NCT04324606.
Antibody Testing
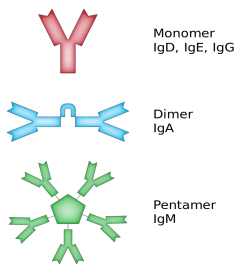
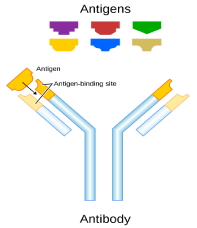
Since the emergence of SARS-CoV-2, serious attention has been paid to antibody tests to identify people who are immune to this virus. Antibodies, also known as immunoglobulins (abbreviated as Ig), are the proteins that are created by our immune system to fight infectious agents. Such proteins recognize the unique characteristics of an infectious agent. There are many types of antibodies, but two of them are most important for human immune system, IgM and IgG, where the former develops within a few days of the onset of an infection and the latter several days or weeks later. Any antibody is of Y-shape and is produced by plasma cells. Antibodies can be monomeric, dimeric, or pentameric, such as shown in figure 2 and thus while IgG is a monomer, IgM is a pentamer. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each antibody contains a paratope, also called an antigen-binding site, which is specific for one particular epitope of an antigen. An epitope, also known as antigenic determinant, is the part of an antigen that is recognized by the immune system, specifically by antibodies, B cells, or T cells. Antibody and antigen interact by spatial lock and key complementarity as shown in figure 3, involving weak and non-specific forces, such as hydrogen bonds, hydrophobic interactions, and van der Waals forces. Thus, the binding between antibody and antigen is usually reversible.
Antibody tests for COVID-19 can help identify people who were previously infected with it and have recovered. Also, antibody tests can conclusively identify people who don’t have antibodies and who are therefore susceptible to COVID-19. Now it is to be seen if antibody tests can confirm immunity to SARS-CoV-2 due to having recovered from the illness or one should wait for a vaccine. According to some immunologists, it’s likely that COVID-19 does induce some degree of immunity in those who recover from it, but this has yet to be proven. It is also not yet established if immunity to COVID-19 might last a few weeks, a few years, or lifelong. Experts believe that even extremely accurate antibody tests cannot ascertain if the person has developed true immunity and if ‘yes’, how long it will last. In fact, the immune system is very complicated.
All such discussions presented above refer to an update of July 9, 2020, by Spencer Blackman [35]. Spencer practices relationship-centered primary care, blending a traditional sensibility with up-to-date clinical knowledge and a strong focus on disease prevention.
A certain group of scientists are trying to find if antibodies against SARS-CoV-2 could be isolated and given as a treatment to others who are infected. The determination of structure and function of different antibodies can be of great help to the development of vaccines. Several potential vaccines now under development are designed to trigger the human body to produce antibodies to the SARS-CoV-2 spike proteins that protrude from the surface of SARS-CoV-2 particles. These spikes are attached to human cells and then undergo a structural change to enable viral membrane to fuse with the cell membrane. This leads the viral genes to enter the host cell to be copied and produce more viruses. Antibodies that can recognize and bind to the spike protein may hopefully block the virus from infecting human cells. The spike protein has a Receptor Binding Domain (RBD) which binds to Angiotensin-Converting Enzyme 2 (ACE2) receptors in human cells [36]. Investigators first isolated antibodies that could bind to this RBD and then put them to the test if they can neutralize SARS-CoV-2-that is, bind to the virus and stop infection [37]. Several potential vaccines now under development are designed to trigger the human body to produce antibodies to the SARS-CoV-2 spike protein.
Convalescent Plasma
The blood plasma from patients who have recovered from COVID-19 contains a high neutralizing antibody. Such patients may be a valuable donor source of Convalescent Plasma (CP) [38]. CP is a classic adaptive immunotherapy and it has been applied to the prevention and treatment of many infectious diseases since long. Therefore, on April 7, 2020, the FDA announced a National Expanded Access Protocol administered through the Mayo Clinic (https://www.uscovidplasma.org) that allows individual physicians to treat a wider range of patients with serious COVID-19 disease with convalescent plasma. This plasma therapy may be more effective if it is given to COVID-19 patients early to eliminate the virus before it causes serious damage in their lungs. Encouraged by the results of studies in China on the use of CP therapy, showing the recovery from SARS-CoV-2 infection, hospitals in New York City are heading towards the use of blood plasma of those recovering from this infection [39,40]. Multiple ongoing clinical trials are investigating the use of convalescent plasma in patients suffering from COVID-19 and positive results are being found, but these results may not be so conclusive in the favor of CP because the patients also receive simultaneously at least one additional therapy, including antivirals, antibiotics or antifungals, and corticosteroids. Thus, the use of CP therapy is an interim approach for the treatment of COVID-19, until the hyperimmune globulin, drug therapies, and vaccines are not available. However, vast majority of patients who recover from COVID-19 illness have been found to develop some level of circulating neutralizing antibodies to various SARS-CoV-2 proteins 2-3 weeks following infection, as detected by ELISA (enzyme-linked immunosorbent assay) or other quantitative assays. Nonetheless, despite some adverse effects and study limitations, the improved outcomes in recipients of CP therapy obtained in two recent studies in China [41,42] support the possibility of investigating this therapy further in adequately designed clinical trials. The CP therapy is within clinical studies or as an emergency compassionate use in the USA and EU/EEA member states [43], carried out according to EC guidance developed in collaboration with ECDC (European Centre for Disease Prevention and Control), national competent authorities and other stakeholders [44]. The EEA (European Economic Area) links the EU member states and three EFTA (European Free Trade Association) states: Iceland, Liechtenstein, and Norway. These studies indicated that CP infusion to COVID-19 patients is safe and effective [45,46], and as of May 29, 2020, 17674 units of CP were infused to COVID-19 patients in the USA [47].
Immune Modulators
In the context of COVID-19, a group of proteins, called cytokines, also drew that attention, as they are a category of signaling molecules that mediate and regulate immunity, inflammation and hematopoiesis. Cytokines are secreted by specific cells of immune system. In some people with COVID-19, the immune system goes into overdrive and releases large amounts of cytokines, and according to some scientists this may be the reason certain people with severe COVID-19 develop Acute Respiratory Distress Syndrome (ARDS) and need to be put on ventilator. Thus, some drugs that act as immune suppressants are under clinical trials to see if they can suppress the cytokine releases and reduce the severity of ARDS. Some researchers in UK claimed that the inexpensive corticosteroid dexamethasone was able to reduce deaths of about one-third of severe COVID-19 patients who were on ventilators and one-fifth of those who needed oxygen support. Other drugs that are under trial include baricitinib and IL-6 inhibitors, used for rheumatoid arthritis treatment, and CM4620-IE, used to treat pancreatic cancer. There also has been a device that can filter cytokines trusted source out of the blood of COVID-19 patients. This device has been approved by FDA.
A recent report (July 20, 2020) came from BBC news [https://www.bbc.com/news/health-53467022] that Southampton-based biotech Synairgen has tried interferon beta, a protein which the body produces when it gets a viral infection, to treat COVID-19 patients. It is expected that this protein can stimulate an immune response if inhaled directly by the patients into the lungs using a nebulizer. Synairgen claimed that with the use of interferon beta the COVID-19 patients were two to three times more likely to recover to the point where they could resume their daily routine activities and could gain very significant reductions in breathlessness, in the average time to be spent in hospital (from an average of nine days to six days), and in the requirement of ventilation (by 79%.). A double-blind trial was performed for this protein involving 101 volunteers who had been admitted for treatment at nine UK hospitals for SARS-CoV-2 infections. Half of the participants were given the drug, the other half got what is known as a placebo - an inactive substance. According to the scientist in charge of the trial, Tom Wilkinson, though the trial was small but benefits observed were unexpectedly quite strong and if the results are confirmed in a lager study, treatment may be “a game changer”. Similarly, Synairgen chief executive Richard Marsden expressed that the results observed were unexpected and may turn to a major breakthrough in the treatment of hospitalized Covid-19 patients and according to Synairgen team, the drug could be even more effective at the early stages of infection. The results of Synairgen got also the supports from other scientists, e.g., a Professor of metabolic medicine at the University of Glasgow expressed his happiness over the results and said that though the work will require a larger trial, even so the results so far obtained are very exciting. However, according to the expert in emergency medicine at the University of Sheffield, Prof Steve Goodacre, these results are not interpretable and require the full details and, perhaps more importantly, the trial protocol which should have been registered be made available before any analysis was undertaken.
Stem Cell Therapy
Mesenchymal Stem Cells (MSCs) constitute a powerful group of immunomodulatory and anti-inflammatory agents, which can normalize the function of immune system altered by COVID-19. Some companies, such as Athersys Inc. (a US based clinical-stage biotechnology company) and Mesoblast Ltd (an Australian-based regenerative medicine company) have entered into a phase II/III clinical trial that will examine whether their stem cell treatment could potentially benefit people with ARDS. The FDA has approved the start of a phase I/IIa trial assessing the use of umbilical cord mesenchymal stem cells for treating patients with severe COVID-19 [48]. China is also conducting a number of trials, which are under the strict supervision of the National Health Commission of China or the National Institutes for Food and Drug Control of China. Crossing China’s border, clinical trials of MSC therapy for COVID-19 are also being conducted by scientists from Brazil, Jordan, France, and India and it is hoped that these efforts combined with candid assessment and regulatory approval will soon provide an effective therapeutic intervention. A recent work of Zhao provided the support to use the MSC therapy for COVID-19 [49].
With all said and done, the conclusion is that there is no sure shot medication for COVID-19 and the only remedy is prevention for which WHO and other organizations have issued some basic guidelines [1], such as (i) wash the hands frequently and carefully, especially after contact with infected people or their environment; (ii) avoid touching your face including mouth, nose and eyes; (iii) cover your mouth and nose when coughing and sneezing; (iv) take social distancing seriously by keeping a distance of 6 ft from other people; and (v) self-quarantine if sick and wear a mask when you need medical care [50,51]. Also the healthcare providers and researchers have been advised to wear necessarily FFP3 (filter capacity at least 99%) or N95 (filter capacity at least 95%) masks and other protective gear when around COVID-19 patients [51].
Conclusions
So far antiviral medication is concerned for COVID-19, no new drug could be designed till today for it, but only drug repurposing strategies have been applied. So far only one drug, remdesivir, has shown some positive effect and because of which FDA issued an emergency use authorization (EUA) for it on May 1, 2020 in United States, but this EUA did not mean that remdesivir is full proof medication for COVID-19, rather it simply meant that doctors may take it for hospitalized patients with severe COVID-19 symptoms. The other two drugs that drew attention for the treatment of COVID-19 are hydroxychloroquine (HCQ) and chloroquine and were approved also by the FDA to be tested against COVID-19, but soon revoked the EUA to use these two drugs because of their several side effects, such as worsening vision, nausea, digestive disorders and risk of heart failure of more severe cases. Rather, an FDA approved anti-parasitic drug ivermectin has created a great hope to be potentially useful for the treatment of COVID-19, if not alone, then in combination with some other drug such as hydroxychloroquine in particular. However, some authors have emphasized that all potential anti-SARS-CoV agents may be effective to SARS-CoV-2 as well, since 3CL proteases of SARS-CoV-2 and SARS-CoV have a sequence identity of 96.1% and the binding-site RMSD of 0.42 Å.
So far the vaccine about COVID-19 is concerned, there are multiple efforts in progress to develop such a vaccine, but till today not a single vaccine has completed its clinical trials. However, Under Warp Speed (OWS), the US government has chosen three vaccine candidates to fund for Phase 3 trials: Modern’s mRNA-1273 in July, The University of Oxford and AstraZeneca’s AZD1222 (formerly known as ChAdOx1 nCoV-19) in August, and Pfizer and BioNTech’s BNT162 in September. However, till any vaccine is final, antibody testing is being tried to recognize unique characteristics of SARS-CoV-2. A certain group of scientists are trying to find if antibodies against SARS-CoV-2 could be isolated from the patients who have recovered from this infection and given as a treatment to others who are infected.
Convalescent Plasma (CP), a classic adaptive immunotherapy and applied to the prevention and treatment of many infectious diseases since long, is also being tried for COVID-19. FDA announced a National Expanded Access Protocol administered through the Mayo Clinic (https://www.uscovidplasma.org) that allows individual physicians to treat a wider range of patients with serious COVID-19 disease with convalescent plasma. The blood plasma from patients who have recovered from COVID-19 contains a high neutralizing antibody. Additionally, immune modulators and stem cell therapy are also on trial. A group of proteins, called cytokines, are a category of signaling molecules that mediate and regulate immunity, inflammation and hematopoiesis. For stem cell therapy, FDA has approved the start of a phase I/IIa trial assessing the use of umbilical cord mesenchymal stem cells for treating patients with severe COVID-19.
A recent communication of South et al. [52] has pointed out that since SARS-CoV-2 has not yet been fully contained and since any potential treatments or vaccine are still months away, a second wave is not impossible. However, the anecdotal reports and clinical observations suggesting a significant risk of thrombotic events, including stroke, in patients hospitalized with COVID-19, is yet to be confirmed.
- Tarek Mohamed Abd El-Aziz, James D Stockand. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)-an update on the status. Infect. Genet. Evol. 2020; 83: 104327. DOI: 10.1016/j.meegid.2020.104327
- Hossam M Ashour, Walid F Elkhatib, Md Masudur Rahman, Hatem A Elshabrawy. Insights into the recent 2019 novel coronavirus (SARS- CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020; 9: 186. DOI: 10.3390/pathogens9030186
- Xiang Xu, Yunqing Liu, Susan Weiss, Eddy Arnold, Stefan G Sarafianos, Jianping Ding. Molecular model of SARS coronavirus polymerase: implications for biochemical functions and drug design. Nucleic Acids Res. 2003; 31: 7117-7130. DOI: 10.1093/nar/gkg916
- John H Beigel, Kay M Tomashek, Lori E Dodd, Aneesh K Mehta, Barry S Zingman, Andre C Kalil, et al. Remdesivir for the treatment of Covid-19 preliminary report. N Engl J Med. 2020. DOI: 10.1056/nejmoa2007764
- Liying Dong, Shasha Hu, Jianjun Gao. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020; 14: 58-60. DOI: 10.5582/ddt.2020.01012
- Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet. 2020. DOI: 10.1016/S0140-6736(20)31180-6
- Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MML, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: A multicentre retrospective matched cohort study. Hong Kong Med J. 2003; 9: 399-406. https://pubmed.ncbi.nlm.nih.gov/14660806/
(b) Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax. 2004; 59: 252-256. DOI: 10.1136/thorax.2003.012658 - Bin Cao, Yeming Wang, Danning Wen, Wen Liu, Jingli Wang, Guohui Fan, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020; 382: 787-799. DOI: 10.1056/NEJMoa2001282
- H. Lopinavir-ritonavir in severe COVID-19. Nat Med. 2020; 26: 465. DOI: 10.1038/s41591-020-0849-9
- Elfiky, AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life
- Sci. 2020; 248: 117477. https://tinyurl.com/y5s4pedf
- https://www.covid19treatmentguidelines.nih.gov/dexamethasone/
- Randomised Evaluation of COVID-19 Therapy (RECOVERY). Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. 2020. https://tinyurl.com/ycsel489
- 16 June 2020, WHO News release. WHO welcomes preliminary results about dexamethasone use.
- Leon Caly, Julian D Druce, Mike G Catton, David A Jans, Kylie M Wagstaff. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020; 178: 104787. DOI: 10.1016/j.antiviral.2020.104787
- Kaifu Gao, Duc Duy Nguyen, Jiahui Chen, Rui Wang, Guo-Wei Wei. Repositioning of 8565 existing drugs for COVID-19. J Phys Chem Lett. 2020; 11: 5373-5382. DOI: 10.1021/acs.jpclett.0c01579
- Linlin Zhang, Daizong Lin, Xinyuanyuan Sun, Ute Curth, Christian Drosten, Lucie Sauerhering, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020; 368: 409-412. DOI: 10.1126/science.abb3405
- Angelo Spinello, Andrea Saltalamacchia, Alessandra Magistrato. Is the rigidity of SARS-CoV‑2 spike receptor-binding motif the hallmark for its enhanced infectivity? Insights from all-atom simulations. J Phys Chem Lett. 2020; 11: 4785-4790. DOI: 10.1021/acs.jpclett.0c01148
- Khan Sharun, Kuldeep Dhama, Shailesh Kumar Patel, Mamta Pathak, Ruchi Tiwari, Bhoj Raj Singh, et al. Ivermectin, a new candidate therapeutic against SARS‑CoV‑2/COVID‑19. Ann Clin Microbiol Antimicrob. 2020; 19: 23. DOI: 10.1186/s12941-020-00368-w
- Virginia D Schmith, Jie Jessie Zhou, Lauren R L Lohmer. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin Pharmacol Ther. 2020. DOI: 10.1002/cpt.1889
- Patri A, Fabbrocini G. Hydroxychloroquine and ivermectin: A synergistic combination for COVID-19 chemoprophylaxis and/or treatment? J Am Acad Dermatol. 2020. DOI: 10.1016/j.jaad.2020.04.017.
- Subissi L, Imbert I, Ferron F, Collet A, Coutard B, Decroly E, et al. SARS-CoV ORF1b-encoded nonstructural proteins 12−16: Replicative enzymes as antiviral targets. Antiviral Res. 2014; 101: 122-130. DOI: 10.1016/j.antiviral.2013.11.006
- Graham RL, Sparks JS, Eckerle LD, Sims AC, Denison MR. SARS coronavirus replicase proteins in pathogenesis. Virus Res. 2008; 133: 88-100. DOI: 10.1016/j.virusres.2007.02.017.
- Xu C, Ke Z, Liu C, Wang Z, Liu D, Zhang L, et al. Systemic in silico screening in drug discovery for coronavirus disease (COVID-19) with an online interactive web server. J Chem Inf Model. 2020; DOI: 10.1021/acs.jcim.0c00821
- Draft landscape of COVID 19 candidate vaccines. World Health Organization. 2020.
- COVID-19 vaccine development pipeline (Refresh URL to update). Vaccine Centre, London School of Hygiene and Tropical Medicine. 2020.
- COVID-19 vaccine tracker (Choose vaccines tab, apply filters to view select data). Milken Institute. 2020.
- Jeff Craven. COVID-19 vaccine tracker. 2020. https://tinyurl.com/yxy2f96s
- Eyal N, Lipsitch M, Smith PG. Human challenge studies to accelerate coronavirus vaccine licensure. J Infec Dis. 2020; 221: 1752-1756. DOI: 10.1093/infdis/jiaa152.
- Callaway E. Should scientists infect healthy people with the coronavirus to test vaccines? Nature. 2020; 580: 17. DOI: 10.1038/d41586-020-00927-3.
- Boodman E. Coronavirus vaccine clinical trial starting without usual animal data. STAT. 2020.
- Cohen J. Speed coronavirus vaccine testing by deliberately infecting volunteers? Not so fast, some scientists warn. Science. 2020. DOI: 10.1126/science.abc0006
- Diamond MS, Pierson TC. The challenges of vaccine development against a new virus during a pandemic. Cell Host Microbe. 2020; 27: 699-703. DOI: 10.1016/j.chom.2020.04.021.
- Tung TL, Zacharias A, Arun K, Raul GR, Stig T, Melanie S, et al. The COVID-19 vaccine development landscape. Nature Rev Drug Discov. 2020; 19: 305-306. DOI: 10.1038/d41573-020-00073-5.
- Folegatti PM, Ewer JJ, Aley PK. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. 2020. DOI: https://doi.org/10.1016/S0140-6736(20)31604-4
- Spencer Blackman. https://www.onemedical.com/blog/live-well/covid-19-antibody-testing
- Satya P Gupta. A Perspective of viruses and the outbreak of a novel coronavirus SARS-CoV-2. J Enz Inhib. 2020; 16: 107-114. DOI: 10.2174/1573408016999200525155302
- Reynolds S. Potent antibodies found in people recovered from COVID-19. 2020. https://tinyurl.com/y7btson6
- Chen L, Xiong J, Bao L, Yuan Shi. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020; 20: 398-400. DOI: 10.1016/S1473-3099(20)30141-9
- Kai Duan, Bende L, Cesheng L, Huajun Zhang, Ting Yu, Jieming Qu, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020; 117: 9490-9496. DOI: 10.1073/pnas.2004168117
- Jawhara S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int J Mol Sci. 2020; 21: 2272. DOI: 10.3390/ijms21072272
- Chenguang Shen, Zhaoqin Wang, Fang Zhao, Yang Yang, Jinxiu Li, Jing Yuan, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020; 323: 1582-1589. DOI: 10.1001/jama.2020.4783
- Kai Duan, Bende Liu, Cesheng Li, Huajun Zhang, Ting Yu, Jieming Qu, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: A pilot study. medRxiv. 2020. DOI: 10.1101/2020.03.16.20036145
- U S Food & Drug Administration (FDA). Investigational COVID-19 convalescent plasma - emergency INDs: FDA. 2020. https://tinyurl.com/y57as9ve
- European Commission- Directorate-General for Health and Food Safety. An EU programme of COVID-19 Convalescent Plasma Collection and Transfusion. Guidance on collection, testing, processing, storage, distribution and monitored use. 2020. https://tinyurl.com/yxqaqq2e
- Michael Joyner, Scott Wright R, DeLisa Fairweather, Jonathon Senefeld, Katelyn Bruno, Stephen Klassen, et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. medRxiv. 2020. DOI: 10.1172/JCI140200
- Sean THL, Hung ML, Ian B, Ania W, Jeffrey PG, Farah R, et al. Convalescent plasma treatment of severe COVID-19: A matched control study. medRxiv. 2020. DOI: 10.1101/2020.05.20.20102236
- COVID-19 expanded access program. Plasma donors needed for treatment protocol: Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19; 2020. https://tinyurl.com/yylwg7yq
- Zhao RC. Stem cell-based therapy for coronavirus disease 2019. Stem Cells Develop. 2020; 29: 679-681. DOI: https://doi.org/10.1089/scd.2020.0071
- Ying HJ, Lin C, Zhen SC, Hong C, Tong D, Yi PF, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Military Med Res. 2020; 7: 4. DOI: 10.1186/s40779-020-0233-6
- Ping W, Neil A, Yang P, Leo P, Carmen C, Nathan Z, et al. The SARS-CoV-2 outbreak: Diagnosis, infection prevention, and public perception. Clin Chem. 2020; 66: 644-651. DOI: 10.1093/clinchem/hvaa080
- Kieron South, Laura MC, Barry WMC, Mitchell SVE, Stuart MA, Craig JS. Preceding infection and risk of stroke: An old concept revived by the COVID-19 pandemic. Int J Stroke. 2020. DOI: 10.1177/1747493020943815

Sign up for Article Alerts