Biological Active Compound Biosynthesis in a Cu2+-Contaminated Culture Media
Anca Roxana Petrovici1*, Irina Stoica2, Irina Volf3 and Valentin I. Popa3
1“Petru Poni” Institute of Macromolecular Chemistry, Centre of Advanced Research in Bionanoconjugates and Biopolymers Department
2SC. Zeelandia SRL, R&D Department, Valea Lupului, 707410, Iasi, Romania
3“Gheorghe Asachi” Technical University of Iasi, “Cristofor Simionescu” Faculty of Chemical Engineering and Environmental Protection
*Address for Correspondence: Anca Roxana Petrovici, “Petru Poni” Institute of Macromolecular Chemistry, Centre of Advanced Research in Bionanoconjugates and Biopolymers Department, 41A Grigore Ghica-Voda Alley, 700487, Iasi, Romania, Tel: +402-322-174-54; Fax: +402-322-112-99; E-mail: petrovici.anca@icmpp.ro
Submitted: 08 November 2019; Approved: 22 November 2019; Published: 22 November 2019
Citation this article: Petrovici AR, Stoica I, Volf I, Popa VI. Biological Active Compound Biosynthesis in a Cu2+-Contaminated Culture Media. Open J Biotechnol Bioeng Res. 2019;3(1): 007-013.
Copyright: © 2019 Petrovici AR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Asclepias syriaca; Carotenoid pigments; Copper ions; Folin-ciocalteu method; Polyphenols; Rhodotorula mucilaginosa
Download Fulltext PDF
In the present study we monitor the effect of copper-contaminated culture media on development and active compound biosynthesis of two Rhodotorula mucilaginosa strains, assigned as R1 and R2. The media was prepared in aqueous extracts of Asclepias syriaca stems and different copper ions concentrations were added. The data reveals that the presence of 10 mg/ L Cu2+ for the Rhodotorula mucilaginosa strain-1 (R1) and 50 mg/ L Cu2+ for the Rhodotorula mucilaginosa strain-2 (R2) strain fermentation increases the biomass yield (24.7 g and 14.8 g wet biomass/ L culture medium, respectively) and biosynthesis of carotenoid pigments (approximately 1.4 μg/ g dry biomass) compared with references. The chelating properties of polyphenol compounds from aqueous extracts of Asclepias syriaca stems was confirmed by detection of 17.9 mg/ L Cu2+ in extracts with 100 mg/ L Cu2+ added, after 24 hours at 4°C. In the residual culture media, we have determines that the Cu2+ concentrations decreased at 14.0 mg/L for Rhodotorula mucilaginosa strain-1 (R1) and 13.8 mg/ L for Rhodotorula mucilaginosa strain-2 (R2) strain fermentation, respectively, which means that the copper was trapped by the yeasts cells. Likewise, the polyphenols are digested and used as a carbon source. These results bring a significant contribution to the possibility of yeast fermentations in a low cost-vegetal polyphenol and copper ions system.
Introduction
Asclepias species has a high potential as an alternative crop. Asclepias syriaca can provide a proper development home for Monarch butterfly Danaus plexippus larval stage and the development of this species in the world [1] beside the economically aspects of providing latex for rubber production and biodiesel from the seed’s oil. The silky seed floss is currently used to produce hypoallergenic pillows, comforters, and insulating fibre that can be used as a substitute for wood in pressed panels [2]. The de-flossed seeds have in turn been used to develop skin care products and nematocides/pesticides and contain potentially valuable health-benefitting lipids (e.g. specifically the sterols and fatty acids) [3].
Milkweed seeds contain 21% oil and 30% crude protein (dry basis). The oil is similar in quality to soybean oil. The dominant protein classes are water-soluble (22%) and salt-soluble (15%). The kernel contains 39% protein, which accounts for 45% of total protein, including 22% albumins which the water-soluble [4]. These results show that milkweed seed proteins have functional properties as thickeners, protein extender in adhesives or emulsifier in paints. Bleached milkweed seed oil consists in predominately unsaturated fatty acids (34% oleic, 50% linoleic, 1% linolenic) and has very low oxidative stability, but the oil could be used as an alternative triglyceride source for biodiesel [5]. Also, some compounds isolated from Asclepias subulata. Have anti-proliferative properties being highly selective to human cancer cells lines [6]. Despite numerous uses listed the entire plant is not completely exploited. In this perspective in previous studies performed in our research group, a crude aqueous extract from the plant’s stems containing polyphenol compounds was used as a plants growth regulator and microorganism mediator. The aqueous extracts modulate some heavy metals bioaccumulation in plants and also the biosynthesis of photosynthesizing pigments, plant growth and development [7].
Copper is an essential micronutrient present in the active centre of some enzymes like Cu/ Zn superoxide dismutase, ascorbate oxidase, polyphenol oxidase and cytochrome C oxidase. Without this element, the plants growth becomes very difficult [8]. Also copper acts as cofactor in the catalytically biological processes [9]. Alternatively high concentrations of copper interfere with the development and metabolic systems of organisms [10]. On the opposite presence of free copper ions in high amounts in natural systems becomes one of the most severe environmental problems today.
Carotenoid pigments have anti-oxidant properties, protecting cells from free radicals, preventing lipid peroxidation and promoting the metabolism homeostasis [11]. The carotenoids are abundantly found in fruits and vegetables such as pomegranate [12], carrots, tomatoes [13], apricots, jackfruits [14], but are also biosynthesised by algae [11] and by microorganisms like Rhodopseudomonas palustris [15], Sporobolomyces ruberrimus [16], Rhodobacter sphaeroides, Dunaliella, Rhodospirillum rubrum, Rhodotorula, Phaffia rhodozyma, Sporidiobolus pararoseus [17] etc. Since the main amounts of carotenoids are provided by chemical synthesis which does not meet population demands for natural foods, cosmetics and vitamin supplements, the biosynthesis of pigments from microbial sources has received greater interest lately.
Therefore the aim of this paper was to assess the synergic influence of an A. syriaca polyphenol aqueous extract and copper ions (Cu2+) on two Rhodotorula mucilaginosa strains (assigned as R1 and R2) growth and development in a stressful media. Also, the carotenoids biosynthesis capacity of the yeasts was monitored in order to increase this biological active compound production.
Materials and Methods
Microorganisms
Two Rhodotorula mucilaginosa yeast strains, assigned R1 and R2 were isolated and selected by Integrated Centre for Research Bioaliment, Biotechnology Applied in Food Industry, “Dunarea de Jos” University of Galati. Molecular identification was achieved in Department of Applied Biology, Section of Microbiology & Industrial Yeasts Collection DBVPG and University of Perugia [18].
Chemicals
All the chemicals used were provided by Sigma-Aldrich.
Aqueous extraction and experimental assay
The Asclepias syriaca biomass was obtained from an energetically crop (2008) in a research program of “Gheorghe Asachi” Technical University of Iasi [7]. The plant stems were grinded to 1 - 2 mm and 15 g of grounded material was extracted with 100 ml distilled water in a simple bath system, at 80°-C for 45 min. The aqueous extraction was repeated until the extract was colourless. All the extraction fractions were collected and cumulated to a final volume of 1000 ml using distilled water.
In the final extract, four different copper sulphate concentrations (10, 25, 50 and 100 mg Cu2+/ L) were added. The solutions were kept at 4°C for 24h and then the Cu2+ and total polyphenol concentrations were evaluated. In order to assess the effect of the polyphenol and Cu2+ on the yeasts development and biosynthesis of carotenoid pigments, the basic and minimal components of yeast culture medium were dissolved in this Cu2+-polyphenol system and the fermentations of the Rhodotorula mucilaginosa yeast strains were performed.
Culture medium preparation and fermentation conditions
Pre-culture medium was obtained following a previously published protocol [19]. The experimental culture medium was prepared in the Asclepias syriaca aqueous extracts containing also (g/ L): glucose - 1; KH2SO4 - 1; (NH3)2SO4 - 1; CaCl2 - 0.05; then it was sterilized for 20 minutes at 110°-C distributed 100 ml each in Erlenmeyer flasks and inoculated. The fermentation process was monitored for four days. Every 24 hours, the wet biomass amount, pH value, the total polyphenol concentration, free copper ions in the culture medium and the concentration of carotenoid pigments extracted was determined.
Aqueous extracts characterization
The aqueous extract was characterized by the total polyphenol content using the Folin Ciocalteu method.
Biomass determination and pH measurements
The wet biomass was separated by centrifugations at 5000 rpm for 20 min and the dry biomass was determined using a protocol adapted from Tinoi et al. [20], by drying at 105°-C until there were no variations in weight. At every 24 hours, the culture media pH was measured.
Cu2+ determination
The Cu2+ concentrations were monitored using a GBS Avanta atomic absorption spectrophotometer, following a protocol reported by Stingu et al. [8]. The wavelength used for the measurement was 324.70 nm and the calibration curves were obtained in a range of 0.02- 5.00 µg/ ml Cu2+. The equation used was y = 0.059x + 0.0016, R2 = 0.9996 and the concentrations were expressed in µg Cu2+/ ml residual culture medium after biomass separation by centrifugation.
Carotenoid pigments extraction and quantification
The carotenoid pigments extraction and quantification were carried out using a previously published protocol [19].
Results and Discussion
Determination of polyphenol concentration in A. syriaca herbal aqueous extract. The polyphenol chelating properties
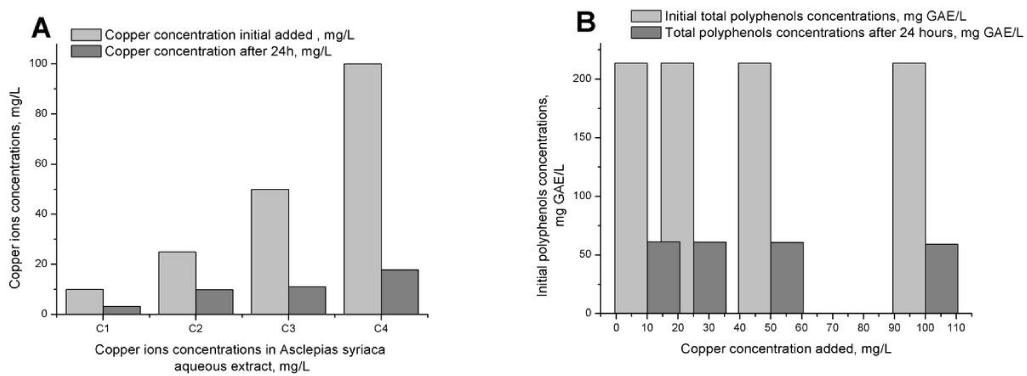
The total polyphenol concentration in the aqueous extract obtained from 15g of Asclepias syriaca plant stems was evaluated as being 213.58 mg GAE/ L of extract. The polyphenol extract has been supplemented with Cu2+ (10, 25, 50 and 100 mg Cu2+/ L) and kept at 4°-C for 24h. After 24h the polyphenol and Cu2+ concentrations were determined. The confirmation of the polyphenol compounds chelating properties was obtained by measuring the polyphenol and Cu2+ concentrations, both having decreased. The sample with the highest copper ions concentration, 100 mg/ L Cu2+, had only 17.892 mg/ L free form Cu2+ after 24 hours (Figure 1a). The same situation was observed for the polyphenol concentrations (Figure 1b).
In agreement with previously published studies, we found that the metal chelation leads to polyphenol redox cycle interruptions by engagement of all the coordination active sites and the formation of insoluble complexes [21]. The complexion efficiencies of metal ions is due to the polyphenol structures and the degree of polymerisation [22] and are also related to the hydroxyl group locations and number on the aromatic ring [23].
Wet biomass measurements
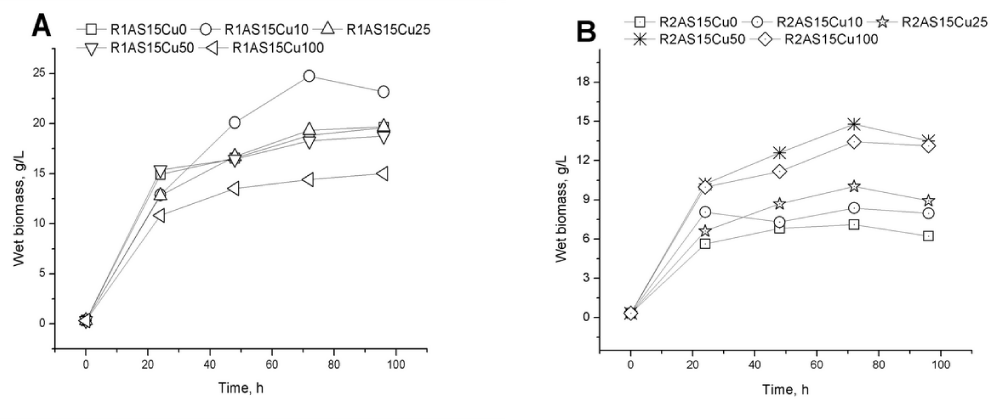
In the synergic fermentation/ bioaccumulation process proposed in this study, wet biomass was evaluated. The wet biomass weigh variations during the bioprocess are presented in (Figure 2a and 2b). The polyphenol presence in the culture medium has a positive influence on the biomass development for the blank fermentation (19.61 g/ L wet biomass). A concentration of 10 mg Cu2+/ L added in the culture medium leads to an increase of biomass for Rhodotorula mucilaginosa- 1 strain (24.7 g/ L wet biomass after 72h fermentation) while high Cu2+ concentrations added (100 mg/ L) inhibit the strain growth. The behaviour of Rhodotorula mucilaginosa- 2 strain in the same conditions is different. The higher amount of wet biomass (14.8 g/ L) was obtained for the fermentation process in which 50 mg Cu2+/ L were added in the medium, but the biomass yield is lower compared with Rhodotorula mucilaginosa- 1 (R1) strain in the same conditions.
pH determination
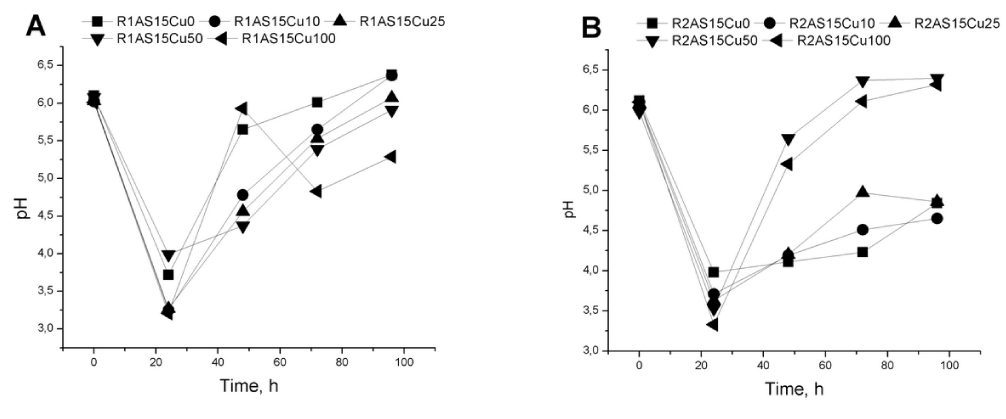
The pH variation in media during the fermentation process was evaluated (Figure 3a and 3b). The pH value significantly decreases from 6 to 3 in the first 24 hours. This change is correlated with the yeast metabolism (formation of acidic metabolites during microbial growth) creating appropriate condition for Cu2+ bioaccumulation.
In the Rhodotorula mucilaginosa- 1 (R1) yeast strain fermentation, the pH increases to around 6 after 24 hours. The increased pH value corresponds to the maximum concentration of biomass. The same observations are consistent for the Rhodotorula mucilaginosa- 2 (R2) strain. High amounts of wet biomass (R2AS15Cu50 and R2AS15Cu100) are obtained when the pH value is around 6.
Variation of total polyphenol concentration from residual fermentative culture medium
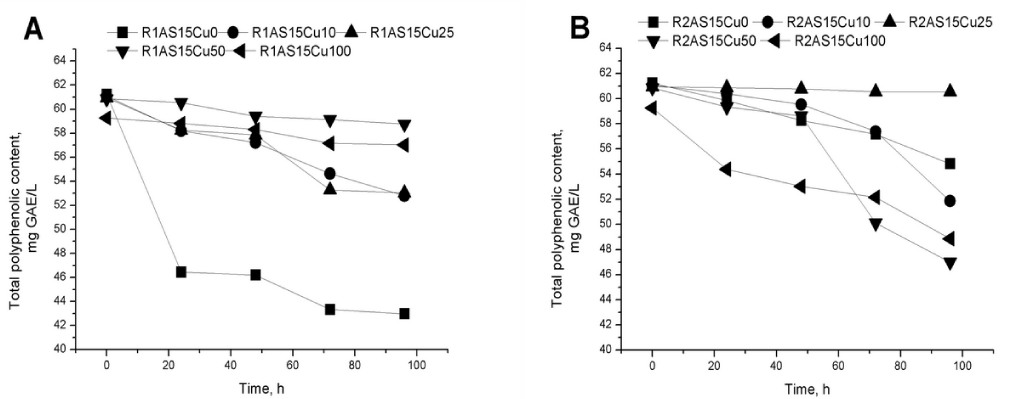
The natural polyphenols from the culture medium have a positive influence on yeast development. High concentrations of polyphenols (approximately 10 mg GAE/ L total polyphenol for R1AS15Cu10 experiment) are used in the Rhodotorula mucilaginosa- 1 (R1) strain fermentation process (Figure 4a). For the Rhodotorula mucilaginosa- 2 (R2) fermentation, (R2AS15Cu50 and R2AS15Cu100), high yields of wet biomass and more evident polyphenol intake from culture medium was observed (Figure 4b).
The data show that the yeast’s enzymatic equipment was adjusted promptly and the metabolic pathways were directed toward polyphenol consumption as carbon source for growth and development in the presence of oxygen.
Variation of copper ions concentrations from residual culture medium
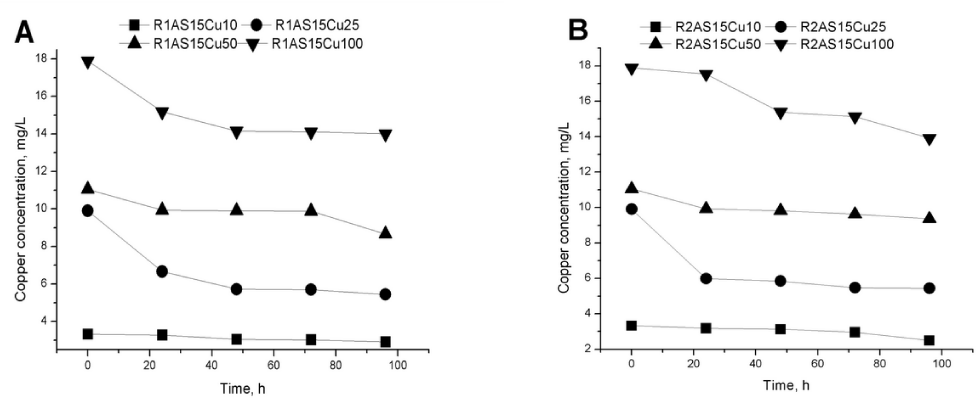
The Cu2+ concentrations were evaluated by analysing the residual culture medium every 24 hours for four days. The obtained data confirm a continuous decrease of Cu2+ concentrations during the fermentation (Figure 5a and 5b).
Some yeasts are very resistant to stressful environment [24]. The Cu2+ from culture medium could be trapped by yeasts through different mechanisms. It can be used as a catalyst in the metabolic processes or adsorbed on the cellular membrane. In the first case, the fate of copper ions depends on the metabolic pathways of the strain. One of the copper uptake mechanisms in yeast is the sequestration of the ions by metathionin due to the high cysteine content, followed by the absorption of cationic ions in the cells [25]. Rhodotorula species adsorbs the heavy metal on the wall cell surface by an independent mechanism [26]. At the same time, the ions can enter into the cells by membrane delivery, internal distribution or extracellular precipitations by excretion of some extracellular metabolites [27,28]. The yeast is a very good absorption system due to high growth rate and unicellular nature [29]. A second advantage of yeasts that the efficiency of copper retention is more pronounced with the expanse of the wet biomass [30]. In this context, the highest Cu2+ amount consumed by Rhodotorula mucilaginosa- 1 (R1) strain was in the R1AS15Cu10 experiment, (22.11% from total Cu2+ added) and for Rhodotorula mucilaginosa- 2 (R2) strain in the R2AS15Cu50 experiment (3.4% from total Cu2+ added) (Figure 5a and 5b).
Regarding the dependence of the Cu2+ amount trapped by the yeast cells, the decrease in culture medium pH value to between 4 and 5 is correlated with the increased amount of copper retained by the yeast [26,29].
The carotenoid pigments biosynthesis
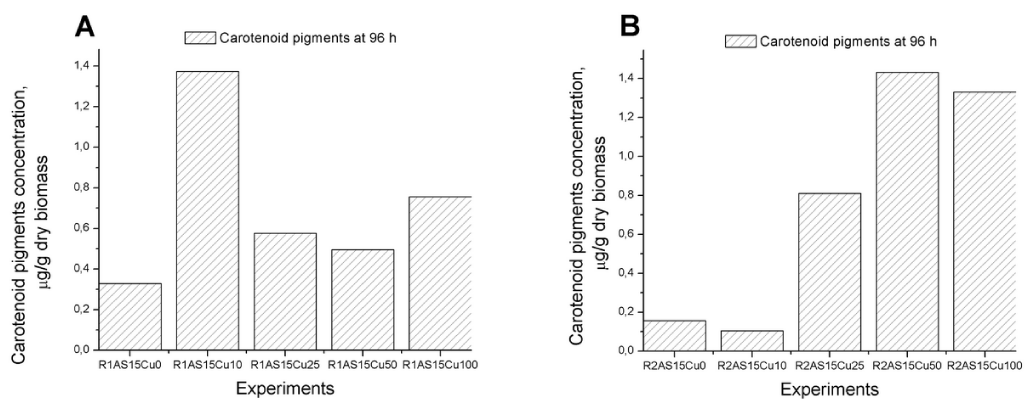
The amount of carotenoid pigments biosynthesised was evaluated in order to highlight the polyphenol and Cu2+ influence at the end of every fermentation process.
The biomass was subjected to carotenoid pigments extraction and the pigments were quantified by UV measurements (Figure 6a and 6b). The presence in the culture medium of only polyphenols (213.50 mg GAE/ L) is not well tolerated (R1As15Cu0 and R2As15Cu0). When 10 mg Cu2+/ L are added, a higher amount of pigment (1.3 μg pigment/ g dry biomass) was biosynthesised by Rhodotorula mucilaginosa-1 (R1). This is explained by the fact that polyphenols bind the copper in the medium and the polyphenol concentrations and available copper ions are important for yeast development and carotenoid pigments biosynthesis. For R1AS15Cu10 fermentation, a higher amount of wet biomass was obtained, while a higher amount of polyphenol was degraded.
The higher carotenoid concentrations obtained for the Rhodotorula mucilaginosa-2 (R2) strain (R2As15Cu50) are correlated with the highest amount of wet biomass obtained and also the highest amount of polyphenol degraded. The carotenoid pigments concentrations are close with those obtained for the Rhodotorula mucilaginosa- R1 strain fermentation, 1.4μg pigments/ g dry biomass.
• On a scale of 1 to 10, did I feel I was able to medically monitor my patients during the Remote Patient Monitoring (RPM) as well as I do when they were in the hospital?
Conclusions
The culture media with Asclepias syriaca stems aqueous extract and 10 mg Cu2+ / L have a positive influence on the wet biomass yield (24.7 g/ L at 72h), the polyphenol compounds degradation and the carotenoid biosynthesis (1.3 μg pigment/ g dry biomass) by a Rhodotorula mucilaginosa- 1 (R1) strain. The higher amount of wet biomass for Rhodotorula mucilaginosa- 2 (R2) strain is obtained for 50 mg Cu2+/ L added in the media (14.8 g/ L) and 1.4 μg pigment/ g dry biomass are biosynthesised. The Cu2+ concentrations retained increase until the end of the experiments, and it is a process that depends on pH variation and biomass yield during fermentations. The polyphenol compounds were digested by the yeast’s enzymatic systems and used in the energetic metabolism. Also the water used in the vegetable extract and culture media preparations may be copper contaminated.
Acknowledgements
This work was supported by a grant of the Romanian Minister of Research and Innovation, CCCDI - UEFISCDI, project number PN – III - P1-1.2 - PCCDI-2017-0697/ 13PCCDI/ 2018, within PNCDI III, and The financial support of European Social Fund for Regional Development, Competitiveness Operational Programme Axis 1-Project “Petru Poni Institute of Macromolecular Chemistry - Interdisciplinary pol for smart specialization through research and innovation and technology transfer in bio(Nano)polymeric materials and (eco)technology”, InoMatPol (ID P_36_570, Contract 142/ 10.10.2016).
Author contributions statement
A. R. Petrovici - conceptualization, methodology, validation, investigation, resources, data duration, writing-original draft preparation, writing-review and editing, supervision, project administration I. Stoica - formal analysis, Irina Volf - visualization, writing - review and editing, Valentin I. Popa - writing-review and editing, supervision, project administration.
- Maclvor JS, Cadotte MW, Livingstone SW, Lundholm JT, Yasui S-LE. Phylogenetic ecology and the greening of cities. J Appl Ecol. 2016; 53: 1470-1476. http://bit.ly/2ObibmM
- Spiridon I. Modifications of Asclepias syriaca fibers for paper production. Ind Crop Prod. 2007; 26: 265-269. http://bit.ly/2XGHaSc
- Schlegel V, Zbasnik R, Gries T, Hyun Lee B, Carr T, Lee JY, et al. Characterization of potential health promoting lipids in the co-products of de-flossed milkweed. Food Chem. 2011; 126: 15-20. http://bit.ly/2sc0TNR
- Hojilla-Evangelista MP, Evangelista RL. Effects of cooking and screw-pressing on functional properties of protein in milkweed (Asclepias spp.) seed meals and press cakes. Ind Crop Prod. 2009; 29: 615-621. http://bit.ly/37vPhFy
- Hojilla-Evangelista MP, Evangelista RL, Wu YV. Characterization of milkweed (Asclepias spp.) seed proteins. Ind Crop Prod. 2009; 29: 275-280. http://bit.ly/35qmEYp
- Rascon VL, Velazquez C, Garibay EA, Medina JLA, Vilegas W, Robles ZRE. Antiproliferative activity of cardenolide glycosides from Asclepias subulata. J Ethnopharmacol. 2015; 171: 280-286. http://bit.ly/2XEU9DB
- Stingu A, Volf I, Popa VI, Gostin I. New approaches concerning the utilization of natural amendments in cadmium phytoremediation. Ind Crop Prod. 2012; 35: 53-60. http://bit.ly/34dzetL
- Stingu A, Volf I, Popa VI. Physiological changes in seedling germination and growth plant under chemical stress conditions. Environ Eng Manag J. 2009; 8: 1309-1313.
- Yuce M, Nazir H, Donmez G. A voltammetric Rhodotorula mucilaginosa modified microbial biosensor for Cu(II) determination. Bioelectrochem. 2010; 79: 66-70. http://bit.ly/33ecJ6R
- Stingu A, Volf I, Popa VI. Study of copper and cadmium accumulation by bean. Environ Eng Manag J. 2009; 8: 1247-1252. http://bit.ly/2OFE4cG
- Gong M, Bassi A. Carotenoids from microalgae: a review of recent developments. Biotechnol Adv. 2016; 34: 1396-1412. http://bit.ly/2KNqSS7
- Goula AM, Ververi M, Adamopoulou A, Kaderides K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason Sonochem. 2017; 34: 821-830. http://bit.ly/33eFj80
- Luengo E, Condon AS, Condon S, Alvarez I, Raso J. Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Sep Purif Technol. 2014; 136: 130-136. http://bit.ly/33eWfLA
- Mai HC, Truong V, Debaste F. Carotenoids purification from gac (Momordica cochinchinensis Spreng.) fruit oil. J Food Eng. 2016; 172: 2-8. http://bit.ly/2satlzE
- Liu S, Zhang G, Zhang J, Li X, Li J. Performance carotenoids yield and microbial population dynamics in a photobioreactor system treating acidic wastewater: Effect of Hydraulic Retention Time (HRT) and Organic Loading Rate (OLR). Bioresource Technol. 2016; 200: 245-252. http://bit.ly/2DbuPvO
- Cardoso LAC, Jackel S, Karp SG, Framboisier X, Chevalot I, Marc I. Improvement of Sporobolomyces ruberrimus carotenoids production by the use of raw glycerol. Bioresource Technol. 2016; 200: 374-379. http://bit.ly/2qC855e
- Valduga E, Rausch Ribeiro AH, Cence K, Colet R, Tiggemann L, Zeni J, et al. Carotenoids production from a newly isolated Sporidiobolus pararoseus strain using agroindustrial substrates. Biocatal Agri Biotechnol. 2014; 3: 207-213. http://bit.ly/35sWZyu
- Hainal AR, Volf I, Popa VI. Influence of copper ions in aqueous extracts of Vitis vinifera grape seeds on the development of Rhodotorula mucilaginosa yeast, Bull Inst Politech Iasi. Section Chemistry and Chemical Engineering. 2011; 57: 205.
- Hainal AR, Ignat I, Volf I, Popa VI. Transformation of polyphenols from biomass by some yeast species. Cell Chem Technol. 2011; 45: 211-219. http://bit.ly/33iXTw4
- Tinoi J, Rakariyatham N, Deming RL. Simplex optimization of carotenoid production by Rhodotorula glutinis using hydrolyzed mung bean waste flour as substrate. Process Biochem. 2005; 40: 2552-2557. http://bit.ly/33dlgXR
- Andjelkovic M, Van Camp J, De Meulenaer B, Depaemelaere G, Socaciu C, Verloo M, et al. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006; 98: 230-231. http://bit.ly/2OIIsrM
- Oo CW, Kassim MJ, Pizzi A. Characterization and performance of Rhizophora apiculata mangrove polyflavonoid tannins in the adsorption of copper (II) and lead (II). Ind Crop Prod. 2009; 30: 152-161. http://bit.ly/37vqaTq
- Vestergaard M, Kerman K, Tamiya E. An electrochemical approach for detecting copper-chelating properties of flavonoids using disposable pencil graphite electrodes: possible implications in copper-mediated illnesses. Anal Chim Acta. 2005; 538: 273-281. http://bit.ly/2XEKpcA
- Lahav R, Fareleira P, Nejidat A, Abeliovich A. The identification and characterization of osmotolerant yeast isolates from chemical wastewater evaporation ponds. Microb Ecol. 2002; 43: 388-396. http://bit.ly/2OCBnJ3
- Lode A, Kuschel M, Paret C, Rodel G. Mitochondrial copper metabolism in yeast: interaction between Sco1p and Cox2p. FEBS Lett. 2000; 485: 19-24. http://bit.ly/2pJqUDj
- Hainal AR, Popa VI, Volf I. Study of interaction between copper ions and aqueous extracts from bark of Picea abies in the culture of Rhodotorula species, proceeding of the international conference-science & technology of biomasses: advances and challenges. 2011; 353-356.
- Fourest E, Roux JC. Heavy metal biosorption by fyngal mycelian by-products: mechanisms and influence of pH. Appl Microbiol Biotechnol. 1992; 37: 399-403. http://bit.ly/2XEXkv1
- Li Z, Yuan H. Characterization of cadmium removal by Rhodotorula sp. Y11. Appl Microbiol Biotechenol. 2006; 73: 458-463. http://bit.ly/2ODr2wA
- Ertugrun S, San NO, Donmez G. Treatment of dye (Remazol Blue) and heavy metals using yeast cells with the purpose of managing polluted textile wastewaters. Ecol Eng. 2009; 35: 128-134. http://bit.ly/2KKtAaQ
- Tsekova KV, Marinov PG, Tsekova AN. Copper accumulation by Aspergillus awamori. Folia Microbiol. 2000; 45: 217-220. http://bit.ly/37ssdrc

Sign up for Article Alerts