Benign TFH-Cell Lymph Node Infiltrate Morphologically Mimicking Angioimmunoblastic T-Cell Lymphoma in a Rheumatoid Arthritis Patient Treated with a TNF Alpha-Inhibitor and Methotrexate: a Case Report and Review of the Literature
Simran Kalsi and Jagmohan S. Sidhu*
Department of Pathology and Laboratory Medicine, United Health Services Hospitals
*Address for Correspondence: Jagmohan S. Sidhu, Department of Pathology and Laboratory Medicine, United Health Services Hospitals, 33-57 Harrison Street, Johnson City, New York, Tel: +607-763-6285; Fax: +607-763-5410; E-mail: jagmohan_sidhu@uhs.org
Submitted: 11 July 2018; Approved: 17 September 2018; Published: 18 September 2018
Citation this article: Kalsi S, Sidhu JS. Benign TFH-Cell Lymph Node Infiltrate Morphologically Mimicking Angioimmunoblastic T-Cell Lymphoma in a Rheumatoid Arthritis Patient Treated with a TNF Alpha-Inhibitor and Methotrexate: a Case Report and Review of the Literature. Int J Cancer Cell Biol Res. 2018; 3(1): 005-008.
Copyright: © 2018 Sidhu JS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Etanercept is a TNFα inhibitor (a biologic agent) and methotrexate is an antimetabolite agent. Both of these drugs are commonly used to treat rheumatoid arthritis. However, etanercept, methotrexate and rheumatoid arthritis are all known to cause increased numbers of T-Follicular Helper (TFH) cells individually. Combined therapy with etanercept and methotrexate is especially known to cause a higher proliferation of TFH cells than either of these drugs used alone. We present a case report where a 63-year-old female with a history of rheumatoid arthritis, which was managed with etanercept and methotrexate, presented with extensive lymphadenopathy raising a clinical suspicion of lymphoma. The morphological examination revealed almost complete effacement of lymph node architecture due to marked proliferation of small T lymphocytes with immunophenotypic features of TFH cells and high endothelial cell venules and therefore, mimicked Angioimmunoblastic T-Cell Lymphoma (AITL). Further immunophenotypic examination and T-Cell Receptor (TCR) gene rearrangement studies provided evidence that this entity did not fit the diagnostic criteria for AITL or any other established T-cell lymphoma. Rather, it is a benign TFH-cell proliferation in the lymph node that mimics AITL. To our knowledge, our case is the first well-supported case of a benign TFH-cell proliferation mimicking AITL in an immunosuppressed patient due to combined etanercept and methotrexate therapy.
Introduction
Immunomodulatory agent therapy is known to contribute to a broad range of lymphoproliferative disorders. Etancercept is one such immunomodulatory agent. It is a dimeric fusion protein that functions to block the effects of TNFα [1]. Methotrexate, an antimetabolite, is a chemotherapeutic agent that is also a disease-modifying antirheumatic drug that has an anti-inflammatory effect in low doses [2]. Etanercept and methotrexate are both known to cause increased TFH cell numbers. Etanercept has been shown to result in a greater increase in TFH cells than methotrexate, but the combined use of these drugs results in a much more significant TFH cell proliferation than etanercept or methotrexate alone [2]. TFH cells are a major subset of nonpolarized effector T cells that “help” B cells in activation, expansion, and differentiation [3].
Rheumatoid Arthritis (RA) is a chronic systemic autoimmune disorder that is often managed with etanercept and methotrexate [2]. RA is known to increase TFH cell numbers and put patients at an increased risk of lymphoproliferative disorders. Such lymphoproliferative disorders are typically non-Hodgkin lymphomas such as diffuse large B-cell lymphoma [1]. This can be partially attributed to the dysfunctional immune response that contributes to the disease pathophysiology. There has also been a link between lymphoproliferative disease and use of TNF inhibitors, which is due to the immunosuppressive effects of this kind of medication [4].
Angioimmunoblastic T-cell lymphoma is a subset of peripheral T-cell lymphoma. Histologically, increased vascularity and a polymorphous inflammatory infiltrate, which may include aggregates of large cells and small cells with clear cytoplasm, are typically found in AITL. Generalized lymphadenopathy is the main presentation of AITL. However, there is evidence of extranodal involvement of the bone marrow, spleen, and lungs. Immunophenotypically, the proliferation of extrafollicular dendritic cells appreciated with CD21, CD23 or CD35 is most characteristic of AITL. The expression of CD3 by the monoclonal proliferation of T cells is another important feature of AITL [5]. Neoplastic cells in AITL are the TFH cells that are normally CD4+, CD57+, CD10+ cells that are typically found in the follicles of the lymph node [5]. Malignant T cells in AITL have been shown to express many markers of TFH cells, including CD4, CD10, CD57, CXCR5, CD40, PD1, BCL6, and CD200 [6,7]. AITLs are rather heterogeneous, which often results in lymphoma cells not expressing all possible TFH markers [6]. T-Cell Receptor (TCR) gene rearrangement studies in AITL provide evidence for clonal T-cell proliferation [5].
We present here a case of benign T-cell lymphoproliferation that was clinically suspected to be a lymphoma due to generalized lymphadenopathy, and was histomorphologically mimicking AITL in a RA patient treated with etanercept and methotrexate.
Case Report
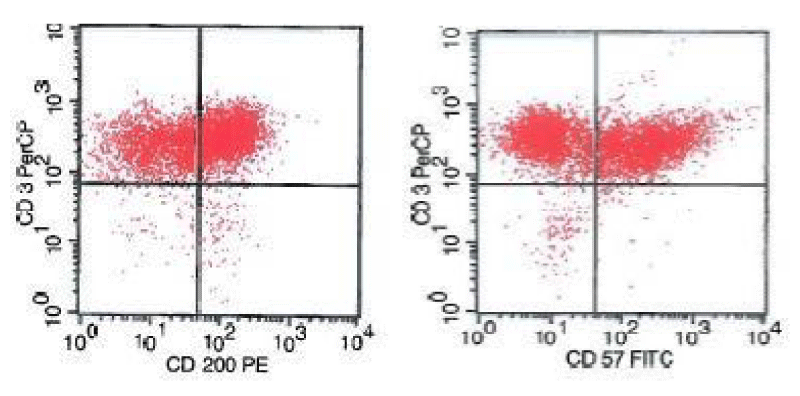
A 63-year-old female with a history of RA, congestive heart failure, coronary artery disease, chronic kidney disease, obesity, diabetes mellitus, dyslipidemia, hypothyroidism, chronic obstructive pulmonary disease, and a 50 pack-year history of smoking presented with progressive, severe shortness of breath for two days. She was taking metolazone, furosemide, amlodipine, carvedilol, rosuvastatin, folic acid, hydralazine, and thyroxine on admission. Additionally, she had been on methotrexate in the past and was taking steroids and etanercept for the management of her rheumatoid arthritis. She had to be intubated due to breathing difficulties brought on by congestive heart failure. Her skin was unremarkable for lesions. CT scans revealed extensive bilateral pulmonary disease with bilateral pleural effusions, presumably pneumonia. Additionally, there was extensive cervical, bilateral axillary, and mediastinal adenopathy present. A bronchial culture for influenza A was positive. The patient developed progressive pancytopenia, with blood count revealing a white blood cell count of 1.7 K/uL, hemoglobin 7.8 gm/dl, hematocrit 25%, and platelets 70 K/uL. Upon discontinuation of etanercept, the patient was still pancytopenic a few weeks later although blood counts increased to a WBC of 2.3 K/uL, hemoglobin 8.5 g/dl, and platelets 103 K/uL. A biopsy was performed of the left axillary lymph node for ruling out a lymphoma. Flow cytometric analysis of the lymph node showed a population of 30% lymphocytes that was CD3+, CD4+, CD200+, CD57+, and TCR alpha-beta+ T cells (Figure 1) and was, therefore, a population of CD4+ T-Follicular Helper (TFH) cells. The CD4:CD8 ratio was 1.4:1.
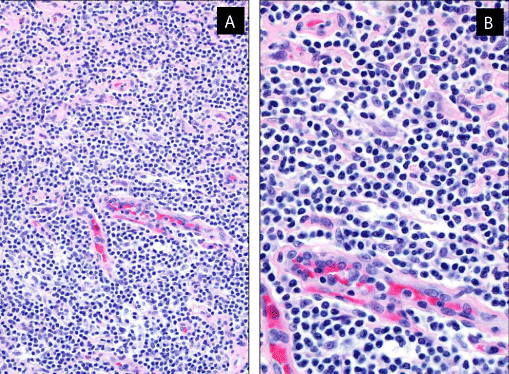
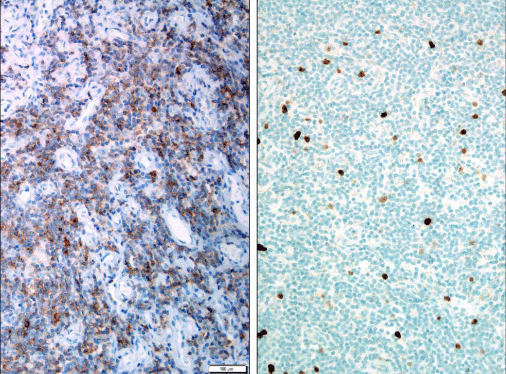
Histologically, the lymph node architecture was almost completely effaced by small lymphocytes with slightly irregular nuclear contours, high cytoplasmic ratio and no prominent nucleoli. There was marked proliferation of high endothelial cell venules (Figure 2A & 2B). No perivenular large cells were seen. A few atrophic follicles were present.
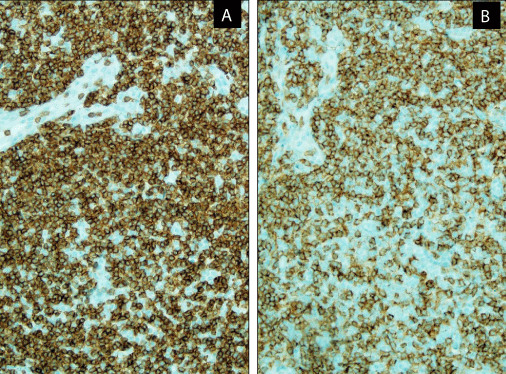
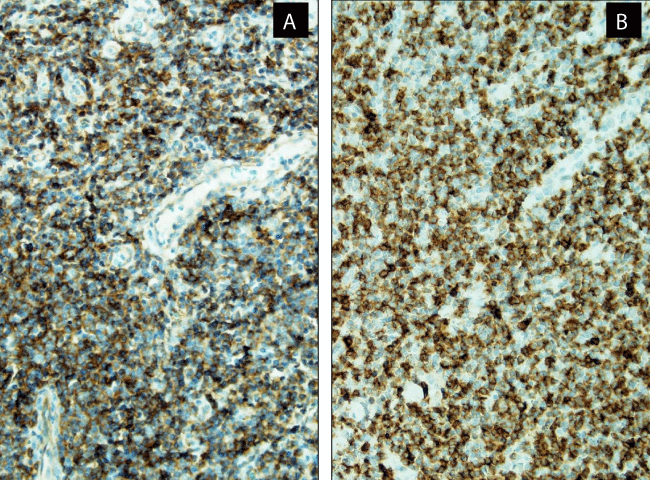
Immunohistochemically, there was an increased number of CD2+, CD5+, CD7+, BCL6+ (focal and dim), CD3+ (Figure 3A), CD4+ (Figure 3B), CD200+ (Figure 4A), CD57+ (Figure 4B) cells, and PD1 (Figure 5A) with only 1% Ki67 labeling index (Figure 5B).
CD10, EBV-LMP1, CD15 and CD30 were negative. Only a few CD20+ B cells and CD8+ T cells were seen. CD21 was staining only a few follicular dendritic reticulum cells in the small number of atrophic follicles. Since the patient was taking immunosuppressive agents, an in-situ hybridization for EBER was performed and was negative. An in-situ hybridization for kappa and lambda light chain mRNA revealed few polytypic plasma cells.
A PCR study for TCR gene rearrangement was performed and showed no evidence for monoclonality. A diagnosis of benign T-follicular helper cell proliferation was made and lymphoma was ruled out. The patient developed multisystem organ failure and passed away 2 days after the biopsy diagnosis.
Discussion
The patient was diagnosed with etanercept and methotrexate-related benign T-cell proliferation of follicular helper-cell-type and small blood vessel (high endothelial cell venule) proliferation in the lymph node mimicking angioimmunoblastic T-cell lymphoma. Additionally, the patient was diagnosed with chronic hypercapneic respiratory failure with chronic hypercapneic respiratory acidosis.
There are a number of established TFH cell lymphoproliferative disorders that have been described in literature. These include AITL, follicular variant of peripheral T-cell lymphoma, primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoproliferative disorder, and some cases of mycosis fungoides (cutaneous T-cell lymphoma) [6,8]. Our case mimicked AITL because of the diffuse proliferation of T-cells with immunophenotypic features of TFH cells. However, features of our case do not fulfill diagnostic criteria of AITL e.g. absence of CD21+ extrafollicular dendritic cell network, focal and dim BCL6 positivity, absence of clear cytoplasm in small lymphocytes, absence of large B lymphoid cells, negativity for EBER, a very low proliferation fraction, and absence of TCR gene rearrangement.
There are a few features of our case that require explanation i.e. lack of significant BCL6 expression on the T lymphocytes and absence of large B cells. Although BCL6 is a common TFH cell marker, circulating TFH cells that are BCL6 negative are increased in earlier stages of RA and express BCL6 in later stages of the disease. This normalization can likely be attributed to cell migration into germinal centers of lymphoid tissue [6]. The lack of expression of some TFH markers in our case can be attributed to the fact that different TFH subsets can be present due to different stages in differentiation. Different subsets may only express some of the TFH surface antigens [3].
Etanercept decreases B-cell numbers by suppressing germinal center B cells and follicular dendritic cells [1]. Methotrexate has been shown to reduce overall B-cell numbers by affecting early cell-developmental stages and differentiation [2]. Methotrexate can also cause the suppression of B-cells by inhibiting purine biosynthesis [9]. Therefore, absence of large B cells and paucity of small B cells in our case is most likely due to etanercept and methotrexate therapy.
Etanercept and methotrexate-related benign T-cell proliferation of follicular helper-cell-type and blood vessel (high endothelial cell venule) proliferation in the lymph node raised a concern of AITL. However, we have shown that it is a non-neoplastic TFH lymphocyte infiltrate that caused lymphadenopathy. A case report of AITL with 80% Ki67 labeling index following treatment of ankylosing spondylitis with etanercept has been published, but this report provides incomplete morphologic detail and inadequate immunophenotypic support for the diagnosis of AITL [10]. Etanercept-related benign T-cell proliferation mimicking peripheral T-cell lymphoma has also been reported previously once in literature [11]. Though methotrexate is often used in the treatment of various T-cell lymphomas, it has been shown to be associated with the development of CD8+ cutaneous T-cell lymphoproliferative disorder [12]. Furthermore, a study compiling 3 cases of methotrexate-associated lymphoproliferations mimicking AITL has been reported in the past [13]. However, TFH phenotype of benign T-cell proliferation and blood vessel (high endothelial cell venule) proliferation mimicking AITL related to etanercept and/or methotrexate has not been reported in English literature to our knowledge.
In conclusion, we have identified a type of etanercept and methotrexate-related lymphadenopathy that is caused by a diffuse infiltrate of TFH cells, can clinically raise a suspicion of immunosuppressive drug-related lymphoma and can histologically masquerade as AITL. A detailed morphologic and immunophenotypic analysis can easily rule out a lymphoma in this type of lymphadenopathy. We suggest including etanercept and methotrexate-related benign T-cell proliferation of follicular helper-cell-type in the list of TFH-related lymphoid disorders/lymphomas.
- Hasserjian RP, Chen S, Perkins SL, de Leval L, Kinney MC, Barry TS, et al. Immunomodulator agent-related lymphoproliferative disorders. Mod Pathol. 2009; 22: 1532-1540. https://goo.gl/FzKfSe
- Glaesener S, Quach TD, Onken N, Weller-Heinemann F, Dressler F, Huppertz HI, et al. Distinct Effects of Methotrexate and Etanercept on the B cell Compartment in Patients with Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2014; 66: 2590-2600. https://goo.gl/N45vTZ
- Ma CS, Deenick EK, Batten M, Tangye SG. The origins, functions and regulation of T follicular helper cells. J Exp Med. 2012; 209: 1241-1253. https://goo.gl/38sjLb
- Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: Twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002; 46: 3151-3158. https://goo.gl/Y64tvW
- Dogan A, Attygalle AD, Kyriakou C. Angioimmunoblastic T-cell lymphoma. British Journal Haematology. 2003; 121: 681-691
- Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol. 2014; 92: 64-71. https://goo.gl/VxQThx
- Dorfman DM, Shahsafaei A. CD200 (OX-2 membrane glycoprotein) is expressed by follicular T helper cells and in angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2011; 35: 76-83. https://goo.gl/G7J9Eo
- Ma H, Qiu S, Lu R, Feng P, Lu C. Methotrexate and etanercept-induced primary cutaneous CD4 positive small/medium-sized pleomorphic T-cell lymphoma. Anais Brasileiros de Dermatologia. 2016; 91: 368-371. https://goo.gl/EWqLdB
- Bohm I. Decrease of B-cells and autoantibodies after low-dose methotrexate. Biomed Pharmacother. 2003; 57: 278-281. https://goo.gl/Gc6u2e
- Xu L. Ankylosing spondylitis combined with angioimmunoblastic T-cell lymphoma. BMJ Case Rep. 2011; 2011. https://goo.gl/1xmwpV
- Hurley MY, George MN, Leonardi CL, Frater JL. A transient benign lymph node-based proliferation of T-cells simulating non-Hodgkin lymphoma in a patient with psoriasis treated with tumor necrosis factor alpha and CD11a antagonists. Diagn Pathol. 2008; 3: 13. https://goo.gl/z32a2L
- Wederson CM, Gibson B, Tse W, Krem M, Grewal J. Methotrexate-associated primary cutaneous CD30-positive cutaneous T-cell lymphoproliferative disorder: a case illustration and a brief review. Am J Blood Res. 2016; 6: 1-5. https://goo.gl/vdsMd1
- Hatanaka K, Nakamura N, Kojima M, Ando K, Irie S, Bunno M, et al. Methotrexate-associated lymphoproliferative disorders mimicking angioimmunoblastic T-cell lymphoma. Pathol Res Pract. 2010; 206: 9-13. https://goo.gl/oA55DH

Sign up for Article Alerts