Micro Vascular and Macro Vascular Disease in Systemic Hypertension: The Role of Cardiac Imaging and Nitric Oxide Synthase Gene Polymorphism
Galal E Nagib Elkilany1*, Abeer Saeed Ghobashi2, Jan Fedacko3, Mai Salama4, Jaipaul Singh5 , Sherif Baath Allah6, Mohammed Elmahal7, Adel H Allam8, Ahmad Adham Raafat9 and Hani Aiash10
1,4Professor and Consultant of Cardiology (SCU), Tanta University, Egypt
2Professor of Radio-Diagnosis, Tanta University, Egypt
3Kosice Medical University, Slovakia
5School of Forensic and Applied Sciences, University of Central Lancashire, Preston, UK
6RAK Medical and Masafi Hospital; Health Science University, UAE
7London University and Masafi Hospital, UAE
8Professor of Cardiology, AL-Azhar University, Egypt
9Alexandria University, Alexandria, Egypt
10SUNY Upstate Medical University, Syracuse, NY, USA
*Address for Correspondence: Galal Eldin Nagib Elkilany, Professor of Cardiology-SCU and Consultant of Cardiology at Tanta University, ARE, 132 Holly Drive, LaPlace, LA , 70068 , USA, Professor and Consultant of Cardiology - SCU and at Tanta University , ARE , Kings College Hospital London , Dubai , UAE and Emirates International Hospital , Sharjah, UAE, Tel: 973-981-7875; ORCID ID: https://orcid.org/0000-0001-8327-7862; E-mail: galal@kilany.org / galal.elkilany@gmail.com
Submitted: 21 January 2020; Approved: 11 February 2020; Published: 13. February 2020
Citation this article: Nagib Elkilany GE, Ghobashi AS, Fedacko J, Salama M, Singh J, et al. Micro Vascular and Macro Vascular Disease in Systemic Hypertension: The Role of Cardiac Imaging and Nitric Oxide Synthase Gene Polymorphism. Int J Clin Cardiol Res. 2020;4(1): 001-008.
Copyright: © 2020 Elkilany GE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Systemic hypertension; Cardiac imaging; Coronary artery disease; Micro vascular disease; Nitric oxide synthase gene allele
Download Fulltext PDF
Systemic Hypertension (HTN) accounts for the largest amount of attributable Cardiovascular (CV) mortality worldwide. There are several factors responsible for the development of HTN and its CV complications. Multicenter trials revealed that risk factors responsible for Micro Vascular Disease (MVD) are similar for those attributable to Coronary Artery Disease (CAD) which include tobacco use, unhealthy cholesterol levels, HTN, obesity and overweight, physical inactivity, unhealthy diet, diabetes, insulin resistance, increasing age and genetic predisposition. In addition, the defective release of Nitric Oxide (NO) could be a putative candidate for HTN and MVD. This study reviewed the risk stratification of hypertensive population employing cardiac imaging modalities which are of crucial importance in diagnosis. It further emphasized the proper used of cardiac imaging to determine patients at increased CV risk and identify the management strategy. It is now known that NO has an important effect on blood pressure, and the basal release of endothelial Nitric Oxide (eNOS) in HTN may be reduced. Although there are different forms of eNOS gene allele, there is no solid data revealing the potential role of the polymorphism of the eNOS in patients with HTN and coronary vascular diseases. In the present article, the prevalence of eNOS G298 allele in hypertensive patients with micro vascular angina will be demonstrated.
This review provides an update on appropriate and justified use of non-invasive imaging tests in hypertensive patients and its important role in proper diagnosis of MVD and CAD. Second, eNOS gene allele and its relation to essential hypertension and angina pectoris are also highlighted.
Introduction
Hereditary factors contributed directly to the occurrence of hypertension, as evidenced by family studies showing that premature onset of hypertension among first degree relatives yielded a remarkable high risk of 3.8 times to develop this disorder [1]. However, it remains unclear how many genes or which genetic determinants might constitute such hereditary background. The gene encoding endothelial Nitric Oxide Synthase (eNOS) is regarded as one of the potentially logical candidate for hypertension, since its enhanced production or enzyme bioavailability can lead to the constitutive release of nitric oxide in endothelial cells, which exerts vaso protective effects in Blood Pressure (BP) regulation [2].
The clinical cluster of typical anginal chest pain, a positive response to stress testing and angiographically normal coronary arteries is relatively common in patients with systemic hypertension. Functional and structural mechanisms affecting the coronary microcirculation are often responsible for micro vascular angina in patients with hypertension.
Capillary rarefaction and left ventricular hypertrophy are common structural abnormalities responsible for micro vascular dysfunction in hypertension. The higher prevalence of micro vascular angina in women than in men, and its relation to menopausal status have led to the suggestion that estrogen deficiency may play a pathogenic role in the subgroup of peri- and post-menopausal women with angina and hypertension [3,4]. Similar considerations also apply to arterial hypertension, the incidence of which increases greatly in women after the menopause. The link between estrogen deficiency and the development of micro vascular angina and hypertension is complex and includes abnormal endothelial function, altered autonomic nervous system responses and the activation of the Renin Angiotensin Aldosterone System (RAAS) [4].
Patients with HTN and micro vascular angina [4,5] often show slow flow and tortuous coronary arteries, suggestive of small vessel “obstruction”. Moreover, there is a complex relation among insulin sensitivity, hypertension, and endothelial function. Although there are few prospective data on the relation between insulin levels and the development of hypertension, there is some evidence that insulin resistance precedes the onset of established hypertension in high risk patients. Because insulin is a vasodilator, it would need to activate a variety of other potential physiologic mechanisms to play a causal role in the pathogenesis of hypertension. There are changes in the arterial wall in patients with hyperinsulinemia, and characteristic decreases in elasticity of the arterial wall have been noted in hypertensive patients with insulin resistance. Hyperglycemia, hyperinsulinemia, and hypertriglyceridemia appear to jointly contribute to increased arterial stiffness. There are, however, ethnic and racial disparities in the association of insulin, insulin sensitivity, and blood pressure, as this relation is not strongly observed in the black population in the United States and elsewhere. This may reflect complex relations among obesity, diabetes, and hypertension, which are more common in patients with African ancestry [6].
Pathogenesis mechanisms
The mechanisms underlying angina pectoris in essential arterial Hypertension Patients (HTN) without obstructive Coronary Artery Disease (CAD) are still largely unknown. Several functional pathophysiological abnormalities have been reported in these patients and have been postulated to represent the pathogenic basis for angina in these patients, including endothelial dysfunction, increased sympathetic tone, micro vascular spasm, estrogen deficiency, psychological disorders, and increased pain sensitivity. Furthermore, hypertensive patients have a higher likelihood of presenting with features of the metabolic syndrome, e.g., hypertension, dyslipidemia, obesity and insulin resistance, compared with the general population (30% versus 8%, respectively) [6,7]. This occurs more frequently in postmenopausal women. Insulin resistance, therefore, may represent an important mechanism for vascular dysfunction in this setting [8]. Structural abnormalities are also important, i.e., capillary rarefaction [9] as well as medial hypertrophy and/or fibrosis of arteriolar vessels. Myocardial ischemia triggered by functional and/or anatomical abnormalities in the coronary microcirculation has been documented in many studies using radionuclide Myocardial Perfusion Imaging (MPI) techniques as well as stress induced alterations of cardiac high energy phosphate, as assessed by magnetic resonance spectrometry [10,11].
In summary, both coronary micro vascular spasm and/or a reduced micro vascular vasodilator capacity have been demonstrated to cause myocardial ischemia and anginal symptoms in patients with hypertension and micro vascular angina.
The pivotal role of endothelial Nitric Oxide Gene (eNOS) Allele
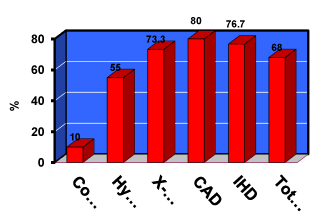
Although there are many genes responsible for the development of HTN and different polymorphisms of eNOS gene, we observed an important polymorphism of eNOS gene since 2004. In this study, Myocardial Perfusion Imaging (MPI) and coronary angiography was performed for 60 consecutive hypertensive patients complaining of chest pain, whom are admitted at our critical care department at Tanta University Hospital11. We revealed that 15 patients had chest pain with true ischemia and reversible myocardial perfusion defects (multiple and mild) but normal epicardial coronary arteries (micro vascular angina), while 15 patients had significant CAD, and 20 hypertensive patients showed normal perfusion scan and coronary angiography. In conclusion; this study revealed that, the prevalence of the NOS G298 allele was higher in the hypertensive group with microvascular angina (documented by MPI) than it was among the control participants (p < 0.005). In addition, we found that eNOS allele was significantly higher in the hypertensive group than in the control participants, but there was no significant difference in homozygote mutants among hypertensive participants, x-syndrome and patients with CAD [11] (figure 1).
Similarly, a recent meta-analyze of three eNOS widely evaluated polymorphisms was demonstrated and they include G894T (rs1799983) in exon 7, 4b/a in intron 4, and T2786C (rs2070744) in promoter region, in association with hypertension from both English and Chinese publications, while addressing between study heterogeneity and publication bias [12]. The investigators of this meta-analysis, ascertained the role of eNOS G894T and 4b/a polymorphisms on hypertension in Asians, and T2786C polymorphism in white population [12].
Micro vascular disease in systemic hypertension
Patients with systemic hypertension often experience chest pain despite the absence of obstructive coronary artery disease, which is caused by coronary micro vascular dysfunction. Structural coronary micro vascular abnormalities such as capillary rarefaction and functional mechanisms such as endothelial dysfunction are common causes for the development of angina pectoris in hypertensive individuals [13].
The presence of endothelial dysfunction in patients with hypertension and microvascular angina has been suggested by a reduced coronary flow response to acetylcholine or atrial pacing and by inappropriate endothelial vasoconstrictor activity, mainly mediated by Endothelin-1 (ET-1) production [10]. Both coronary and peripheral micro- vascular dysfunction have been demonstrated in hypertensive patients with chest pain, normal appearing coronary angiograms, and no left ventricular hypertrophy by echocardiography. Angina may occur in patients with arterial hypertension in the absence of epicardial coronary artery disease due to an abnormally elevated resistance of the coronary microvasculature [4,13].
Microvascular function and structure are generally considered to be the consequence of high blood pressure levels, there is evidence that micro vascular changes, i.e., capillary rarefaction, and endothelial dysfunction may anticipate the clinical onset of arterial hypertension. This is supported by the finding of decreased capillary density in borderline hypertensive subjects and even in the normotensive offspring of hypertensive parents as well as the presence of endothelial dysfunction in the normotensive offspring of hypertensive patients [13].
Hypertension and coronary artery disease (Macro Vascular Disease)
High blood pressure is a major modifiable risk factor for all clinical manifestations of Coronary Artery Disease (CAD). In people without known cardiovascular disease, the lowest systolic (down to 90-114 mmHg) and the lowest diastolic (down to 60-74 mmHg) pressures are associated with the lowest risk for developing CAD. Although diastolic blood pressure is the strongest predictor of CAD in younger and middle aged people, this relationship becomes inverted and pulse pressure shows the strongest direct relationship with CAD in people above 60 years of age [14].
Pathophysiological mechanisms of blood pressure as a risk factor for CAD are complex and include the influence of blood pressure as a physical force on the development of the atherosclerotic plaque, and the relationship between pulsatile hemodynamics/arterial stiffness and coronary perfusion. Treatment of arterial hypertension has been proven to prevent coronary events in patients without clinical CAD. Recent studies suggest that a lower systolic blood pressure may be appropriate, whereas caution is advised with diastolic blood pressure below 60 mmHg [14].
The risk of CAD events in hypertensive patients further increase in the presence of dyslipidemia. High cholesterol level, the major modifiable risk factor for heart disease, has both an environmental as well as a genetic component (mutations in the LDL receptor, ApoB, PCSK9, and ApoE genes) [15]. Familial Hypercholesterolemia (FH) is characterized by isolated elevation of plasma low density lipoprotein cholesterol and is associated with high risk of premature cardiovascular disease. Premature CAD in the presence of hypercholesterolemia might be due to an unhealthy lifestyle alone or due to genetic factors in combination with an unhealthy lifestyle [15].
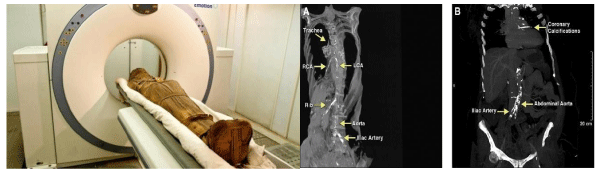
Atherosclerosis in the eras of ancient Egypt
Definite or probable atherosclerosis was present in mummies who lived during virtually every era of ancient Egypt, which published recently in JACC as the “Horus study” [16], a time span of 2,000 years. The investigators performed whole body, Multi Slice Computed Tomography Scanning (MSCT) on 52 ancient Egyptian mummies from the Middle Kingdom to the Greco Roman period to identify cardiovascular structures and arterial calcifications. The estimated age at the time of death was measured from the computed tomography skeletal evaluation. Adel Alam, et al. [16] reported forty-four of 52 mummies had identifiable Cardiovascular (CV) structures, and 20 of these had either definite atherosclerosis (defined as calcification within the wall of an identifiable artery, n = 12) or probable atherosclerosis (defined as calcifications along the expected course of an artery). Calcifications were found in the aorta as well as the coronary, carotid, iliac, femoral, and peripheral leg arteries. The 20 mummies with definite or probable atherosclerosis were older at time of death (mean age 45.1 +/- 9.2 years) than the mummies with CV tissue but no atherosclerosis (mean age 34.5 +/- 11.8 years, p = 0.002). Two mummies had evidence of severe arterial atherosclerosis with calcifications in virtually every arterial bed. Definite coronary atherosclerosis was present in 2 mummies, including a princess who lived between 1550 and 1580 BCE. This finding represents the earliest documentation of coronary atherosclerosis in a human [16] (Figure 2).
Interestingly, the investigators of Horus study [16] demonstrated an evidence of atherosclerosis in almost all the dynastic eras of ancient Egypt. The prevalence of modern day risk factors for atherosclerosis in ancient Egypt is challenging to estimate. Tobacco was unavailable, and without modern transportation, an active lifestyle was likely, but the incidence of hypertension and diabetes mellitus is unknown. Although the diet of a particular ancient Egyptian with or without atherosclerosis is difficult to ascertain, hieroglyphic inscriptions on Egyptian temple walls indicate that beef, sheep, goats, wildfowl, bread, and cake were regularly consumed suggested that the ancient Egyptian diet may have been atherogenic (high in saturated fat), particularly among the clergy who consumed the ritual feasts left by families mourning their deceased relatives.
Cardiac imaging in systemic hypertension complaining of chest pain
Identification of myocardial ischemia among hypertensive population is recommended to reclassify patients who are at risk. Non-invasive CV imaging is progressively being used and continues to provide new technological opportunities to CAD (Macro vascular) and micro vascular dysfunction evaluation at early stage.
Echocardiography and deformation imaging resting study
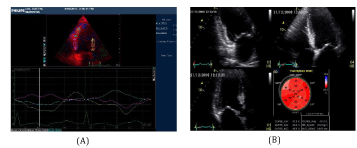
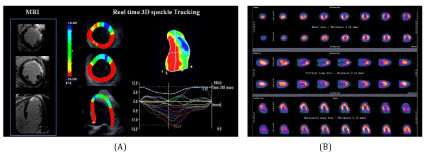
Diabetes and hypertension are two major risk factors which are often associated with the impairment of Global Longitudinal Systolic Strain (GLS), GLS are also common findings in patients with Heart Failure with Preserved Ejection Fraction (HFpEF) and it is now well known that Left Ventricular Hypertrophy (LVH) is the key structural change of HTN. Probably, repetitive ischemic insults due to macro-vascular and micro-vascular abnormalities and interstitial fibrosis cause an early intrinsic depression of subendocardial longitudinal fiber contractility in these patients, especially in those who have hypertrophic hearts. For this reason, longitudinal myocardial performance is impaired at an early stage in patients with HTN. In addition, in the first stage of the disease the sparing of circumferential fibers results in Ejection Fraction (EF) remaining within the normal range [17] (Figure 3).
Thus, GLS provides additional information in the evaluation of LVH and myocardial ischemia in HTN, and regional changes in longitudinal strain seem to identify specific myocardial deformation patterns for some forms of myocardial hypertrophy [18]. It is clear nowadays that myocardial fiber mechanical dysfunction precedes histopathological changes such as fibrosis and hypertrophy in essential hypertension which can be detected in the preclinical stage of the disease, through a reduced tissue Doppler derived systolic and diastolic velocities and depressed GLS either before or even without the development of LVH [17-19].
Stress imaging studies in HTN patients with suspected CAD
Myocardial Perfusion Imaging (MPI); Single Photon Emission Computed Tomography (SPECT); Stress Echocardiography (SE); Cardiac Magnetic Resonance Imaging (CMR).
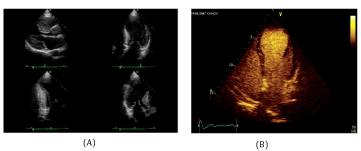
Myocardial ischemia ESC guidelines, recommend the search for myocardial ischemia in hypertensive patients with suspected history of CAD. Diagnosis of myocardial ischemia in hypertensive patients is particularly challenging because HTN substantially lowers the specificity of exercise ECG and perfusion scintigraphy [19,20]. When exercise ECG is either uninterpretable or ambiguous, an imaging test of inducible ischemia by functional study such as perfusion scintigraphy [21], SE [22], or stress CMR [23], is warranted. Among the imaging tests, stress echo has been shown to have higher specificity but reduced sensitivity compared with SPECT imaging [24]. In fact, stress induced wall motion abnormalities are highly specific for detecting epicardial coronary artery stenosis angiographic ally, whereas myocardial perfusion abnormalities are frequently detected in hypertensive patients with normal coronary arteries and coronary micro vascular disease angiographically (Figures 4,5). Use of dual echo imaging of regional wall motion and Coronary Flow Reserve (CFR) (normal values > 2) on left anterior descending artery to distinguish epicardial coronary stenosis from isolated coronary microcirculatory dysfunction (reduced CFR without wall motion abnormalities) has been proposed. Stress CMR is a valuable option to assess myocardial ischemia in HTN patients and should be considered when stress echocardiography is expected to be sub optimal due to poor window [25]. Figure 5 shows SPECT Myocardial Perfusion Imaging, STE and CMR.
MSCT coronary angiography
Coronary artery calcium is recognized as an independent predictor of CV events and mortality, whereas absence of coronary calcium is associated with a very high negative predictive value [26]. Yet the role for risk stratification of uncomplicated HTN is not well defined. In fact, the inclusion of coronary calcium into prediction models mainly improves risk stratification of hypertensive patients at intermediate risk (SCORE risk between 5 and 10%), whereas little value has been demonstrated in patients at low risk [27].On the other hand, the limited availability, costs, and radiation exposure (±1 mSv) represent substantial limitations to wide spread implementation of coronary calcium evaluation in clinical practice.
Clinical implications of cardiac imaging on health care and “HTN patients” management
The correct diagnosis of hypertension and precise assessment of cardiovascular risk are essential to give proper treatment in patients with hypertension. Risk stratification in HTN patients is of crucial importance to properly manage patients at increasing risk and prevent adverse events. A symptomatic involvement of different organs in HTN patients represents an independent determinant of CV risk and the identification of target organ damage is recommended to further reclassify patient’s risk.
Although echocardiography and allied techniques (MSCT, CMR, MPI) are the second line study in the evaluation of hypertensive patients, it gives many clues suggesting poor prognosis associated with hypertension, including LVH, decreased LV systolic function, impaired LV diastolic function, and increased left atrial size and impaired systolic function. Along with conventional echocardiographic methods, tissue Doppler imaging, three dimensional echocardiography, SE and GLS speckle tracking echocardiography are emerging echocardiographic modalities in the evaluation of hypertensive patients in the current echocardiographic laboratories. Understanding conventional and newer echocardiographic parameters is important in the diagnosis and assessment of cardio vascular risk in hypertensive patients.
Recommendations of echocardiography in the current hypertension guidelines
In the 2013, ESH/ESC Guidelines for the management of arterial hypertension, echocardiography is the second line study based on medical history, physical examination, and findings from routine laboratory tests [28]. The guidelines recommended performing echocardiographic examination in patients who are suspected with having Left Ventricular Hypertrophy (LVH), Left Atrial (LA) dilatation, or Concomitant Heart Diseases (CAD).
Advances in echocardiography and CMR techniques over the last decade have provided new insights into the morphological and pathophysiological changes associated with Hypertensive Heart Disease (HHD). Comprehensive assessment of systolic and diastolic function provides prognostically relevant data. CMR is a particularly attractive imaging technique, provides the most accurate and reproducible measures of LV function and mass, and enables myocardial fibrosis assessment which could identify patients at risk of serious cardiac arrhythmias and sudden arrhythmic death. In addition, CMR is the most reliable means of distinguishing hypertension related LVH from other causes and can also be used to screen for secondary causes of systemic hypertension [29].
Cardiac impact of hypertension therapy LVH regression
LVH represents an important end organ consequence of hypertension. Population based studies using echocardiography have demonstrated hypertrophy to be closely linked with adverse events [30], including stroke, renal impairment, left ventricular dysfunction, atrial and ventricular arrhythmias, and sudden cardiac arrhythmia or premature death [31]. The eventual development of complications from LVH represents long term effects that are too final to guide clinical therapy, and too slow as a research outcome. Therefore, LVH has been proposed as a surrogate marker of outcome. LVH has been shown to be reversed or prevented by a variety of haemodynamic, non haemodynamic and pharmacological factors [32]. While reductions in the ventricular mass have been associated with improved outcome across populations, in studies which identify regression of hypertrophy on an individual basis, large populations are required to overcome the variability of these measurements. Thus, while the association between LVH regression and improved outcome has now been recognized in a number of studies [33]. This role of echocardiography in accurate detection of LVH and its regression is great and definitely may be improved by the enhancement and clinical use of 3DE, which has been validated against CMR [34].
Moreover, LVH occurs in 36-41% of hypertensive subjects [35], but hypertension is not the only cause of this problem. Hypertrophy may be influenced by obesity, diabetes, the metabolic syndrome, and renal impairment, among other etiologies. Progression of the condition may lead to ischemia, both due to concurrent CAD as well as failure of vascular proliferation to match myocardial proliferation, vascular compression, and the effect of raised LV pressure on subendocardial flow (relative ischemia) or even MVD. Hence, the role of non-invasive cardiac imaging is crucial in order to identify the etiology of myocardial ischemia in such patient’s population.
Investigation of chest pain symptoms
Chest pain in patients with hypertension may signify concurrent CAD or may simply reflect sub endocardial ischemia due to LV hypertrophy and increased afterload. The diagnosis of CAD has particular challenges in this setting, because ‘false positive’ results may occur when sub endocardial ischemia causes abnormal stress ECG or myocardial perfusion scan in the absence of flow limiting epicardial coronary disease [36]. A normal stress electrocardiogram, performed to a high workload, has a high negative predictive value, but an abnormal or ambiguous test warrants further evaluation. There is some evidence in favor of preferential use of stress echocardiography for this purpose, because stress induced wall motion abnormalities are highly specific for CAD, while perfusion defects (MPI) in hypertensive patients may arise from abnormal myocardial flow reserve not due to epicardial coronary disease [37].
Role of echocardiography in decision to initiate treatment
Effects of antihypertensive agents on Left Ventricular Mass (LVM) and other echocardiographic surrogate endpoints (e.g. LA size and diastolic function) have been extensively studied. Several large studies sponsored by the National Institutes of Health and the US Veterans Administration Cooperative Studies program have evaluated the effects of antihypertensive mono therapy. In general, it appears likely that there are differences between the efficacy of antihypertensive drugs and their effects on LVH. LVH regression does not adversely affect cardiac function and may be associated with improvements in diastolic function. However, although the finding of increased LVM on echocardiography could potentially guide selection of initial or intensity of therapy in hypertensive patients, JNC 7 recommendations do not risk stratify patients for treatment on the basis of target organ damage. Current guidelines recommend the use of combination treatment to get blood pressure to goal, thus blood pressure remains the primary target of therapy. A part of the problem with getting a more central role for echocardiography to guide therapy is that despite the adverse prognosis associated with LVH in hypertension, there are inconsistent data from numerous studies that have evaluated the comparative efficacy of specific antihypertensive agents in LVH regression, as well as survival benefits associated with LVH regression. In a meta-analysis of trials of antihypertensive therapy, angiotensin converting enzyme inhibitors were the most effective agents, leading to a 13.3% reduction in LVM compared with 9.3% for calcium channel blockers, 6.8% for diuretics, and 5.5% for beta blockers [38]. However, in a comparison of Enalapril and long acting nifedipine in patients with essential hypertension, the PRESERVE (Prospective Randomized Enalapril Study Evaluating Regression of Ventricular Enlargement) trial, systolic and diastolic pressures, as well as LVM were reduced to a similar degree with both agents [39]. On the other hand, the LIFE (Losartan Intervention For Endpoint Reduction in Hypertension) trial echocardiographic sub study demonstrated superior LVM reduction (21.7 g/m2 ) in patients treated with the angiotensin receptor blocker losartan compared with those treated with the beta blocker atenolol (17.7 g/m2 ) [40]. Finally, despite a 20% incidence of LVH regression with placebo, diuretic therapy with chlorthalidone and hydrochlorthiazide, respectively, demonstrated greater LVH regression over alternative agents in both the TOMHS (Treatment of Mild Hypertension Study) and Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents [41,42].
In summary, echocardiography may be helpful in several scenarios. Patients with hypertensive heart disease who become symptomatic require follow-up echocardiography to evaluate systolic and diastolic function. Dissociation between blood pressure measurements and LV hypertrophy is an indication for further testing. On the other hand, stress echocardiography or MPI showed be performed for evaluation of the nature of chest pain in hypertensive patients with LVH.
Genetic testing in HTN patients
Studies involving adoptive families and twins have demonstrated the genetic basis of hypertension and shown that genetic factors account for about 40% of the variance in blood pressure among individuals. Arterial hypertension is genetically complex: Multiple genes influence the blood pressure phenotype through allelic effects from single genes and gene interactions. Moreover, environmental factors also modify the blood pressure phenotype. This complexity explains why the identification of the underlying genes has not been as successful in hypertension as in other diseases (such as type 1 and type 2 diabetes mellitus). The identification of the genetic determinants of hypertension has been most successful in endocrine forms of hypertension, which have well defined phenotypes that permit a precise patient stratification into homogeneous cohorts [43].
Research has shown that genetic factors contribute significantly to the susceptibility of developing hypertension [44-47]. A study of Caucasian and African American children revealed the T235 allele on the angiotensinogen gene to be more common in African American children compared to Caucasian. This is meaningful such that the T235 allele is positively correlated with increased serum angiotensinogen levels and hypertension in African American boys and girls, when compared to Caucasian children (p < 0.01) [48]. Other groups addressing African Americans have found that Single Nucleotide Polymorphisms (SNPs) on SLC4A5, a sodium bicarbonate transporter gene found on chromosome 2, were also significantly associated with hypertension [49,50].
A promising area for the application of genetic testing to personalized medicine is the prediction of responses and adverse reactions to antihypertensive drugs.
Conclusion
Patients with systemic hypertension often experience chest pain despite the absence of obstructive coronary artery disease, which is caused by coronary micro vascular dysfunction. eNOS gene polymorphism is proved to be an important etiology in micro vascular angina (x-syndrome) among hypertensive patients. In addition, the eNOS mutant gene showed a significant increase in isolated HPN and in patients with CAD as well. The major cardiovascular risk factors responsible for small vessel disease (micro-vascular) are similar for those attributable to CAD (macro-vascular) in systemic hypertension. Repetitive ischemic insults in HTN patients due to macro-vascular and micro-vascular abnormalities and interstitial fibrosis can cause an early intrinsic depression of sub endocardial longitudinal fiber contractility and this can be detected clearly non-invasively by GLS speckle tracking echocardiography. Similarly, microvascular dysfunction can be demonstrated with great certainty by MPI. Currently, non-invasive cardiac imaging is being increasingly used, and innovative imaging techniques are on the way that might further refine risk stratification of hypertensive patients and provide opportunities to better target therapeutic strategies.
- Williams RR, Hunt SC, Hasstedt SJ, Hopkins PN, Wu LL, Berry TD, et al. Are there interactions and relations between genetic and environmental factors predisposing to high blood pressure? Hypertension. 1991; 18: I29-I37. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1889856
- Zhuo ML, Huang Y, Chen JZ, Sun LH, Yang RF, Chen HZ, et al. Endothelium specific overexpression of human IC53 down regulates endothelial nitric oxide synthase activity and elevates systolic blood pressure in mice. Cardiovasc Res. 2009; 84: 292-299. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19541669
- Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. 2012; 14: 254-260. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22427070
- Brush JE Jr, Cannon RO, Schenke WH, Bonow RO, Leon MB, Maron BJ, et al. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med. 1988; 319: 1302-1307. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3185633
- Hamasaki S, Al Suwaidi J, Higano ST, Miyauchi K, Holmes DR Jr, Lerman A. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy. J Am Coll Carl. 2000; 35: 1654-1660. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10807473
- Kwame Osei. Insulin resistance and systemic hypertension. Amer J Cardiol. 1999; 84: 33-36. https://bit.ly/31GS8J6
- Agrawal S, Mehta PK, Bairey Merz CN. Cardiac syndrome x: update 2014. Cardiol Clin. 2014; 32: 463-478. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25091971
- Godsland IF, Crook D, Stevenson JC, Collins P, Rosano GM, Lees B, et al. Insulin resistance syndrome in postmenopausal women with cardiological syndrome x. Br Heart J. 1995; 74: 47-52. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7662453
- Antonios TF, Kaski JC, Hasan KM, Brown SJ, Singer DR. Rarefaction of skin capillaries in patients with anginal chest pain and normal coronary arteriograms. Eur Heart J. 2001; 22: 1144-1148. https://bit.ly/2UIiJEq
- Kaski JC, Aldama G, Cosin Sales J. Cardiac syndrome X. Diagnosis, pathogenesis and management. Am J Cardiovasc Drugs. 2004; 4: 179-194. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15134470
- Nagib El Kilany, GE Nayel, E r Hazzaa S. Nitric oxide synthase gene G298 allele: Is it a marker for microvascular angina in hypertensive patients? Cardiovas Radiat Medi. 2004; 5: 113-118. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15721845
- Wenquan N, Yue Q. An Updated meta-analysis of endothelial nitric oxide synthase gene: Three well-characterized polymorphisms with hypertension. PLoS ONE. 2011; 6: e24266. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21912683
- Kaski JC, Vitale C. Microvascular angina and endothelial dysfunction. E J Cardiol Pract. 2016; 14: https://bit.ly/2OFLtK0
- Weber T, Lang I, Zweiker R, Horn S, Wenzel RR, Watschinger B, et al. Hypertension and coronary artery disease: Epidemiology, physiology, effects of treatment, and recommendations. A joint scientific statement from the Austrian Society of Cardiology and the Austrian Society of Hypertension. Wiener klinische Wochenschrift J. 2016; 128: 467-479. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27278135
- AK Soutar, RP Naoumova. Mechanisms of disease: Genetic causes of familial hypercholesterolemia. Nature Clin Pract Cardiovasr Med. 2007; 4: 214-225. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17380167
- Adel H Allam, Randall C Thompson, L Samuel Wann, Michael I Miyamoto, Abd el Halim Nur el Din, Gomaa Abd el Maksoud, et al. Atherosclerosis in ancient Egyptian mummies the horus study. Cardiovasc Imaging: JACC. 2011; 4: 315-27. https://bit.ly/2w2N0Dq
- Todaro MC, Khandheria BK, Longobardo L, Zito C, Cusma Piccione M, Di Bella G, et al. New diagnostic perspectives on heart failure with preserved ejection fraction: Systolic function beyond ejection fraction. J Cardiovas Med J Cardiovasc Med (Hagerstown). 2015; 16: 527-537. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25469729
- Luis SA, Chan J, Pellikka PA. Echocardiographic assessment of left ventricular systolic function: An overview of contemporary techniques, including speck-le-tracking echocardiography. Mayo Clin Proc. 2018: 94: 125-138. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30611439
- Picano E, Palinkas A, Amyot R. Diagnosis of myocardial ischemia in hypertensive patients. J Hypertens. 2001; 19: 1177-1183. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11446706
- Gargiulo P, Dellegrottaglie S, Bruzzese D, Savarese G, Scala O, Ruggiero D, et al. The prognostic value of normal stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: A meta-analysis. Circ Cardiovasc Imaging. 2013; 6: 574-582. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23771988
- Schulman DS, Francis CK, Black HR, Wackers FJ. Thallium-201 stress imaging in hypertensive patients. Hypertension. 1987; 10: 16-21. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2954904
- Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, et al. Stress echocardiography expert consensus statement- executive summary: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2008; 9: 415-437. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18579481
- Maceira AM, Mohiaddin RH. Cardiovascular magnetic resonance in systemic hypertension. J Cardiovasc Magn Reson. 2012; 14: 28. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22559053
- Gargiulo P, Petretta M, Bruzzese D, Cuocolo A, Prastaro M, D Amore C, et al. Myocardial perfusion scintigraphy and echocardiography for detecting coronary artery disease in hypertensive patients: A meta-analysis. Eur J Nucl Med Mol Imaging. 2011; 38: 2040-2049. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21814850
- Chin D, Battistoni A, Tocci G, Passerini J, Parati G, Volpe M. Non-invasive diagnostic testing for coronary artery disease in the hypertensive patient: Potential advantages of a risk estimation based algorithm. Am J Hypertens. 2012; 25: 1226-1235. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22785407
- Valenti V, O Hartaigh B, Heo R, Schulman Marcus J, Cho I, Kalra DK, et al. Long-term prognosis for individuals with hypertension undergoing coronary artery calcium scoring. Int J Cardiol. 2015; 187: 534-540. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25863296
- Mallikethi Reddy S, Rubenfire M, Jackson LA, Brook RD. Coronary artery calcium in hypertension: A review. J Am Soc Hypertens. 2015; 9: 993-1000. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26489731
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013; 31: 1281-1357. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23817082
- Hoey ET, Pakala V, Teoh JK, Simpson H. The role of imaging in hypertensive heart disease. Int J Angiol. 2014; 23: 85-92. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25075160
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the framingham heart study. N Engl J Med. 1990; 322: 1561-1566. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2139921
- Verdecchia P, Angeli F, Achilli P, Castellani C, Broccatelli A, Gattobigio R, et al. Echocardiographic left ventricular hypertrophy in hypertension: Marker for future events or mediator of events? Curr Opin Cardiol. 2007; 22: 329-334. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17556886
- Gosse P. Left ventricular hypertrophy as a predictor of cardiovascular risk. J Hypertens Suppl. 2005; 23: S27-S33. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15821448
- Nadour W, Biederman RW. Is left ventricular hypertrophy regression important? Does the tool used to detect it matter? J Clin Hypertens (Greenwich). 2009; 11: 441-447. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19695032
- Shimada YJ, Shiota T. Meta-analysis of accuracy of left ventricular mass measurement by three-dimensional echocardiography. Am J Cardiol. 2012; 110: 445-452. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22541420
- Cuspidi C, Sala C, Negri F, Mancia G, Morganti A. Prevalence of left ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012; 26: 343-349. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22113443
- Picano E, Palinkas A, Amyot R. Diagnosis of myocardial ischemia in hypertensive patients. J Hypertens. 2001; 19: 1177-1183. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11446706
- Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, et al. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001; 103: 102-107. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11136693
- Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A meta-analysis of randomized double blind studies. JAMA. 1996; 275: 1507-1513. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8622227
- Devereux RB, Palmieri V, Sharpe N, De Quattro V, Bella JN, de Simone G, et al. Effects of once-daily angiotensin-converting enzyme inhibition and calcium channel blockade-based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension: The prospective randomized enalapril study evaluating regression of ventricular enlargement (preserve) trial. Circulation. 2001; 104: 1248-1254. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11551875
- Okin PM, Devereux RB, Nieminen MS, Jern S, Oikarinen L, Viitasalo M, et al. Electrocardiographic strain pattern and prediction of cardiovascular morbidity and mortality in hypertensive patients. Hypertension. 2004; 44: 48-54. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15173125
- Liebson PR, Grandits GA, Dianzumba S, Prineas RJ, Grimm RH Jr, Neaton JD, et al. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995; 91: 698-706. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7828296
- Gottdiener JS, Reda DJ, Massie BM, Materson BJ, Williams DW, Anderson RJ. Effect of single-drug therapy on reduction of left ventricular mass in mild to moderate hypertension: comparison of six antihypertensive agents. The department of veterans affairs cooperative study group on antihypertensive agents. Circulation. 1997; 95: 2007-2014. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9133508
- Rossi GP, Ceolotto G, Caroccia B,Lenzini L .Genetic screening in arterial hypertension. Nat Rev Endocrinol. 2017; 13: 289-298. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28059156
- Taylor J, Sun Y, Chu J, Mosley T, Kardia S. Interactions between metallopeptidase 3 polymorphism rs679620 and BMI in predicting blood pressure in African-American women with hypertension J Hypertens. 2008; 26: 2312-2318. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19008710
- Taylor J, Sun Y, Hunt SC, and Kardia SL. Gene-environment interaction for hypertension among African American women across generations. Biol Res Nurs. 2010; 12: 12149-12155. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20591971
- Smith JG, Magnani JW, Palmer C, Meng YA, Soliman EZ, Musani SK, et al. Genome-wide association studies of the PR interval in Afri-can Americans. PLoS Genet. 2011; 7: e1001304. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21347284
- Fox ER, Young JH, Li Y, Dreisbach AW, Keating BJ, Musani SK, et al. Association of genetic variation with systolic and diastolic blood pressure among African Americans: the candidate gene association resource study. Hum Mol Genet. 2011; 20: 2273-2284. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21378095
- Bloem L, Manatunga A, Tewksbury D, Pratt J. The serum angiotensinogen concentration and variants of the angiotensinogen gene in white and black children. J Clin Invest. 1995; 95: 948-953. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7883995
- Barkley RA, Chakravarti A, Cooper RS, Ellison RC, Hunt SC, Province MA, et al. Positional identification of hypertension susceptibility genes on chromosome 2. Hypertension. 2004: 43; 477-482. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14732741
- Cooper RS, Luke A, Zhu X, Kan D, Adeyemo A, Rotimi C, et al. Genome scan among Nigerians linking blood pressure to chromosomes 2, 3, and 19. Hypertension. 2002: 40; 629-633. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12411454

Sign up for Article Alerts