Long-Term Follow-Up of Atrial Septal Defect Closure after Percutaneous Balloon Mitral Valvuloplasty by Transesphageal Echocardiography?
Samir Rafla* and Tarek Bishay
Alexandria University, Faculty of Medicine, Cardiology Department and National Heart Institute, Embaba, Cairo
*Address for Correspondence: Samir Rafla, Alexandria University, Faculty of Medicine, Cardiology Department and National Heart Institute, Embaba, Cairo, Tel: +010-014-95577, E-mail: smrafla@yahoo.com
Submitted: 21 February 2019; Approved: 03 April 2019; Published: 04 April 2019
Citation this article: Rafla S, Bishay T. Long-Term Follow-Up of Atrial Septal Defect Closure after Percutaneous Balloon Mitral Valvuloplasty by Transesphageal Echocardiography. Int J Cardiovasc Dis Diagn. 2019;4(1): 001-007.
Copyright: © 2019 Rafla S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Iatrogenic atrial septal defect; Balloon mitral valvotomy; Transesophageal echocardiography
Download Fulltext PDF
Percutaneous Balloon Mitral Valvuloplasty (PBMV) involves atrial septostomy during the procedure. One of the consequences of transseptal puncture is the creation of an Atrial Septal Defect (ASD). Transesophageal Echocardiography (TEE) can detect Left to Right (L-R) shunts too small to be detected by other methods. The aim of this study was to evaluate the 3 years follow-up of ASD closure after PBMV by TEE. 200 consecutive patients with rheumatic Mitral Stenosis (MS) who underwent successful PBMV by using the Inoue balloon catheter were studied prospectively. ASD with small L-R atrial shunting occurred in all the patients (100%) immediately after PBMV. Total study 200 patients. All the ASDs were small in size (≤ 5 mm). The puncture site (ASD site) occurred in the fossa ovalis (Fo.Ov) in 120 patients (60%), while it occurred outside the Fo.Ov (either in the superior limbus or in the inferior limbus of the Interatrial Septum (IAS)) in the other 80 patients (40%). 180 patients presented at 6 month follow-up. ASD was closed in 117 patients (65%), while it was persisted in 63 patients (35%). 95 patients presented at 3 years follow-up. ASD was closed in 76 patients (80%) (Group I), while it was persisted in 19 patients (20%) (Group II). All the 74 patients who had ASD immediately after PBMV in the Fo.Ov, presented with ASD closure at 3 years follow-up. Only 2 patients who had ASD immediately after PBMV outside the Fo.Ov, presented with ASD closure at 3 years follow-up. All the 19 patients who presented at 3 years follow-up with ASD persistence had ASD immediately after PBMV outside the Fo.Ov (14 in the superior limbus and 5 in the inferior limbus). No patient presented at 3 years follow-up with ASD persistence, had ASD immediately after PBMV in the Fo.Ov Large LAD, high total Echocardiographic (echo) score of the Mitral Valve (MV), thick Fo.Ov, thick superior limbus, thick inferior limbus and ASD site immediately after PBMV outside the Fo.Ov were significant predictors of ASD persistence at 3 years follow-up.
In conclusion, ASD with L-R atrial shunting occurs in all the patients after PBMV by using the Inoue balloon catheter. ASD after PBMV persists in 20% of the patients at 3 years follow-up. Predictors of ASD persistence at 3 years follow-up are: large LAD, high total echo score of the MV, thick Fo.Ov, thick superior limbus, thick inferior limbus and ASD site immediately after PBMV outside the Fo.Ov. ASD closes at 3 years follow-up in all the patients who had ASD in the Fo.Ov immediately after PBMV. All the patients with ASD persistence at 3 years follow-up had ASD outside the Fo.Ov after PBMV. It is recommended that operators doing transseptal puncture during PBMV by using the Inoue balloon catheter should aim to do it in the Fo.Ov.
Abbreviations
PBMV: Percutaneous Balloon Mitral Valvuloplasty; IASD: Iatrogenic Atrial Septal Defect; CFI; Color Flow Doppler Imaging; TTE: Transthoracic Echocardiography; IAS: Interatrial Septum; TEE: Transesophageal Echo; MVA: Mitral Valve Area; MR: Mitral Regurgitation; TS: Transseptal.
Introduction
Rheumatic heart disease is a chronic manifestation of rheumatic carditis, which occurs in 20% of cases of rheumatic fever (increases to 80% if the heart is already affected from first attack) [1-4]. The endocardial lesion most often leaves permanent sequela resulting in valvular regurgitation, stenosis, or both. Before the advent of PBMV, most patients with symptomatic MS were treated with surgical mitral commissurotomy, either open or closed.
Percutaneous Balloon Mitral Valvuloplasty (PBMV) is a safe and effective procedure for relief of severe mitral stenosis. Indications for PBMV: The management of symptomatic and severe MS is described comprehensively in recent guidelines published by the European Society of Cardiology (ESC) [1].
Contraindications to PBMV: Persistent left atrial or left atrial appendage thrombus, more than moderate mitral regurgitation, massive or bicommissural calcification.
During PBMV, a transseptal puncture is made. A hole is created in the atrial septum, so that dilating balloons can be advanced across the septum to the MV. Thus, an atrial communication is an obligatory part of the procedure [5-12]. The knowledge of iatrogenic Atrial Septal Defect (iASD) is vital.
Persistent iatrogenic Atrial Septal Defects (iASD) after structural TS interventions are not uncommon especially when larger TS sheaths are used (25%-50% with 22 Fr sheaths) [6-13]. The optimal management strategy of postprocedural iASD is currently unknown. In the absence of societal recommendations with regards to iASD, the decision to close iASD and the timing of the closure pose a clinical dilemma to the interventionist caring for these patients [5].
If a very sensitive method to detect an L-R shunt is used, a shunt through the atrial septum should be detected immediately after PBMV. TEE with color Flow Doppler Imaging (CFI) can detect L-R shunts too small to be detected by other methods [5-11].
In this study, we evaluated the factors affecting the development of iatrogenic atrial septal defect and searched for the factors that can minimize undue or exaggerated defect.
Aim of the Work
To evaluate the 3 years follow-up of ASD closure after PBMV by TEE; and to try to find means to minimize the effect of this iatrogenic shunt; and to define Predictors of ASD persistence.
Patients and Methods
200 consecutive patients with rheumatic MS who underwent successful PBMV were studied prospectively at the NHI during the period between January 2008 and November 2011.
Exclusion criteria:
• Patients with intracardiac masses on thrombi
• Patients with significant MR (moderate or severe MR)
• Patients with ASD, patent foramen ovale or interatrial septal aneurysm
• Patients with previous PBMV or surgical mitral commissurotomy
• Patients with multiple atrial septal punctures during the procedure
• Patients who developed restenosis at 6 month follow-up after the procedure
Every patient was subjected to:
1. Complete history taking
2. Clinical examination
3. Chest X-Ray
4. ECG
5. Transthoracic Echocardiography (TTE)
6. PBMV procedure: was done by using the Inouye balloon catheter technique [1].
7. TEE:
• TEE was done by using multiplane probe.
• TEE was done before the procedure, within 24 hours after the procedure, at 6 month follow-up and at 3 years follow-up.
• Standard transverse and longitudinal views were used for precise examination and measurements of the interatrial septum.
• The IAS thickness was measured at its thinnest part which is the Fo.Ov and at the superior and the inferior limb of the IAS.
• ASD was evaluated after PBMV, at 6 month follow-up and at 3 years follow-up. L-R atrial shunting was determined by CFI and PW Doppler.
• The MV was scored by using the criteria described by Wilkins et al. [8].
6 Month follow-up
• TTE and TEE were done to the patients who presented at 6 month follow-up.
• Restenosis: Echocardiographic restenosis was defined at follow-up as a loss of 50% of the initial gain and a valve area < 1.5 cm2 [9].
3 Years follow-up
• TTE and TEE were done to the patients who presented at 3 years follow-up.
• Informed consent was obtained from the patients.
Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale are followed [1].
Statistical Analysis
· Quantitative data were expressed as mean ± standard deviation
· Student’s t test was used to compare quantitative data between 2 groups
· Discrete variables were compared with Chi squared and Fisher’s exact test
· A value of p < 0.05 was considered statistically significant. P < 0.01 was considered highly significant
Results
200 consecutive patients with MS who underwent successful PBMV were studied prospectively.
Immediate results
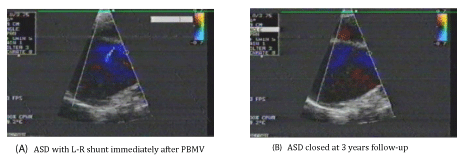
ASD occurred in all the patients (100%) after PBMV (Iatrogenic ASD). Small L-R atrial shunting occurred in all the patients determined by CFI (Figures 1-3) and PW Doppler (Figure 4). All the ASDs were small in size (≤ 5 mm). The diameter of the ASDs (ASD size) ranged from 2.0 to 5.0 mm with a mean of 3.02 ± 0.94 mm.
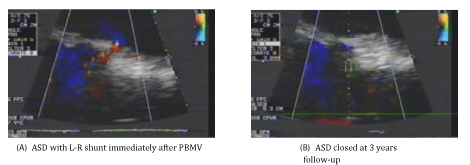
The puncture site (ASD site) occurred in the Fo.Ov in 120 patients (60%) (Figure 1-A), while it occurred outside the Fo.Ov in 80 patients (40%). In 30 patients (37.5%) of these 80 patients, it occurred in the superior limbus (Figure 2-A), while in the other 50 patients (62.5%), it occurred in the inferior limbus (Figure 3-A).
6 Month follow-up results
• 180 patients presented at 6 months after PBMV.
• ASD was closed in 117 patients (65%). In 88 patients (75.2%) of these 117 patients, ASD immediately after PBMV (before closure) was present in the Fo.Ov, while it was present outside the Fo.Ov in 29 patients (24.8%). In 11 patients (38%) of these 29 patients, it was in the superior limbus, while in the other 18 patients (62%); it was in the inferior limbus.
• ASD persisted in 63 patients (35%). All of them had L-R atrial shunting with the same degree as that immediately after PBWV. All the ASDs were small in size (≤ 5 mm) and with the same size as that immediately after PBMV in all the 63 patients. ASD site was in the Fo.Ov in 18 patients (28.6%), while it was outside the Fo.Ov in 45 patients (71.4%). In 17 patients (37.8%) of these 45 patients, it was in the superior limbus, while in the other 28 patients (62.2%); it was in the inferior limbus.
• 18 patients (10%) developed restenosis of the MV. So, they were excluded from subsequent follow-up study.
• No patients developed severe MR.
3 years follow-up results
• 95 patients presented at 3 years after PBMV. ASD was closed in 76 patients (80%) (Figure 1,3), while it was persisted in 19 patients (20%) (Figure 2).
• The patients were classified into 2 groups:
Group I: Included 76 patients (80%) with closed ASD. Their age ranged from 25 to 52 years old with a mean of 39.30 ± 10.46 years. 49 patients (64.5%) were females and 27 patients (35.5%) were males. 50 patients (65.8%) were in sinus rhythm and 26 patients (34.2%) were in Atrial Fibrillation (AF). LAD ranged from 4.0 to 6.4 cm with a mean of 5.31 ± 0.96 cm. Total echo score of the MV ranged from 6.0 to 9.0 with a mean of 7.16 ± 0.99. Mitral Valve Area (MVA) before PBMV ranged from 0.6 to 1.1 cm2 with a mean of 0.85 ± 0.19 cm2. The IAS thickness at the Fo.Ov (Fo.Ov thickness) ranged from 1.0 to 2.0 mm with a mean of 1.27 ± 0.19mm. The IAS thickness at the superior limbus (superior limbus thickness) ranged from 2.0 to 6.0 mm with a mean of 3.32 ± 1.16 mm. The IAS thickness at the inferior limbus (inferior limbus thickness) ranged from 2.0 to 8.0 mm with a mean of 3.12 ± 1.12 mm. In 74 patients (97.4%) of these 76 patients, the ASD immediately after PBMV (before closure) was present in the Fo.Ov, (Figure 1), while it was present outside the Fo.Ov in 2 patients (2.6%). In these 2 patients, it was in the inferior limbus (Figure 3).
• 18 patients (23.7%) developed restenosis at 3 years follow-up.
• 15 patients (19.7%) developed severe MR at 3 years follow-up.
Group II: Included 19 patients (20%) with ASD persistence. All of them had L-R atrial shunting with the same degree as that immediately after PBMV. All the ASDs were small in size (≤ 5.0 mm) and with the same size as that immediately after PBMV in all the 19 patients. Their age ranged from 30 to 54 years old with a mean of 41.47 ± 10.88 years. 12 patients (63.2%) were females and 7 patients (36.8%) were males. 12 patients (63.2%) were in sinus rhythm and 7 patients (36.8%) were in Atrial Fibrillation (AF). LAD ranged from 4.4 to 7.1 cm with a mean of 6.22 ± 1.19 cm. Total echo score of the MV ranged from 6.0 to 10.0 with a mean of 8.63 ± 1.67. Mitral Valve Area (MVA) before PBMV ranged from 0.6 to 1.0 cm2 with a mean of 0.80 ± 0.18 cm2. The IAS thickness at the Fo.Ov (Fo.Ov thickness) ranged from 1.0 to 2.1 mm with a mean of 1.71 ± 0.15 mm. The IAS thickness at the superior limbus (superior limbus thickness) ranged from 2.0 to 7.0 mm with a mean of 4.26 ± 1.76 mm. The IAS thickness at the inferior limbus (inferior limbus thickness) ranged from 2.0 to 8.0 mm with a mean of 5.84 ± 1.89 mm. The ASD site was outside the Fo.Ov in all the 19 patients (100%). In 14 patients (73.7%) of these 19 patients, it was in the superior limbus (Figure 2), while in the other 5 patients (26.3%); it was in the inferior limbus. So, no patients who had ASD in the Fo.Ov immediately after PBMV had ASD persistence.
• So, in all the 74 patients who had ASD site in the Fo.Ov immediately after PBWV, the ASD was closed at 3 years follow-up.
• 5 patients (26.3%) developed restenosis at 3 years follow-up.
• 4 patients (21%) developed severe MR at 3 years follow-up.
- No significant difference was found between the 2 groups as regards restenosis at 3 years follow-up
(Table 1).
- No significant difference was found between the 2 groups as regards severe MR at 3 years follow-up (Table 1).
Predictors of ASD persistence
• Several factors were compared in both groups (Table 2).
Patients with ASD persistence (Group II) at 3 years follow-up had:
1. A larger LAD (P < 0.01).
2. A higher total echo score of the MV (P < 0.001).
3. A thicker Fo.Ov of the IAS (P < 0.001).
4. A thicker superior limbus of the IAS (P < 0.5).
5. A thicker inferior limbus of the IAS (P < 0.001).
6. ASD site immediately after PBMV outside the Fo.Ov (100%).
Discussion
PBMV involves atrial septostomy during the procedure. One of the consequences of transseptal technique is the creation of an ASD [6-11].
The present study was designed to evaluate the 3 years follow-up of ASD closure after PBMV by TEE.
In the present study, 200 consecutive patients with MS who underwent successful PBMV were studied prospectively. ASD occurred in all the patients (100%) after PBMV. Small L-R atrial shunting occurred in all the patients and all the ASDs were small in size. The same result was reported by Rittoo et al. [11] and El-Kady et al. [14] and Arora et al. [15] detected ASD after PBMV in 92% of their series. They suggested that evaluation of the ASD flow probably could be difficult in some patients in their series. Yoshida et al. [6] detected ASD after PBMV in 87% of their series, however, they used single plane TEE probe for detecting ASD, while multiplane probe was used in the present study which is more sensitive than single plane probe for detecting ASD.
In the present study, the puncture site (ASD site) occurred in the Fo.Ov in 120 patients (60%), while it occurred outside the Fo.Ov (either in the superior limbus or in the inferior limbus) in the other 80 patients (40%). Rittoo et al. [11], reported ASD in the Fo.Ov in 89% of their patients, while it was outside the Fo.Ov in 11% of their series. El-Kady et al. [10], reported ASD in the Fo.Ov in 71.4% of their patients, while it was outside the Fo.Ov in the other 28.6% of their patients.
In the present study, 180 patients presented at 6 month follow-up. ASD was closed in 117 patients (65%), while it was persisted in 63 patients (35%). Yoshida et al. [6], reported spontaneous closure of ASD in 80% of their series at 6 month follow-up after PBMV. This may be related to the lower incidence of ASD immediately after PBMV in their series.
95 patients presented at 3 years after PBMV in the present study. ASD was closed in 76 patients (80%) (Group I), while it was persisted in 19 patients (20%) (Group II).
All the 74 patients who had ASD immediately after PBMV in the Fo.Ov, presented with ASD closure at 3 years follow-up. Only 2 patients who had ASD immediately after PBMV outside the Fo.Ov (in the inferior limbus), presented with ASD closure at 3 years follow-up.
All the 19 patients (20%) who presented at 3 years follow-up with ASD persistence had ASD immediately after PBMV outside the Fo.Ov (14 in the superior limbus and 5 in the inferior limbus). No patient presented at 3 years follow-up with ASD persistence, had ASD immediately after PBMV in the Fo.Ov
To determine the factors predicting ASD persistence at 3 years follow-up, several factors were compared in both groups. Large LAD, high total echo score of the MV, thick Fo.Ov, thick superior limbus, thick inferior limbus and ASD site immediately after PBMV outside the Fo.Ov were significant predictors of ASD persistence at 3 years follow-up in the present study.
Large LAD was a significant predictor of ASD persistence in the present study. This could be explained that larger LAD could make directing the balloon catheter towards the MV more difficult. El-Kady et al. [14] reported the same result.
High total echo score of the MV was a significant predictor of ASD persistence in the present study. This result is in agreement with the results of Casale et al. [9], and El-Kady et al. [14], Casale et al. [9], reported that patients with MS who have high MV score, present the most technical difficulties at PBMV. Prolonged manipulations of the balloons may be necessary to correctly position them across the MV. In addition, they reported that when commissural splitting of the MV is not easily accomplished, the balloons tend to move toward the atrial septum as they are inflated, which produces a sawing motion of the balloon shafts and may enlarge the ASD [5].
Thick Fo.Ov, thick superior limbs and thick inferior limbus of the IAS were significant predictors of ASD persistence at 3 years follow-up in the present study. This result is in agreement with the results of El-Kady et al. [14], Hung et al. [15], Hung et al. [16] reported that the risk of creating a significant interatrial shunting logically depends on the stress exerted on the IAS. They found that IAS resistance was an independent predictor of significant interatrial shunting after PBMV. They reported that septal thickness in an indicator of septal resistance which in turn determines the propensity to withstand the stress exerted on the transseptal access site. So, thick IAS results in some degree of septal resistance which can lead to ASD persistence at follow-up.
ASD site after PBMV outside the Fo.Ov was a significant predictor of ASD persistence at 3 years follow-up in the present study. This result is in agreement with the result reported by El-Kady et al. [14]. This could be explained that the Fo.Ov is the thinnest part of the IAS, while either the superior or the inferior limbus of the IAS are much thicker than the Fo.Ov so, the chance for spontaneous closure of the ASD in the Fo.Ov is much easier.
Arora et al. [15] reported that the Valsalva maneuver may produce Right to Left (R-L) shunting in a patient with an iatrogenic ASD. Also known is that a high incidence of interatrial communication is seen in patients with an unexplained cerebrovascular accident, therefore, realization of a persistent ASD after PBMV, however small, becomes important, especially if open heart surgery for MV disease is undertaken at a later date in these patients when these iatrogenic defects should be closed.
There are other publications on the subject of iatrogenic ASD that shows how important is this subject [17-28].
Conclusions and Recommendations
1. ASD with L-R atrial shunting occurs in all the patients after PBMV by using the Inoue balloon catheter.
2. ASD after PBMV persists in 20% of the patients at 3 years follow-up.
3. Predictors of ASD persistence at 3 years follow-up are: large LAD, high total echo score of the MV, thick Fo.Ov, thick superior limbus, thick inferior limbus and ASD site immediately after PBMV outside the Fo.Ov
4. ASD closes at 3 years follow-up in all the patients who had ASD in the Fo.Ov immediately after PBMV.
5. All the patients with ASD persistence at 3 years follow-up had ASD outside the Fo.Ov after PBMV.
It is recommended that operators doing transseptal puncture during PBMV by using the Inoue balloon catheter should aim to do it in the Fo.Ov. Serial TEE follow-up for patients with factors predicting ASD persistence is recommended. Also, in patients requiring MV surgery who had undergone PBMV before, the surgeon should be aware of a possible ASD and should assess the IAS at the time of surgery.
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017; 38: 2739-2791. https://bit.ly/2G1cWlu
- Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, Hijazi ZM, et al. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the American society of echocardiography and society for cardiac angiography and interventions. J Am Soc Echocardiogr. 2015; 28: 910-958. https://bit.ly/2Vt9Vzu
- Nobuyoshi M, Arita T, Shirai S, Naoya Hamasaki , Hiroyoshi Yokoi , Masashi Iwabuchi, et al. Percutaneous balloon mitral valvuloplasty: A Review. Circulation. 2009; 119: e211-e219. https://bit.ly/2FQiwWv
- Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease incidence and progression in the northern territory of australia, 1997 to 2010. Circulation. 2013; 128: 492-501. https://bit.ly/2CYjCPl
- Alkhouli M, Sarraf M, and Holmes D. Iatrogenic atrial septal defect. Circ Cardiovasc Interv. 2016; 9: 4. https://bit.ly/2WDKXxM
- Yoshida K, Yoshikawa J, Akasaka T, Yamaura Y, Shakuda M, Hozumi T, et al. Assessment of left-to-right atrial shunting after percutaneous mitral valvuloplasty by transesophageal color Doppler flow mapping circulation. 1989; 80: 1521- 1526. https://bit.ly/2CR94BF
- Kronzon I, Tunick OA, Glassman E, Slater J, Schwinger M, Freedberg RS. Transesophageal echocardiography to detect clots in candidates for percutaneous transseptal mitral balloon valvuloplasty. J Am Coll Cardiol. 1990; 16: 1320-1322. https://bit.ly/2HZbUZx
- Ishikura F, Nagata S, Yasuda S, Yamashita N, Miyatake K. Residual atrial septal perforation after percutaneous transvenous mitral commissurotomy with Inoue balloon catheter. Am Heart J. 1990; 120: 873-878. https://bit.ly/2I3YsDR
- Casale P, Block P, O’Shea JP, Palacios IF. Atrial septal defect after percutaneous mitral balloon valvuloplasty: immediate results and follow-up. J Am Coll Cardiol. 1990; 15: 1300-1304. https://bit.ly/2ON0oB2
- Eid Fawzy M, Ribeiro PA, Dunn B, Galal O, Muthusamy R, Shaikh A, et al. Percutaneous mitral valvotomy with the Inoue balloon catheter in children and adults: Immediate results and early follow-up. Am Heart J. 1992; 123: 462-465. https://bit.ly/2Uitbn5
- Rittoo D, Sutherland GR, Currie P, Starkey IR, Thomas RD. The comparative value of transesophageal and transthoracic echocardiography before and after percutaneous mitral balloon valvotomy: a prospective study. Am Heart J. 1993; 125: 1094-1105. https://bit.ly/2YKVr06
- Wilkins GT, Weyman AE. Abascal VM, Block P, Palacios IF. Percutaneous balloon dilatation of the mitral dilatation of the mitral value area: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988; 60: 299. https://bit.ly/2HWTTLd
- Thomas MR, Monaghan MJ, Michalis LK, Jewitt DE. Echocardiographic restenosis after successful balloon dilatation of the mitral valve with the Inoue balloon: experience of a United Kingdom Center. Br Heart J. 1993; 69: 418.
- El-Kady T, Bishay TA, Ghaffar TA, Salahuddin A, Ibrahim M. Closure pattern of atrial septal defect developed during percutaneous balloon mitral valvuloplasty at one year follow-up A TEE study. Medical Journal of Teaching Hospitals and Institutes. 2005; 64: 17.
- Arora R, Jolly N, Kalra GS, Khalilullah M. Atrial septal defect after balloon mitral valvuloplasty: a transesophageal Echocardiographic study. Angiology. 1993; 44: 217-221. https://bit.ly/2WJEu4g
- Hung JS, Lau KW, Lo PH, Chen MS, Wu JJ. Complications of Inoue balloon mitral commissurotomy: impact of operator experience and evolving technique. Am Heart J. 1999; 138: 114-121. https://bit.ly/2UdqSl7
- McGinty PM, Smith TW, Rogers JH. Transseptal left heart catheterization and the incidence of persistent iatrogenic atrial septal defects. J Interv Cardiol. 2011; 24: 254-263. https://bit.ly/2CXlHLg
- Schueler R, Öztürk C, Wedekind JA, Werner N, Stöckigt F, Mellert F, et al. Persistence of iatrogenic atrial septal defect after interventional mitral valve repair with the MitraClip system: a note of caution. JACC Cardiovasc Interv. 2015; 8: 450-459. https://bit.ly/2IcLQt9
- Singh SM, Douglas PS, Reddy VY. The incidence and long-term clinical outcome of iatrogenic atrial septal defects secondary to transseptal catheterization with a 12F transseptal sheath. Circ Arrhythm Electrophysiol. 2011; 4: 166-171. https://bit.ly/2OKxktK
- Devarakonda SB, Mannuva BB, Durgaprasad R, Velam V, Akula VS, Kasala L. Real time 3D echocardiographic evaluation of iatrogenic atrial septal defects after percutaneous transvenous mitral commissurotomy. J Cardiovasc Thorac Res. 2015; 7: 87-95. https://bit.ly/2HVZDoB
- Zanchetta M, Onorato E, Rigatelli G, Dimopoulos K, Pedon L, Zennaro M, et al. Use of Amplatzer septal occluder in a case of residual atrial septal defect causing bidirectional shunting after percutaneous Inoue mitral balloon valvuloplasty. J Invasive Cardiol. 2001; 13: 223-226. https://bit.ly/2G1aXhe
- Harikrishnan S, Titus T, Tharakan JM. Septal defects after percutaneous mitral valvotomy–all are not innocent. J Am Soc Echocardiogr. 2005; 18: 183-184. https://bit.ly/2U0kPvq
- Nakao M, Ch’ng JK, Sin YK, Chua YL, Lee CY. Acquired right-to-left shunt through an atrial septal perforation with cyanosis after percutaneous transvenous mitral commissurotomy. J Thorac Cardiovasc Surg. 2008; 135: 690-691. https://bit.ly/2K4LqHV
- Pitcher A, Schrale RG, Mitchell AR, Ormerod O. Percutaneous closure of an iatrogenic atrial septal defect. Eur J Echocardiogr. 2008; 9: 294-295. https://bit.ly/2I3PJS7
- Sur JP, Pagani FD, Moscucci M. Percutaneous closure of an iatrogenic atrial septal defect. Catheter Cardiovasc Interv. 2009; 73: 267-271. https://bit.ly/2FZxsCV
- Aznaouridis K, Hobson N, Rigg C, Bragadeesh T. Emergency percutaneous closure of an iatrogenic atrial septal defect causing right-to-left shunt and severe refractory hypoxemia after pulmonary vein isolation. JACC Cardiovasc Interv. 2015; 8: e179-e181. https://bit.ly/2FO71Pm
- Huntgeburth M, Müller-Ehmsen J, Baldus S, Rudolph V. Postinterventional iatrogenic atrial septal defect with hemodynamically relevant left-to-right and right-to-left shunt as a complication of successful percutaneous mitral valve repair with the Mitra Clip. Int J Cardiol. 2013; 168: e3-e5. https://bit.ly/2WNN8Pr
- Rich ME, Tseng A, Lim HW, Wang PJ, Su WW. Reduction of iatrogenic atrial septal defects with an anterior and inferior transseptal puncture site when operating the cryoballoon ablation catheter. J Vis Exp. 2015: e52811. https://bit.ly/2IcTeF0



Sign up for Article Alerts