Ablation of Atrioventricular Nodal Tachycardia through a Point by Point Analysis of the Stability of an Irrigated Catheter?
Paolo Sabbatani*, Alessandro Corzani, Beatrice Gardini and Marco Marconi
Bufalini Hospital Cesena, Italy
*Address for Correspondence: Paolo Sabbatani, Bufalini Hospital Cesena, Italy, Tel: +390-547-352832; E-mail: sabbatani@alice.it
Submitted: 21 March 2019; Approved: 04 May 2019; Published: 07 May 2019
Citation this article: Sabbatani P, Corzani A, Gardini B, Marconi M. Ablation of Atrioventricular Nodal Tachycardia through a Point by Point Analysis of the Stability of an Irrigated Catheter. Int J Cardiovasc Dis Diagn. 2019;4(1): 008-014.
Copyright: © 2019 Sabbatani P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: AVNRT; 3D mapping system; Irrigated tip catheter; Ablation; Zero X ray
Download Fulltext PDF
Introduction: Radiofrequency Ablation (RF) of Nodal Reentry Tachycardia (AVNRT) requires precision to avoid AV block. 3D Electro-Anatomic Mapping (EAM) systems allowed to reduce radiological exposure. We sought to evaluate safety and efficacy of AVNRT ablation, analyzing tip stability with a EAM system aiming a Minimal Fluoroscopic Approac (MFA).
Methods: Consecutive patients (pts) with AVNRT were submitted to ablation using an EAM system. Ablation was performed with a flexible-tip Irrigated Catheter (IAC,) whose stability was quantified by the SD of the catheter coordinates in 3 axes of space (X,Y,Z).
Results: 50 pts with AVNRT were treated with RF (12 males, age 52,5 ± 16,6 years). The success rate was 100%, with a mean procedure time of 134 ± 40 min, a mean fluoroscopy (fluo) time of 0,63 ± 1,97 sec and a very low mean fluo dosage (166 cGy/ cm2). In 44 pts (88%) no fluo was used. The mean distance between RF and fast pathway was 14.9 ± 5.3 mm, while the average SD of the position of the ablation catheter during RF was 0.75 ± 0.50, 1.17 ± 0.78, 1.06 ± 0.54 mm respectively in the X-Y-Z axes, confirming a great stability of ablation catheter. After a mean FU of 12 ± 6,4 months 48 patients (96%) showed no recurrence. No complications occurred.
Conclusion: The MFA using a IAC is a safe, cost-effective, feasible alternative to a manual approach for AVNRT ablation. EAM allowed for the first time the analysis of the exact tip position in 3D-axes, ensuring adequate stability of ablation catheter, minimizing the fluo time and without compromising success rates or safety.
Introduction And Purpose Of The Study
Atrioventricular Nodal Re-Entrant Tachycardia (AVNRT) is the most common Supraventricular Tachycardia (SVT) in adults, accounting for approximately 70% of paroxysmal SVT [1,2].
Older studies reported initial success rates of catheter ablation of the AV nodal slow pathway as high as 97% with 5% rate of recurrence over 2 years [3].
On the other hand, prior series showed rates of complete AV block requiring pacemaker implantation of 0.8-1.3% [3-5]. The conventional approach to catheter ablation of AVNRT was described more than 25 years ago and involves empiric Radiofrequency (RF) ablation of the slow pathway site using fluoroscopic anatomical landmarks and specific intra-cardiac electrograms. More recently, alternative ablation strategies have been introduced including the use of cryoablation, irrigated radiofrequency ablation, and 3D Electro-Anatomic Mapping (EAM) with the aim of reducing procedural complications and radiation exposure [6-9].
Fluoroscopy during RF ablation of SVT exposes patients and operators to ionizing radiation.
The demand for reducing radiation exposure by optimizing fluoroscopy or by the use of advanced technologies during these procedures is particularly important, following the ALARA (as low as reasonably achievable) principle and aiming the Minimal Fluoroscopic Approach (MFA ) [9-12].
It is well known that radiation increases the lifetime risk of certain tumors, via stochastic and non-stochastic effects. The latent period between radiation exposure and cancer presentation implies that younger patients are more susceptible to this risk (because in elderly patients this latent period is more likely to exceed the patient’s life expectancy). Many patients undergoing SVT ablation are quite young, and SVT ablation is common also in the paediatric population [13].
Patients are at risk, but operators too; a growing evidence shows that in physicians who perform fluoroscopic-guided procedures radiation exposure is related to tumours of the brain and neck [14], vascular disease [15], cognitive impairment [16].
Given these well-recognized hazards, it is very important to develop and encourage zero- or near-zero-fluoroscopic approaches in EP laboratory, in order to minimize such risks. These are especially important in high-risk populations, including children, young people and pregnant women. The benefits of using 3D EAM systems in the EP lab have been documented in several recent reports [12,17]. The advent of EAM allowed to reduce the radiological exposure and to monitor the stability of the catheter during the procedure.
The purpose of the present study is to assess safety and efficacy of ablation procedure for AVNRT and to evaluate the stability of flexible-tip Irrigated Ablation Catheter (IAC) during procedure, driven by an EAM system, aiming a zero or near-zero fluoroscopic approach.
Methods
Electrophysiology study
Informed written consent was obtained prior to all procedures. Being a prospective study, all consecutive patients with SVT deemed to perform AVNRT ablation were included without selection bias. Diagnosis, procedural strategies, and treatment decisions were specified by protocol. For all electrophysiology studies, vascular access was obtained through both femoral veins and electrode catheters were then advanced under electroanatomic mapping guidance avoiding fluoroscopy from femoral veins to the target site. The ablation procedure was performed using a FlexAbilityTM - sensor-enabledTM (St Jude Medical/Abbott Inc) irrigated ablation catheter with a 4 mm tip, with Ensite Precision (Abbott Inc) magnetic and impedance-based 3D EAM system to create an electroanatomic cardiac shell.
The procedure was guided by the EAM, allowing the measurement of fast and slow pathway location. The AH intervals were measured during pacing from different sites of Koch triangle, in order to mark slow and fast pathway locations and identify the effective RF site. IAC was used to obtain right atrium EAM, with particular concern in Koch’s triangle. All sites with His Bundle potentials were annotated with the EAM.
The ablation catheter was placed in the posteroseptal slow pathway region. RF energy was applied to the lowest part of the Koch’s triangle showing a local slow pathway electrogram (multiphasic atrial component) and/or an A-V amplitude ratio from 1:4 to 1:2.
At target sites, RF energy applications with IAC, ranging from 15W (close to the HIS Bundle) to 30w at 40°C temperature, were delivered. During RF, stability of the tip of ablation catheter was analyzed and continuously checked in all 3 axes by evaluating the mean distance from target site during RF delivery and the mean distance from fast pathway.
Real-time position of the catheter tip was recorded and tracked by the EAM.
Catheter stability during RF delivery was quantified by the Standard Deviation (SD) of the catheter tip coordinates. The presence of irritative junctional rhythm during RF delivery and the corresponding catheter tip location were also recorded and considered for stability analysis.
The presence of junctional beats during ablation was judged as indicative of a correct ablation site. If no junctional beats occurred, RF was stopped after 20-30 s and another site was checked If AVNRT was still inducible, the ablation catheter was moved to more superior sites without His bundle electrograms or near the ostium of coronary sinus.
The endpoint of ablation was non-inducibility of AVNRT without evidence of AV nodal slow pathway conduction (i.e. slow pathway ablation) or jump with just only single nodal echo beat (i.e., slow pathway modulation) both at basal condition (programmed stimulation protocol) and with isoproterenol infusion during programmed stimulation protocol, with a waiting period after ablation of at least 30 minutes.
Study endpoint and Clinical management
3The primary study endpoint was ablation success (including acute success until 7 days post-procedure and midterm success at least 6 months after procedure or at the last available cardiologic visit or clinic record), and catheter stability evaluation in 3 axes during radiofrequency ablation.
Secondary study endpoints were safety profile (accounting major and minor complications), procedure duration, total fluoroscopy time and fluoroscopy dosage for the patients.
Patients attended cardiological visit at our ambulatory 2-3 months after procedure, 1 year after procedure and then every year. A procedure was considered to be acutely successful if a previously inducible arrhythmia was rendered non-inducible at the end of the case or if there was elimination of dual AV nodal physiology in a patient with a documented history of SVT that proved non-inducible at the end of the procedure. Mid-term success was defined as no documented recurrence of SVT based on the last available primary care or cardiology clinic record.
Major complications were defined as death, stroke, vascular access complications requiring surgical intervention or blood transfusion, heart block requiring a permanent pacemaker, or pericardial effusion requiring an intervention within thirty days of the procedure. Minor complications were defined as transient AV block (without need of permanent pacemaker) or hematoma requiring watchful observation with at least 1 adjunctive day of hospitalization.
Statistical Analysis
Categorical variables were summarized by number and percentage. Continuous variables were summarized as a mean and standard deviation. This study was approved by the local ethics committee affiliated with hospital Institution.
Results
All patients were prospectively recruited at our Centre from March 2017 to October 2018. Two operators performed the ablation procedures: one is well experienced and the other one is in training.
The study included 50 consecutive patients with AVNRT, targeted for ablation; 49 patients (98%) showed a typical slow-fast AVNRT, 1 slow-slow AVNRT. No patients were excluded from our study. The study included 2 pregnant women and both procedures were performed without fluoroscopy, preserving their safety and their babies’ one.
The average age was 52,5 ± 16,6 years, 12 patients (24%) were male, 4 (8%) showed a structural heart disease (1 ischemic cardiomyopathy, 2 idiopathic dilated cardiomyopathy, 1 with previous myocarditis) of whom 3 showed a reduced ejection fraction < 50%. Before ablation procedure, 8 patients (16%) took antiarrhythmic drugs (6 Beta blockers, 1 amiodarone, 1 calcium channel blockers). After ablation procedure no patients took antiarrhymic drugs for the index arrhythmia.
The average procedure time was 134 ± 40 min, the average RF delivery time was 10,7 ± 7 minutes.
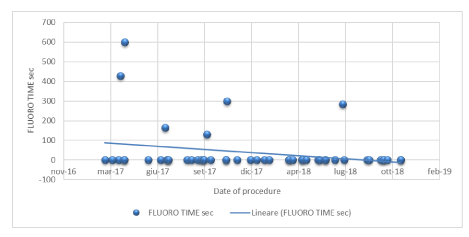
Of 50 patients treated, 44 (88%) underwent a complete zero-fluoroscopic ablation procedure, and 6 patients (12%) were treated with a low fluoroscopic dose of 1390 ± 852 cGy/cm2 with an average fluoroscopy time of 5,3 ± 2,9 minutes (that is 317 ± 174 seconds) –with mean data just considering 6 procedures.
Considering all 50 procedures, the average fluoroscopy time of 0,63 ± 1,97 minutes (that is 38 ± 118 seconds) and an average fluoroscopy dosage of 166 ± 531 cGy/cm2.
Both acute (peri-procedural) and mid-term AVNRT ablation success was satisfactory: all 50 procedures (100%) were acutely successful and 48 patients (96%) showed no recurrences after an average follow-up of 12,0 ± 6,4 months. 2 patients (4%) after few weeks unfortunately experienced the index arrhythmia recurrence and required a new procedure. Considering the close proximity between fast and slow pathway, in both cases a successful cryo-ablation was performed due to the proximity of slow pathway to His bundle.
Overall, no major complications occurred (0%), and just 1 patient (2%) had a minor complication showing a transient AV high degree block during ablation with a rapid recovery of sinus rhythm after few minutes (pacemaker was not required). Clinical variables and procedural characteristics are summarized in table 1.
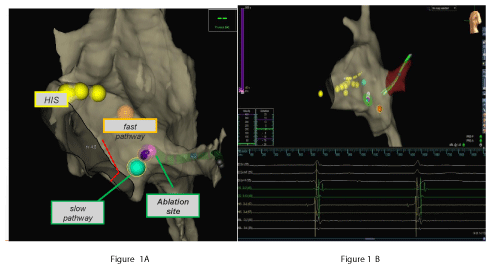
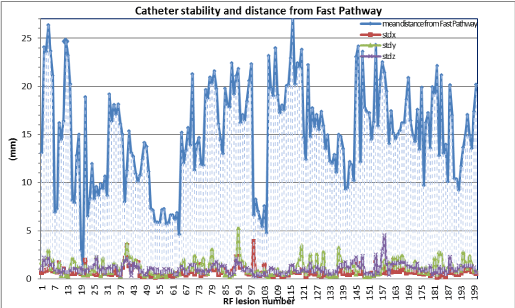
Figure 1A and 1B show 3D EAM during ablation and real time position of the catheter tip during RF delivery respectively. The mean distance between successful RF application and fast pathway was 14,9 ± 5.3mm standard deviation of the ablation catheter position during RF, as a measure of catheter stability, was 0.75 ± 0.50, 1.17 ± 0.78, 1.06 ± 0.54 mm respectively on x, y, z axes, confirming stability of catheter and adequate distance from fast pathway (Figure 2).
It has also been noted that, even in conditions of similar conduction times through slow and fast pathway (Δ < 30 ms), the SD of the position has maintained, on average, very low values (in 91,3% RF application, < 2 mm), thus ensuring stability also in conditions of potentially higher risk of AV block. Slow pathway ablation was achieved in 40 patients (80%), whereas in the remaining patient’s slow pathway modulation was performed.
The procedure was guided by EAM, allowing the measurement of fast and slow pathway location. Catheters were placed by the aid of the mapping system using one bipole on the coronary sinus catheter as a reference. The geometry was created by the sensor-enabled ablation catheter. The AH intervals were measured during pacing from different sites of Koch triangle, in order to mark slow and fast pathway locations and identify the effective RF site. To avoid damage, the his region was tagged with yellow dots. The orange tag represents the fast pathway, while green tag represents slow pathway region and violet tag is the ablation site target (Figure 1A).
Catheter stability during RF delivery was quantified by the Standard Deviation (SD) of the catheter tip coordinates. The presence of irritative junctional rhythm during RF delivery and the corresponding catheter tip location were also recorded and considered for stability analysis (Figure 1B)
Discussion
Our study showed 5 notable findings: 1-AVNRT ablation without fluoroscopic or a MFA provided similar results compared to traditional fluoroscopic approach. Our study reported a very low fluoroscopic exposure, with high rate of acute (100%) and mid-term success (96%) and no major complications.
According to international guidelines [2] patients with frequent symptomatic episodes of AVNRT should strongly be offered the option of catheter ablation, which is the gold standard treatment with high rate of success (higher than 95-100%) with a recurrence of 4-5% and very low rates of major complications [3,18-20].
In fact, other options such chronic antiarrhythmic therapies, are ineffective and poorly tolerated, showing a failure rate in more than 70% of patients in a long 5 years follow up [21].
Our study reported a very low fluoroscopic exposure, with high rate of acute (100%) and mid-term success (96%) and no major complications (0%). Ultimately, our findings are comparable to previous [3-5] and recent [9,11,20] studies reporting long-term success rates of approximately 94-97% for ablation of typical AVNRT, and very low major complication rates (0-1,2%) [11,20,22,23].
3D EAM with or without MFA showed non inferiority compared to traditional procedure; many studies reported similar complication rates for MFA (or EAM-alone) and traditional ablation strategies [6,8,11,20,24].
In our study slow pathway ablation was achieved in the large majority of patients. Currently, non-inducibility of AVNRT with and without isoproterenol infusion in patients without residual evidence of dual Atrioventricular Node (AVN) pathways such as AVN echoes is considered as an acceptable endpoint for RF ablation. In Nikoo et al study [25] the recurrence rate of AVNRT in patients with a non-inducible AVNRT accompanied by postablation inducible single AV echo beats over a wide echo zone is not higher than those with resultant slow pathway elimination (no AVN echoes) or modification with single AV echo beats over a narrow echo zone. This finding conforms with the results from other studies [20] and a meta-analysis [26] indicating that further ablation in patients who have evidence of dual AV node physiology but are non-inducible for AVNRT is not routinely required.
2-A completely fluorless AVNRT ablation was achieved in the great majority of patients (88%) with a great decrease in patients’ and physicians’ exposure. It has been estimated that an interventional cardiologists presents a median radiation exposure per year equivalent to 250 chest X-rays (5 mSv) [12], and this has recently been related to an increased risk of cognitive impairment and brain malignancy [14-16]. Ionizing radiation is known to be teratogenic and carcinogenic; the reduction in its use has been a focus for many years. The advent of non-fluoroscopic technologies guarantees X-rays exposure reduction for both patient and operator during catheter ablation procedure. The use of a MFA with the EnSiteTMNavXTM navigation system is associated with a significant reduction in total fluoroscopy time without any significant difference in terms of success and complication rates [27-29]. In this regard, a recent Italian multicentre trial (NO PARTY) [17] randomized 262 patients with SVTs to conventional approach or the EnSiteTMNavX ™ navigation system with minimal fluoroscopy. Zero-fluoroscopy was achieved in 72% of patients in the minimal fluoroscopy group, with significant overall reduction of the radiation dose. According to ALARA principle (As Low as Reasonably Achievable), we reached a zero-fluoroscopy procedure in 88% (Figure 3). Just few cases (6/50 patients, 12%) required low dose of fluoroscopy, mainly due to 3 reasons: electro-anatomic abnormality of Koch triangle (3 cases), a very difficult femoral vascular access (2 patients), procedure performed with a subclavian access only (1 patient). In cases with variations in anatomy (a.e. kinking of the vessels, wide coronary sinus ostia) it can be impossible to get the catheters in place or the ablation side cannot easily be found with electro anatomy information alone. Here fluoroscopy together with the electro anatomic information still plays an unreplaceable role. Nevertheless reducing fluoroscopy time is important and necessary due to increasing procedure numbers. The results in our study are consistent with those exposed in the Magma study [11], which compared manual traditional catheter ablation to remote magnetic navigation. Our research showed very low fluoroscopy time (mean time of 38 sec that is 0,63 minutes) and very low fluoroscopic dosage (166 cGy/cm2), even lower than those reported in the Magma study (respectively 6 min and 425 cGy/cm2). Otherwise our study revealed longer procedure times (134 minutes) compared to MAGMA study (88 minutes) [11] but shorter procedures times compared to the study of Chrispin, et al (154-181 min) [20].
3-To our knowledge, this is the first study reporting a three dimensional analysis about stability of the catheter tip during RF delivery. Our results showed a great stability of ablation catheter: the stable tip position with an average motion of about 1 mm in all 3 axes ensured high precision and an adequate distance between ablation target zone and fast pathway (14,9mm). Furthermore, 3D EAM allowed a continuous point-by-point testing of tip stability during RF, ensuring a continuous check of the catheter position and tip stability also in conditions of higher risk of AV block.
We can postulate that the advantage of MFA, is the ability to specifically mark the exact anatomical location of the His bundle to avoid radiofrequency delivery in His bundle proximity, thereby potentially reducing the frightening risk of iatrogenic AV block.
Chrispins, et al [20] showed that the use of modern technologies such irrigated radiofrequency ablation catheters and 3D EAM were not associated with long-term success rates or complications. In absence of a direct comparison about this specific issue, we believe that this one could be an opportunity for further investigations. In manual ablation, it is often harder to achieve stability in this region due to significant wall motion. 3D EAM allows to check second-to-second and pont-by-point stability of the tip. Experienced clinicians utilize various methods to achieve stability at the AV junction during manual ablation including fine tactile feedback, use of long sheaths and continuous fluoroscopy. However, for the reasons just mentioned, EAM without the need for continuous fluoroscopy, could be safer.
4-Even in relatively difficult or small anatomies (short distance between fast and slow pathway) and without fluoroscopic guidance, the use of IAC ensures adequate stability and optimal conditions for mapping and ablation of the slow pathway. The magnetic navigation system can really facilitate during mapping and ablation with up to one-millimetre precision. The length of Koch’s triangle has been shown to be approximately 18 mm (distance from the CS Os to His) [30].
The compact AV node which is of 5-7 mm length is located in the upper segment of the Koch’s triangle and the His bundle at its apex [31].
In 25% of patients the His bundle could be located within 5 mm of CS upper lip [30]. Significant variability exists in the relative location of the His bundle with respect to the slow pathway [32], hence precise three-dimensional mapping of the His bundle in relation to potential ablations target is of great importance. In some studies, the location of the successful site of ablation was very variable, ranging from 25 mm from the lowest His position [32] to less than 10 mm [33].
There is not a standard in RF ablation of AVNRT; with a 4mm tip ablation catheter the modulation of the slow pathway of the AV node with 30 and 50 W both have very high short- and long-term success rates with low risks of adverse events.
Modern tools including 3D EAM and irrigated catheters – whose use is increasing [20]- are not associated with a decrease in complication rate or improvement in long-term success [6,8].
RF ablation was performed with a 4mm IAC, with a 1-4-1 mm spacing and 1 mm band electrodes which enhance EGM signals and reduce far-field sensing. In animal models this catheter reduced overall procedural risk with lower incidence of steam pops compared to ThermoCool SF, and lower incidence of charring and coagulum formation in a beating heart [34].
The study [33] showed that FlexAbility catheter is safe and effective with average atrial lesions with a superficial diameter of 7,5 ± 2,4 mm and 4,2 ± 2,9 in depth at 35 W, without significant difference in lesion sizes compared to both standard ThermoCool and ThermoCool SF.
5-This experience on a cohort of adults confirms feasibility and cost-effectiveness of AVNRT ablation with MFA. A cost-effectiveness analysis was also performed in No-PARTY study [17], with a recommendation on acceptable extra-costs in the same series. Moreover, compared to conventional approach, in NO Party trial in MFA the decrease in patients’ exposure shows a 96% reduction in the estimated risks of cancer incidence and mortality, with an important reduction in estimated years of life lost and years of life affected. At a rough economical analysis, the increase in life expectancy and in the period of cancer-free life makes the MFA economically affordable in most European countries (NO PARTY) [17].
Regarding economic considerations about the minimally fluoroscopic approach, there are no studies providing a cost-effectiveness analysis. Data deriving from NO PARTY study show that the extra cost related to the EAM can be considered economically affordable.
Limitations
Our study has some limitations. This is a single-center study (with 2 operators), it is not randomized because all patients were prospectively recruited to MFA, and with a limited number of patients. Moreover, it was therefore not intended to establish comparative safety and efficacy for widespread applicability. A randomized control trial would be needed to determine safety and efficacy in comparison to traditional fluoroscopic approach.
Conclusions
This experience demonstrates the feasibility, safety and acute efficacy of ablation treatment driven by a EAM in patients with AVNRT. Even in relatively difficult anatomies and without fluoroscopy, the use of this catheter ensured adequate stability for mapping and ablation of the slow pathway. MFA showed optimal acute and midterm success without complications, with similar results compared to traditional approach but with a mean very low negligible dose exposition, enabling the physicians to check continuously the stability of the tip and allowing the great majority of procedures to be performed without fluoroscopy.
For the first time the analysis of the exact tip position in 3D-axes was performed using an EAM system, ensuring a greater safety of ablation procedure, minimizing radiation exposure time and without compromising success rates or safety.
- Brembilla PB, Houriez P, Beurrier D, Claudon O, Burger G, Vanc¸on AC, et al. Influence of age on the electrophysiological mechanism of paroxysmal supraventricular tachycardias. Int J Cardiol. 2001; 78: 293-298. https://bit.ly/2vEQMQ6
- Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, et al. 2015 ACC/ AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol, 2016; 67: e27-115. https://bit.ly/2DVSWzf
- Calkins H, Yong P, Miller JM, Olshansky B, Carlson M, Saul JP, et al. Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation. 1999; 99: 262-270. https://bit.ly/2VO3HOg
- Jackman WM, Beckman KJ, McClelland JH, Wang X, Friday KJ, Roman CA, et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry, by radiofrequency catheter ablation of slow-pathway conduction. N Engl J Med. 1992; 327: 313-318. https://bit.ly/2DSCtMn
- Clague JR, Dagres N, Kottkamp H, Breithardt G, Borggrefe M. Targeting the slow pathway for atrioventricular nodal reentrant tachycardia: initial results and long-term follow-up in 379 consecutive patients. Eur Heart J. 2001; 22: 82-88. https://bit.ly/2H849yR
- Alvarez M, Tercedor L, Almansa I, Ros N, Galdeano RS, Burillo F, et al. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm. 2009; 6: 1714-1720. https://bit.ly/2H6pMj1
- Hanninen M, Yeung-Lai-Wah N, Massel D, Gula LJ, Skanes AC, Yee R, et al. Cryoablation versus RF ablation for AVNRT: a meta-analysis and systematic review. J Cardiovasc Electrophysiol. 2013; 24: 1354-1360. https://bit.ly/2J4DepK
- Miyake CY, Mah DY, Atallah J, Oikle HP, Melgar ML, Alexander ME, et al. Nonfluoroscopic imaging systems reduce radiation exposure in children undergoing ablation of supraventricular tachycardia. Heart Rhythm. 2011; 8: 519-525. https://bit.ly/2VhAGLo
- Walsh KA, Galvin J, Keaney J, Keelan E, Szeplaki G. First experience with zero-fluoroscopic ablation for supraventricular tachycardias using a novel impedance and magnetic-field-based mapping system. Clinical Research in Cardiology. 2018; 107: 578-585. https://bit.ly/30423qS
- Kleemann T, Brachmann J, Lewalter T, Andresen D, Willems S, Spitzer SG, et al. Development of radiation exposure in patients undergoing pulmonary vein isolation in Germany between 2007 and 2014: great potential to minimize radiation dosage. Clin Res Cardiol. 2016; 105: 858-864. https://bit.ly/2V4ylhX
- Reents T, Jilek C, Schuster P, Nolker G, Koch-Buttner K, Ammar- Busch, S et al. Multicenter, randomized comparison between magnetically navigated and manually guided radiofrequency ablation of atrioventricular nodal reentrant tachycardia (the MagMa- AVNRT-trial). Clin Res Cardiol. 2017; 106: 947-952. https://bit.ly/2H5zQZq
- Gaita F, Guerra PG, Battaglia A, Anselmino M. The dream of near-zero X-rays ablation comes true. Eur Heart J. 2016; 37: 2749-2755. https://bit.ly/2V6XYP4
- Krause U, Backhoff D, Klehs S, Kriebel T, Paul T, Schneider HE. Catheter ablation of pediatric AV nodal reentrant tachycardia: results in small children. Clin Res Cardiol. 2015; 104: 990-997. https://bit.ly/2VNl6q9
- Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013; 111: 1368-1372. https://bit.ly/2J7pdr9
- Andreassi MG, Piccaluga E, Gargani L, Sabatino L, Borghini A, Faita F, et al. Subclinical carotid atherosclerosis and early vascular aging from long-term low-dose Ionizing radiation exposure: a genetic, telomere, and vascular ultrasound study in cardiac catheterization laboratory staff. JACC: Cardiovasc Interventions. 2015; 8: 616-627. https://bit.ly/301eILl
- Marazziti D, Tomaiuolo F, Dell’Osso L, Demi V, Campana S, Piccaluga E, et al. Neuropsychological testing in interventional cardiology staff after long-term exposure to ionizing radiation. J Int Neuropsychol Soc. 2015; 21: 670-676. https://bit.ly/2Y8Kcxj
- Casella M, Dello Russo A, Pelargonio G, Del Greco M, Zingarini G, Piacenti M, et al. Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias: the NOPARTY multicentre randomized trial. Europace. 2016; 18: 1565-1572. https://bit.ly/2PPVweS
- Bohnen M, Stevenson WG, Tedrow UB, Michaud GF, John RM, Epstein LM, et al. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm. 2011; 8: 1661-1666. https://bit.ly/2DWCsXM
- Brembilla-Perrot B, Sellal JM, Olivier A, Manenti V, Beurrier D, de Chillou C, et al. Recurrences of symptoms after AV node re-entrant tachycardia ablation: a clinical arrhythmia risk score to assess putative underlying cause. Int J Cardiol. 2015; 179: 292-296. https://bit.ly/2vR5Ha5
- Chrispin J, Misra S, Marine JE, Rickard J, Barth A, Kolandaivelu A, et al. Current management and clinical outcomes for catheter ablation of atrioventricular nodal re-entrant tachycardia. Europace. 2018; 20: e51-e59. https://bit.ly/2DRCtfu
- Katritsis DG, Zografos T, Katritsis GD, Giazitzoglou E, Vachliotis V, Paxinos G, et al. Catheter ablation vs. antiarrhythmic drug therapy in patients with symptomatic atrioventricular nodal re-entrant tachycardia: a randomized, controlled trial. Europace. 2017; 1; 19: 602-606. https://bit.ly/2H5X5T2
- Razminia M, Cameron Willoughby, Demo H, Keshmiri H, Wang T, D'Silva OJ, et al. Fluoroless Catheter Ablation of Cardiac Arrhythmias: a 5-Year experience. PACE. 2017; 40: 425-433. https://bit.ly/2PRxb8k
- Wasmer C, Dechering DG, Köbe J, Leitz P, Frommeyer G, Lange PS, et al. Patients' and procedural characteristics of AV-block during slowpathway modulation for AVNRT-single center 10 year experience. International Journal of Cardiology. 2017; 244: 158-162. https://bit.ly/2DURAon
- Mah DY, Miyake CY, Sherwin ED, Walsh A, Anderson MJ, Western K, et al. The use of an integrated electroanatomic mapping system and intracardiac echocardiography to reduce radiation exposure in children and young adults undergoing ablation of supraventricular tachycardia. Europace. 2014; 16: 277-283. https://bit.ly/3042W2G
- Nikoo MH, Attar A, Pourmontaseri M, Jorat MV, Kafi M. Atrioventricular nodal echoes over a wide echo window as a therapeutic end point for the catheter-guided radiofrequency ablation of atrioventricular nodal reentrant tachycardia: a prospective study. Europace. 2018; 20: 659-664. https://bit.ly/2H3oOUu
- Stern JD, Rolnitzky L, Goldberg JD, Chinitz LA, Holmes DS, Bernstein NE, et al. Meta-analysis to assess the appropriate endpoint for slow pathway ablation of atrioventricular nodal reentrant tachycardia. Pacing Clin Electrophysiol. 2011; 34: 269-277. https://bit.ly/2J1ct5q
- Drago F, Silvetti MS, Di Pino A, Grutter G, Bevilacqua M, Leibovich S. Exclusion of fluoroscopy during ablation treatment of right accessory pathway in children. J Cardiovasc Electrophysiol. 2002; 13: 778-782. https://bit.ly/2V58rdR
- Scaglione M, Ebrille E, Caponi D, Blandino A, Di Donna P, Siboldi A, et al. Single center experience of fluoroless AVNRT ablation guided by electroanatomic reconstruction in children and adolescents. PACE. 2013; 36: 1460-1467. https://bit.ly/2J71k3k
- Hummel JD, Strickberger SA, Man KC, Daoud E, Niebauer M, Morady F. A quantitative fluoroscopic comparison of the coronary sinus ostium in patients with and without AV nodal reentrant tachycardia. Journal of Cardiovascular Electrophysiology. 1995; 6: 681-686. https://bit.ly/2VMt4jl
- Widran J, Lev M. The dissection of the atrioventricular node bundle and bundle branches in the human heart. Circulation. 1951; 4: 863-867. https://bit.ly/2DU1lU1
- Bhaskaran A, Albarri M, Ross N, Sara Al Raisi, Rahul Samanta, Leonette Roode, et al. Slow pathway radiofrequency ablation using magnetic navigation: a description of technique and retrospective case analysis heart, lung and circulation. 2017; 26: 1297-1302. https://bit.ly/2H5F0EG
- Yamaguchi T, Tsuchiya T, Nagamoto Y, Miyamoto K, Sadamatsu K, Tanioka Y, et al. Anatomical and electrophysiological variations of Koch’s triangle and the impact on the slow pathway ablation in patients with atrioventricular nodal reentrant tachycardia: a study using 3D mapping. Journal of Interventional Cardiac Electrophysiology. 2013; 37: 111-120. https://bit.ly/2J71ac6
- Winterfield JR, Jensen J, Gilbert T, Marchlinski F, Natale A, Packer D, et al. Lesion size and safety comparison between the novel flex tip on the flexability ablation catheter and the solid tips on the thermocool and thermocool SF ablation catheters. J Cardiovasc Electrophysiol. 2016; 27: 102-109. https://bit.ly/2DShqt7

Sign up for Article Alerts