A Study to Compare Serum Electrolytes Concentrations of Normal Individuals with Valvular Heart Disease and Myocardial Infarction Patients
Ruqaiya Hasan1,2*, Abdul Halim Salim Serafi1, Aisha Javed2, Samia Mushtaq2 and Najmus Sahar2
1Department of Physiology, Faculty of Medicine, Umm Al- Qura University, Makkah, KSA
2Department of Physiology, University of Karachi, Karachi, Pakistan
*Address for Correspondence: Ruqaiya Hasan, Department of Physiology, Faculty of Medicine, Umm Al- Qura University, Makkah, KSA & University of Karachi, Karachi, Pakistan, Tel: +009-665-920-87074; E-mail:ruqaiya55@gmail.com
Submitted: 02 July 2019; Approved: 17 July 2019; Published: 19 July 2019
Citation this article: Hasan R, Salim Serafi AH, Javed A, Mushtaq S, Sahar N. A Study to Compare Serum Electrolytes Concentrations of Normal Individuals with Valvular Heart Disease and Myocardial Infarction Patients. Int J Cardiovasc Dis Diagn. 2019;4(1): 022-027.
Copyright: © 2019 Hasan R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Cardiac diseases; Myocardial infarction; Valvular heart disease; Electrolytes imbalance
Download Fulltext PDF
Electrolyte abnormalities in cardiovascular emergencies are widely studied worldwide as they are mostly found to be associated with cardiovascular morbidity and mortality. The objective of this study was to compare the serum sodium. potassium, calcium and magnesium concentrations of normal healthy individuals with first time diagnosed patients of valvular heart disease and myocardial infarction as well as to evaluate the prognostic value in the severity and outcome of valvular heart disease and myocardial infarction. Following biochemical tests, the mean serum sodium concentrations in both valvular heart disease and myocardial infarction patients were significantly (p ˂ 0.05) higher than normal healthy persons. The mean potassium and calcium concentrations in valvular heart disease and myocardial patients were significantly (p ˂ 0.05) high and low respectively when compared with normal healthy individuals. In comparison to normal healthy persons, respective groups of valvular heart disease and myocardial infarction patients showed a non-significant (p = 0.6123) and a significant (p ˂ 0.05) reduction in mean serum magnesium concentrations. Moreover, comparative analysis of mean serum electrolytes among valvular heart disease and myocardial infarction patients showed a significant low sodium, high potassium, calcium and magnesium concentrations in contrast to significant high sodium, low potassium, calcium and magnesium concentrations respectively.
Introduction
Cardiovascular Disease (CVD) is one of the leading causes of morbidity and mortality across the world. World Health Organization (WHO) has declared cardiovascular disease as a modern epidemic [1]. Even though worldwide, the number of people dying from CVD was lower in 2016 than the previous years but prevalence of CVD reported in 2019 showed a significant increase, mainly driven by the way high blood pressure is defined. The 2017 American Heart Association/ American College of Cardiology hypertension guidelines updated the definition of high blood pressure as a reading of 130/ 80 mm Hg, from the previous definition of 140/ 90 mm Hg [2]. It has been shown that risk factors for CVD such as unhealthy diet, physical inactivity, obesity, tobacco use, diabetes, raised blood pressure and abnormal blood lipids are largely similar in high-income countries as in low- and middle-income countries [3].
Valvular Heart Disease (VHD) is an important cause of morbidity and mortality [4]. It is caused by either damage or defect in one of the four heart valves, aortic, mitral, tricuspid or pulmonary. Defects in these valves can be congenital or acquired. The most common causes of VHD today are calcific aortic stenosis in the elderly, cardiomyopathy, postinterventional therapy, infective endocarditis, patients with connective tissue disorders and rheumatic fever. Considering the likely increase of the aging population worldwide, the incidence of acquired VHD is expected to increase [5]. Early detection and treatment are advocated to allow appropriate timing of intervention and is expected to save lives, however, a significant proportion of patients with valve disease present late, when the long-lasting benefits of intervention are less certain [6].
Myocardial Infarction (MI) is one of the five main manifestations of Coronary Heart Disease (CHD), namely stable angina pectoris, unstable angina pectoris, MI, heart failure and sudden death [7,8] associated with high morbidity and mortality in hospitalised patients older than 65 years, at a rate of 3 million per year [9]. MI is the manifestation of myocardial cell necrosis due to significant and sustained ischemia [3]. The prevalence of MI is higher in men in all age- specific groups than women. Excluding the industrialized nations partly, the rates are rising in the developing countries including South Asia, parts of Latin America and Eastern Europe [10].
Electrolytes such as Sodium (Na+), Potassium (K+), Calcium (Ca++), Magnesium (Mg++) and Phosphate (PO4- -) play important roles in cellular metabolism, energy transformation and in the regulation of cellular membrane potentials, especially those of muscle and nerve cells [11]. Life threatening electrolyte abnormalities have been known to affect the prognosis and outcome of the disease status, in different clinical settings. Alterations in the levels of serum electrolytes have also been associated with increased cardiovascular morbidity and mortality.
Serum Na+, K+, and Ca++ are the major determinants of electrophysiological properties of myocardial membrane [12,13]. The Na+-K+ ATPase pump is responsible for preserving the intracellular K+. Aldosterone and vasopressin stimulate the K+ secretion by up-regulating the luminal Na+-K+ ATPase pump and opening the luminal Na+ and K+ channels [14].
Ca++ ions play a vital role in excitation-contraction of the cardiac muscle fibres and they are essential in both the cardiac and systemic vasculature [15].
Serum Mg++ has been known to influence endothelial function, inflammation, blood pressure and diabetes but a direct relation with CHD risk has not been established [16], however, Liao et al. [17] had reported an inverse relation between serum and dietary Mg++ with CHD risk.
As imbalances of these electrolytes due to different causes can lead to a significant cardiac life threatening events [18], the present study was conducted with the aim to assess the serum Na+, K+, Ca++ and Mg++ levels in VHD and MI patients to evaluate the prognostic value and understanding the underlying electrolyte abnormalities at a very early stage that could be helpful in the management and prevention of complications in these patients.
Materials and Methods
The present comparative study was conducted in the Department of Physiology, University of Karachi, Karachi, Pakistan, included total 60 male subjects, aged between 40-75 years. 20 of them were healthy controls, 20 were patients of VHD and remaining 20 were MI cases; visited first time to the Cardiac Ward, Civil Government Hospital, Karachi. A written informed consent was taken from the participants and ethical approval was taken from the Institutional Ethical Committee.
Inclusion Criteria: First - time Patients diagnosed clinically by Physician based on Clinical examination and ECG changes.
Exclusion Criteria: Patients with diabetes mellitus, chronic muscle disease, renal disease, recent surgery, implanted pacemaker, autoimmune disease, arthritis, any inflammatory disease.
Blood samples of all the participants of study were collected from antecubital vein with all aseptic precautions into heparinized collecting tubes. Blood samples were allowed to clot at room temperature for overnight. The serum separated was used for the estimation of Serum electrolytes (Na+, K+, Ca++ and Mg++) by using biochemical kits.
The data was analysed with the help of SPSS software, version 22. The descriptive analysis results were interpreted as mean and standard error. The independent t -test was applied to compare the difference between controls and cases, p value <0.05 was considered significant.
Results
Na+
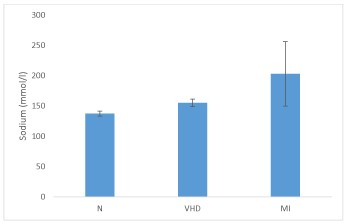
Table 1 shows concentrations of serum Na+ in normal healthy individuals, VHD and MI patients. Serum Na+ concentration in normal individuals ranged from 131.42 - 144.44 mmol/ l with mean 137.27 ± 4.11mmol/ l. Whereas, in VHD and MI patients its concentration ranged from 139 - 164.5 and 133.5 - 312.0 mmol/ l respectively.
The mean serum Na+ concentrations of both VHD and MI patients i.e. 155.20 ± 6.07 and 203.0 ± 53.12 mmol/ l, were significantly higher (p < 0.05) when compared with mean Na+ concentration of normal persons (Figure 1).
However, VHD patients showed a significant low serum Na+ concentration as compared to MI patients (p < 0.05) (Figure 1).
K+
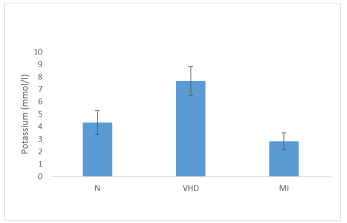
Serum K+ concentrations of normal healthy persons, VHD and MI patients are given in table 2. The range of serum K+ concentration in normal healthy individuals was 3.1-6.1 mmol/ l with mean 4.34 ± 0.96 mmol/ l, while serum K+ concentration between a range of 4.51 - 9.47 mmol/ l with mean value of 7.65 ± 1.16 mmol/ l was measured in VHD patients. In MI patients this range was from 1.2 - 3.7 mmol/ l with mean 2.82 ± 0.68 mmol/ l.
In VHD patients mean serum K+ concentration was significantly higher (p < 0.05) in contrast to MI patients where this value was significantly lower (p < 0.05) when compared with normal group (Figure 2).
Whereas, VHD patients’ mean serum K+ concentration in comparison to MI patients was significantly high (p < 0.05) (Figure 2).
Ca++
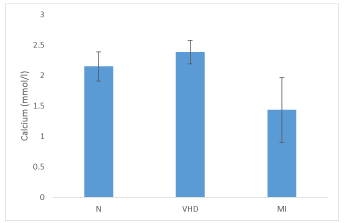
Table 3 shows concentrations of serum Ca++ of normal healthy individuals, VHD and MI patients. In contrast to normal individuals with a range of serum Ca++ concentration from 1.79 - 2.76 mmol/ l with mean value of 2.15 ± 0.24 mmol/ l, VHD and MI patients showed this concentration from 1.89 - 2.64 mmol/ l (with mean 2.38 ± 0.19 mmol/ l) and 0.63 - 2.27 mmol/ l (with mean 1.44 ± 0.53 mmol/ l) respectively.
The mean serum Ca++ concentration of normal persons in comparison to mean Ca++ concentrations of VHD and MI patients were significantly higher and lower respectively (p < 0.05) (Figure 3).
Whereas, VHD patients showed a significantly high (p < 0.05) mean serum Ca++ concentration when compared with MI patients (Figure 3).
Mg++
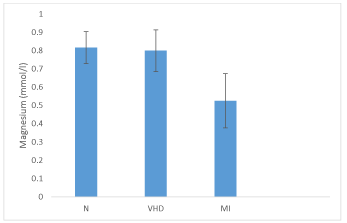
Table 4 shows serum Mg++ concentrations of normal healthy individuals, VHD and MI patients. In normal individuals, serum Mg++ concentrations were measured between 0.69 - 0.98 mmol/ l with mean value of 0.82 ± 0.09 mmol/ l whereas, in VHD patients this concentration ranged from 0.41 - 0.97 mmol/ l (mean value0.80 ± 0.11 mmol/ l) and MI patients showed serum Mg++ concentration between 0.29 - 0.72 mmol/ l (mean value 0.53 ± 0.15 mmol/ l).A comparison of mean serum Mg++ concentrations of VHD and MI patients to mean serum Mg++ concentration of normal persons were non - significantly (p = 0.6123) and significantly (p < 0.05) lower respectively (Figure 4).
However, VHD patients showed a significant high mean serum Mg++ concentration in comparison to MI patients (Figure 4).
Discussion
Electrolyte abnormalities in cardiovascular emergencies mostly found to be associated with cardiovascular morbidity and mortality. These cardiovascular effects may occur in the absence of specific electrocardiographic changes. Moreover, when more than one electrolyte is deficient the effects may be cumulative.
Despite the fact that hypernatremia developed in geriatric patients [19], the alterations in serum Na+ concentration may be a useful indicator of CVD risk [20]. In contrast to previous studies by Hariprasad and Basavaraj [8] and Mandole et al. [21], where both VHD and MI patients showed hyponatremia (serum Na+ concentration less than 135 mmol/ l), present study data indicates hypernatremia in VHD and MI patients’ groups i.e. 155.20 ± 6.07 and 203.0 ± 53.12 mmol/ l when compared with normo-natremia group because with advancing age, not only the ability to dilute and concentrate urine decreases but a fall in Glomerular Filtration Rate (GFR) resulted in a pronounced development of hypernatremia [22]. Present study is in accordance with the studies of Liamis et al. [23] and Naganathan and Al- Dhahir [24], suggesting iatrogenic cause of significant high level of Na+ with the use of osmotic diuretics; further supported by Wali and Yatiraj [25] observed hypernatremia in Acute Myocardial Infarction (AMI) patients who were smokers.
Regarding to serum K+ level in MI patients, present study is in agreement with the studies of Hariprasad and Basavaraj [8] and Rathore et al. [26] where significant decreased levels of Na+ and K+ were found in AMI patients as compared to control subjects; because of activation of the sympathetic nervous system leading to an influx of K+ from the extracellular to the intracellular body fluid compartments [27,28]. However, conversely the VHD patients showed a significant high level of serum K+ in comparison to MI patients and normal group indicating extracellular K+ shift or decreased renal K+ excretion [29].
A large number of clinical and In vitro studies have provided strong evidence that depletion of Ca++, Mg++ and PO4 - - can adversely affect outcome, especially in patients with CVD. Significant low levels of serum Ca++ and Mg++ in MI patients of our data are supported by the studies of Ramasamy et al. [30] and Kughapriya et al. [31]; indicating an association between hypomagnesemia and hypokalemia by various mechanisms involving Na+-K+ ATPase and Renal Outer Medullary Potassium (ROMK) channels in the kidneys [32].
On the other hand, relative hypercalcemia and hypomagnesemia in VHD patients of our study can be attributed to the findings of Zeng et al. [5], who explained the cell-dependent mechanisms and signalling pathways resulted in valve biomineralization and the work of Silva et al. [33] where hypomagnesemia was found to be an independent predictor of mitral valve calcification and cardiovascular mortality.
To summarize the above discussion present data showed a significant high serum Na+, K+; significant low serum Ca++ and non- significant low serum Mg++ levels in VHD patients in comparison to normal subjects. Whereas, MI patients had significant high serum Na+, Ca++; significant low levels of K+ and Mg++ when compared with normal group. However, with reference to the aim of present work, a significant low serum Na+ and significant high serum K+, Ca++ and Mg++ concentrations observed among VHD patients in comparison to MI patients.
Conclusions
Present investigation showed significant changes of serum Na+, K+, Ca++ and Mg++ levels in valvular heart disease and myocardial infarction patients involving several pathophysiological mechanisms. A constant check and correction of serum electrolyte imbalances in valvular heart disease and myocardial infarction patients could improve the prognosis and treatment protocols at a very early stage of heart diseases.
- Park K. Park’s textbook of preventive and social medicine, 22nd ed. Jabalpur: Bhanot Publishers. 2013; p 338. https://bit.ly/2xZmaK1
- American Heart Association. Nearly half of all adult Americans have cardiovascular disease. Science Daily. 2019. https://bit.ly/2D4GMT k
- Myoishi M, Kitakaze M. A role of magnesium: magnesium in the therapy for cardiovascular diseases. Clin Calcium. 2005; 15: 265-270.
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129: 2440-2492. https://bit.ly/2P5mvC1
- Zeng Y, Sun R, Li X, Liu M, Chen S, Zhang P. Pathophysiology of valvular heart disease (Review). Experimental and Therapeutic Medicine. 2016; 11: 1184-1188. https://bit.ly/2O1tsYC
- Chambers JB, Ray S, Prendergast B, Taggart D, Westaby S, Grothier L, et al. Specialist valve clinics: recommendations from the British Heart Valve Society working group on improving quality in the delivery of care for patients with heart valve disease. Heart. 2013; 99: 1714-1716. https://bit.ly/30Fw8wK
- Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, Kapeliovich M. Prognostic importance of hyponatremia in acute ST elevation myocardial infarction. Am J Med. 2004; 117: 242-248. https://bit.ly/2xSgGAX
- Hariprasad S, Basavaraj M. Electrolyte dysfunction in myocardial infarction patients. Int J Adv Med 2018; 5: 1172-1176. https://bit.ly/2LXKcNZ
- Jeldsen KK. Hypokalemia and sudden cardiac death. Exp Clin Cardiol. 2010; 15: 96-99. https://bit.ly/2Sm8hyX
- Jayaraj JC, Karapet Davatyan K, Subramanian SS, Priya J. Epidemiology of myocardial infarction, myocardial infarction, burak pamukçu, IntechOpen. 2018. https://bit.ly/2SkKorM
- Ducceschi V, D'Andrea A, Liccardo B, Sarubbi B, Ferrara L, Romano GP, et al. Ventricular tachyarrhythmias following coronary surgery: predisposing factors. Int J Cardiol. 2000; 73: 43-48. https://bit.ly/2GhoMYz
- Mati E, Krishnamurthy N, Ashakiran S, Sumathi ME, Prasad R. Dyselectrolytemia in acute myocardial infarction-a retrospective study. J Clin Biomed Sci. 2012; 2: 167-174.
- Patil S, Gandhi S, Prajapati P, Afzalpurkar S, Patil O, Khatri M. A study of electrolyte imbalance in acute myocardial infarction patients at a tertiary care hospital in western Maharashtra. Inrter J Contemp Med Resear. 2016; 3: 3568-3571. https://bit.ly/2Z0qDs2
- Mac Donald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients. J Am Coll Cardiol. 2004; 43: 155-161. https://bit.ly/2GhihoD
- Opie LH. Mechanisms of cardiac contraction and relaxation. In: Braunwald E (ed). Heart disease: a textbook of cardiovascular medicine. WB Saunders, Philadelphia. 1996; 360-393.
- Chiuve SE, Sun Q, Curhan GC, Taylor EN, Donna S, Willett WC et al. Dietary and plasma magnesium and risk of coronary heart disease among women. Journal of the American Heart Association: cardiovascular and cerebrovascular disease. 2013; 2: e000114. https://bit.ly/2xV22cc
- Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1998; 136: 480-490. https://bit.ly/2JOTtoK
- Tada Y, Nakamura T, Funayama H, Sugawara Y, Ako J, Ishikawa S, et al. Early development of hyponatremia implicates short and long term outcomes in ST elevation acute myocardial infarction. Circ J. 2011; 75: 1927-1933. https://bit.ly/2YcEMVM
- Long CA, Marin P, Bayer AJ, Shetty HGM, Pathy MSJ. Hypernatraemia in an adult in-patient population. Postgrad Med J. 1991; 67: 643-645. https://bit.ly/2YYdCiH
- Cole NI, Suckling RJ, Swift PA, He FJ, MacGregor GA, Hinton W, et al. The association between serum sodium concentration, hypertension and primary cardiovascular events: a retrospective cohort study J Hum Hypertens. 2019; 33: 69-77. https://bit.ly/2XXnqZ1
- Mandole MB, Howale DS, Mamatha MT, Sharma D, Gamit D, Pandit DP. Evaluation of renal function tests and serum electrolytes in patients with acute myocardial infarction. Int J Biomed Res. 2016; 7: 676-679. https://bit.ly/2O2dopu
- Nur S, Khan Y, Nur S, Boroujerdi H. Hypernatremia: correction rate and hemodialysis. Case Reports in Medicine. 2014. https://bit.ly/2GcKY63
- Liamis G, Milionis HJ, Elisaf M. A review of drug-induced hypernatraemia. NDT Plus. 2009; 2: 339-346. https://bit.ly/30EpCpO
- Naganathan S, Al-Dhahir MA. Hypernatremia. In: StatPearls. Treasure Island (FL): StatPearls Publishing. 2019.
- Wali V, Yatiraj S. Study of serum sodium and potassium in acute myocardial infarction. J Clin Diag Res. 2014; 8: 7-9. https://bit.ly/32waNHG
- Rathore V, Singh N, Mahat RK. Electrolyte imbalance in patients of acute myocardial infarction: a study from central India. JMSCR. 2018; 6: 753-756. https://bit.ly/2XPbxJ9
- Verma S, Agarwal YB, Sharma SK, Doifode DV. Dyselectrolytaemia in acute myocardial infarction. JIACM. 2015; 16: 201-203. https://bit.ly/2XPDmB3
- Shlomai G, Berkovitch A, Pinchevski KS, Bornstein G, Leibowitz A, Goldenberg I, et al. The association between normal-range admission potassium levels in Israeli patients with acute coronary syndrome and early and late outcomes. Medicine. 2016; 95: e3778. https://bit.ly/2JTju6i
- Mushiyakh Y, Dangaria H, Qavi S, Ali N, Pannone J, Tompkins D. Treatment and pathogenesis of acute hyperkalemia. J community Hosp Intern Med Perspect. 2012; 1. https://bit.ly/2xQWI9E
- Ramasamy R, Murugaiyan SB, Gopal N, Shalini R. The prospect of serum magnesium and an electrolyte panel as an adjuvant cardiac biomarker in the management of acute myocardial infarction. JCDR. 2013; 7: 817-820. https://bit.ly/2JHhspK
- Kughapriya P, Ponnudhali D, Evangeline J. Evaluation of serum electrolytes in Ischemic heart disease patients. National J Basic Med Sci. 2016; 6: 163-167. https://bit.ly/2O0oGuA
- Aggarwal HK, Gupta T, Jain D, Yadav RK. Refractory hypokalemia: a rare presentation of hypomagnesemia. JIMSA. 2013; 26: 107-108. https://bit.ly/2LXxmzb
- Silva AP, Gundlach K, Büchel J, Jerónimo T, Fragoso A, Silva C, et al. Low magnesium levels and FGF-23 dysregulation predict mitral valve calcification as well as intima media thickness in predialysis diabetic patients. Int J Endocrinol. 2015; 308190. https://bit.ly/2xSeIjQ

Sign up for Article Alerts