Arterial Wall Hypoxia Promotes Atherosclerosis in Hyperglycemic Apolipoprotein E-Deficient Mice
Lexy Huize Zhong1, Alexandra Bruton1, Christian Araja1, Vi Dang1, Daniel Venegas-Pino1, Yuanyuan Shi1 and Geoff H Werstuck1,2*
1Thrombosis and Atherosclerosis Research Institute, Hamilton, Ontario, Canada
2Department of Medicine, McMaster University, Hamilton, Ontario, Canada
*Address for Correspondence: Geoff H Werstuck, Thrombosis and Atherosclerosis Research Institute 237 Barton Street East, Hamilton General Campus, McMaster University Hamilton, Ontario, Canada, L8L 2X2, Tel: +1-905-521-2100; ext: 40747; FAX: +1-905-575-2646; E-mail: Geoff.Werstuck@taari.ca
Submitted: 31 July 2020; Approved: 08 August 2020; Published: 10 August 2020
Citation this article: Zhong LH, Bruton A, Araja C, Dang V, Venegas-Pino D, et al. Arterial Wall Hypoxia Promotes Atherosclerosis in Hyperglycemic Apolipoprotein E-Deficient Mice. Int J Cardiovasc Dis Diagn. 2020;5(1): 003-010.
Copyright: © 2020 Zhong LH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Atherosclerosis; Hyperglycemia; Hypoxia; Inflammation; Vasa Vasorum
Download Fulltext PDF
Background: Hypoxia within the artery wall is known to promote atherosclerosis; however the underlying mechanisms remain unclear. We have previously shown that hyperglycemic apolipoprotein E-deficient mice have impaired neovascularization of the vasa vasorum, which is associated with the accelerated development of atherosclerosis. The objective of this study is to investigate the relationship between oxygen supply and demand in the arterial wall by examining aortic hypoxia, vasa vasorum neovascularization, vascular inflammation, and atherogenesis.
Methods: Atherosclerosis was induced in apolipoprotein E-deficient mice by glucosamine supplementation, streptozotocin injection, or incorporation of the Ins2+/Akita mutation. Subsets of mice were treated with the chemical chaperone 4-phenylbutyric acid. Mice were harvested at 15 weeks of age and atherosclerotic lesions at the aortic sinus and ascending aorta were quantified and characterized for the presence of hypoxia, vasa vasorum density, and NLRP3 inflammasome expression.
Results and Conclusions: All three models of accelerated atherosclerosis present with lesional hypoxia, impaired vasa vasorum neovascularization, vascular endoplasmic reticulum stress, and inflammation. In each model, treatment with 4-phenylbutyric acid reduced lesion size, lesional hypoxia, endoplasmic reticulum stress, and NLRP3 expression, but did not rescue the reduction in vasa vasorum density. Reduction of inflammation and hypoxia by 4-phenylbutyric acid treatment is sufficient to attenuate hyperglycemic accelerated atherosclerosis.
Abbreviations
ApoE-/-: Apolipoprotein E-deficient; CHOP: C/EBP Homologous Protein; ER: Endoplasmic Reticulum; EPO: Erythropoietin; GlcN: Glucosamine; GRP78/94-78/94-kDa: Glucose-Regulated Proteins; HIF-1α: Hypoxia-Inducible Factor1-Alpha; iNOS: Inducible Nitric Oxide Synthase; IFN-γ: Interferon Gamma; KDEL: Lysine-Aspartic Acid Glutamic Acid-Leucine; LYVE-1: Lymphatic Vessel Endothelial Receptor 1; LDLR-/-: Low Density Lipoprotein Receptor-Deficient; NLRP3: NOD-, LRR- and Pyrin Domain Containing Protein 3; 4PBA: 4-Phenylbutyric Acid; STZ: Streptozotocin; VEGF: Vascular Endothelial Growth Factor; NF-κB: Nuclear Factor Kappa B
Introduction
Hypoxia is a condition that occurs when there is an imbalance between oxygen supply and demand within a cell or tissue. Indications of hypoxia have been observed in human and murine atherosclerotic lesions, which are absent in non-diseased regions of the artery wall [1,2]. Both oxygen supply and demand can be affected in atherosclerosis. Oxygen supply to the cells of the atherosclerotic lesion can be significantly decreased when the diseased artery wall thickens beyond the diffusion limit of O2 (~100μm arterial wall thickness) [3]. Oxygen demand has also been reported to increase with the accumulation of metabolically active inflammatory cells, such as macrophages and T cells, within the artery wall [4].
Evidence in recent years suggests that hypoxia is not just a symptom of atherogenesis, but can also actively promote specific pro-atherogenic processes [5]. For example, apolipoprotein E-deficient (ApoE-/-) mice subjected to intermittent hypoxia have shown indications of accelerated atherosclerosis relative to mice maintained in normoxic conditions [6,7]. In addition, Western diet fed low density lipoprotein receptor-deficient (LDLR-/-) mice exposed to a hyperoxic environment have been reported to develop atherosclerotic lesions with reduced necrotic areas [2].
Hypoxia-inducible factor 1-alpha (HIF-1α), which is stabilized in low oxygen environments, has been implicated as a potential driver of atherosclerosis. It forms a transcriptional activation complex with HIF-1β and p300, which has been shown to drive the expression of over 70 different genes associated with angiogenesis, erythropoiesis, cell metabolism and inflammation [8], including vascular endothelial growth factor (VEGF) [9], erythropoietin (EPO) [10], and inducible nitric oxide synthase (iNOS) [11]. Furthermore, alterations in HIF-1α activity have been shown to impact atherogenic processes. Genetic deletion of smooth muscle HIF-1α reduced vascular inflammation and atherosclerosis in ApoE-/- mice with transverse aortic constriction-induced lesions [12]. Myeloid-specific deletion of HIF-1α attenuated aortic atherosclerosis and reduced the number of lesional inflammatory M1 macrophages [13]. Overexpression of HIF-1α in lymphocytes decreased interferon gamma (IFN-γ) expression and reduced atherosclerotic lesion size. These findings suggest that the effects of HIF-1α on atherosclerosis are cell-type specific [14].
Previously, we reported an increase in hypoxia in the atherosclerotic lesions of streptozotocin (STZ)-injected hyperglycemic ApoE-/- mice that was associated with a significant reduction in the microvessel density of the vasa vasorum [15]. This finding suggests that restricted O2 supply to the artery wall is the cause of low oxygen concentration and may promote atherosclerosis. Interestingly, despite the presence of arterial hypoxia and an increase in lesional HIF-1α, no corresponding increase in VEGF expression was observed in this model, suggesting that hyperglycemia impairs the normal response (VEGF signaling and angiogenesis) to angiogenic stimuli (hypoxia and HIF-1α) and thereby exacerbates hypoxia and accelerates atherosclerosis. In a related study, examination of the left anterior descending coronary artery from 57 autopsies revealed that human patients with diabetes had a lower vasa vasorum density than those without diabetes [16]. Taken together, these findings are the first evidence that; i) hyperglycemia is associated with attenuated neovascularization of the vasa vasorum during atherogenesis and, ii) expansion of the vasa vasorum is not required to support (or promote) accelerated atherosclerosis.
In this study, to better understand the underlying mechanisms that may link hypoxia to atherosclerosis, we utilized two different mouse models of hyperglycemia-accelerated atherosclerosis [17,18] and a glucosamine-supplemented ApoE-/- mouse that develops accelerated atherosclerosis without alterations in glucose or insulin levels [19]. In addition, we investigated the effects of treatment with 4-phenylbutyric acid (4PBA), a chemical chaperone known to reduce vascular endoplasmic reticulum (ER) stress and attenuate atherosclerosis [20,21]. Herein, we specifically investigate the effects of 4PBA on vasa vasorum density, inflammation, and arterial wall hypoxia in order to elucidate the molecular mechanisms by which hyperglycemia promotes pro-atherosclerotic processes.
Animal models
Five-week-old female ApoE-/- mice (B6.129P2-ApoEtm1Unc) (Jackson Laboratories, Bar Harbor, ME) were randomly divided into three groups (n = 16 per group). The first group was untreated controls. The second group received multiple low-dose 40-50 mg/kg body weight per day intraperitoneal injections of STZ (Sigma-Aldrich, Oakville, ON) to induce hyperglycemia, as previously described [17]. The third group received drinking water supplemented with 5% (w/v) glucosamine (Sigma-Aldrich) [19]. Five-week-old female ApoE-/- Ins2+/Akita mice (n = 16) made up the fourth group of mice [18]. The Ins2+/Akita mutation impairs insulin folding and secretion, resulting in hyperglycemia.
Subsets of the control ApoE-/-, STZ-injected ApoE-/-, glucosamine (GlcN)-supplemented ApoE-/-, and ApoE-/- Ins2+/Akita mice (n = 8 per group) were supplemented with 4PBA (1 g/kg per day) (Scandinavian Formulas Inc., Sellersville, PA) in the drinking water. This dose has previously been shown to attenuate atherosclerosis in mice [20,21]. All mice were given unrestricted access to water and the standard chow diet (TD92078) (Harlan Teklad, Madison, WI) and were maintained on a 12-hour light/dark cycle throughout the study. All animal procedures were pre-approved by and performed in accordance with the McMaster University Animal Research Ethics Board and conform to the guidelines of the Canadian Council on Animal Care.
Metabolic measurements
All mice were sacrificed at 15 weeks of age after a 6 hour fast. Fasting blood glucose was measured using an UltraMini blood glucose meter (OneTouch Ultra) (Life Scan, Burnaby, BC). Mice were anaesthetized with 3% isoflurane prior to blood collection by cardiac puncture and then euthanized by cervical dislocation. The vasculature was then flushed with saline via cardiac perfusion and tissues were collected and prepared for analyses. Fasting plasma insulin was measured using enzyme-linked immunosorbent assay kits (Crystal Chem, Downers Grove, IL). Plasma total cholesterol and triglycerides were measured using Infinity reagents (Thermo-Scientific, Middletown, VA).
Quantification of atherosclerotic lesions
Hearts were harvested and fixed in formalin. The aortic sinus was processed and sectioned (5 µm per section), as previously described [22]. Atherosclerotic lesions were visualized by staining serial sections with Masson’s Trichrome (Sigma-Aldrich). Stained sections were imaged using a Leitz LABORLUX S microscope (Leica Microsystems, Concord, ON) connected to a DP71 Olympus camera (Olympus Imaging, Center Valley, PA). Lesion areas were quantified using Image J (1.48v) software. Lesion volume was computed as area under the curve of lesion area versus distance from the aortic sinus.
Characterization of aortic lesions
Micro vessels were visualized by immunostaining with an antibody against the endothelial marker, von Willebrand Factor (vWF) (Aligent Technologies Inc., Santa Clara, CA), as previously described [15]. Positively stained vasa vasorum micro vessels residing within the intima, media, and adventitia of the aortic lesions were counted. Vasa vasorum density was determined as the total number of micro vessels within the defined region per aortic cross-section. Lymphatic vessels were visualized by immunostaining with an antibody against lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) (R+D Systems, Oakville, ON). Positively stained lymphatic vessels were counted and quantified analogous to the vasa vasorum quantification described above.
For other immunofluorescent and immunohistochemical staining, aortic cross-sections were deparaffinized and immunostained with primary antibodies against Grp78/94 (anti-KDEL) (Enzo Life Sciences, Farmingdale, NY), CHOP (Santa Cruz Biotechnology, Santa Cruz CA), VEGF (BosterBio, Pleasanton, CA), HIF-1α (NovusBio, Oakville, ON), NLRP3 (Abcam, Toronto, ON) or pimonidazole hydrochloride (HypoxyprobeTM, Burlington, MA). Primary antibodies were detected and visualized using appropriate fluorescently-conjugated secondary antibodies (Thermo-Scientific). Immunofluorescently stained sections were counterstained with DAPI nuclear stain (Sigma-Aldrich). Negative controls were stained with pre-immune IgG instead of primary antibodies to control for non-specific staining. Positively immune-stained areas were quantified using Image J (1.48v) and normalized to the total lesion area.
To detect tissue hypoxia, a subset of 10-week-old ApoE-/- and STZ-injected ApoE-/- mice were injected with 60 mg/kg pimonidazole hydrochloride two hours prior to sacrifice. Tissues were harvested and prepared as described above.
Statistical analysis
Two-way ANOVA followed by Bonferroni’s test for multiple comparisons were performed using GraphPad Prism (v6.01). All data were normally distributed and expressed as arithmetic means ± standard deviation. For all experiments, a probability value of 0.05 or less was considered statistically significant.
Results
All mice were sacrificed at 15 weeks of age and metabolic parameters, including blood glucose levels, plasma insulin, cholesterol, and triglyceride levels, were quantified (Table 1). STZ-injected ApoE-/- and ApoE-/- Ins2+/Akita mice had significantly elevated blood glucose levels and significantly reduced plasma insulin levels whereas glucosamine supplementation had no effect on glucose or insulin, as expected [19,23]. Total plasma cholesterol and triglyceride levels were not significantly changed by any of the treatments. Supplementation with 4PBA did not have any detectable effect on any of the metabolic parameters that were measured in any of the experimental groups.
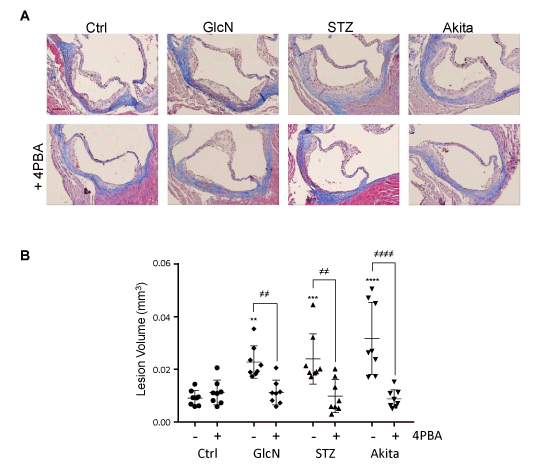
Atherosclerotic volumes were quantified at the aortic sinus and ascending aorta by multiplying lesional areas, measured in Masson’s Trichrome stained serial cross sections, by lesion thicknesses (Figure 1, Supplemental Figure 1) [22]. Hyperglycemic (STZ and Ins2+/Akita) and glucosamine-supplemented ApoE-/- mice presented with significantly larger lesion volumes relative to the ApoE-/- control group. The addition of 4PBA to the drinking water reduced lesional area/volume in each experimental group to levels similar to the control ApoE-/- mice (Figure 1, Supplemental Figure 1).
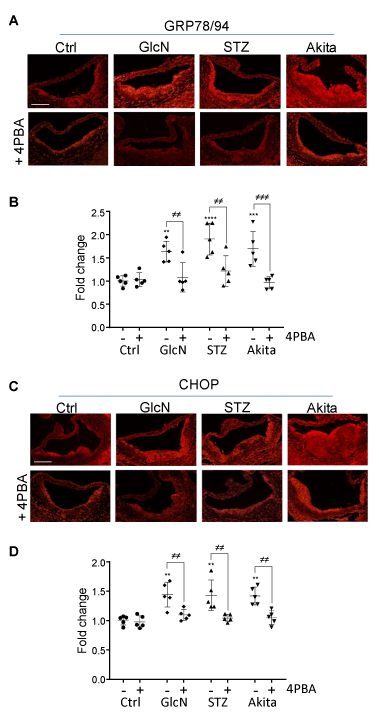
4PBA is a chemical chaperone that assists in protein folding in the ER, thereby reducing ER stress. To determine if 4PBA affected ER stress levels in the atherosclerotic lesions, cross sections of the aortic sinus were immunostained for ER stress markers, Grp78/94 and CHOP (Figure 2). Hyperglycemic and glucosamine-supplemented mice showed significantly elevated levels of both ER stress markers in their atherosclerotic lesions. Treatment with 4PBA reduced lesional ER stress marker expression in each of the experimental groups to levels comparable to controls.
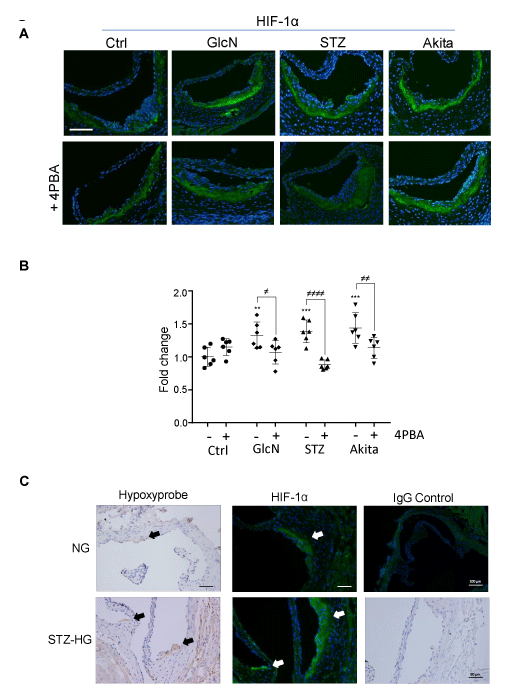
To examine the levels of hypoxia in the atherosclerotic models, cross sections of the aortic sinus were immunostained with an antibody against HIF-1α (Figure 3A & 3B). Hyperglycemic (STZ and Ins2+/Akita) and glucosamine-supplemented ApoE-/- mice had enhanced levels of HIF-1α staining, predominantly in lesional macrophages. Treatment with 4PBA reduced HIF-1α staining to levels comparable to controls. To further validate the presence of hypoxia and to determine if hypoxia is present early in the development of atherosclerosis, 10-week-old normoglycemic and STZ-injected hyperglycemic ApoE-/- mice were injected with pimonidazole hydrochloride to detect regions of relative oxygen depletion. Sections were immunostained with an antibody against the pimonidazole adducts present in hypoxic cells [24]. Hypoxia was evident in regions of the aortic sinus where there were early lesions forming (fatty streaks) (Figure 3C). Staining appear to localize to macrophage foam cells in the subintima and was much more prominent in hyperglycemic ApoE-/- than normoglycemic ApoE-/- mice. These results were confirmed in serial sections that were stained with an anti-HIF-1α antibody (Figure 3C).
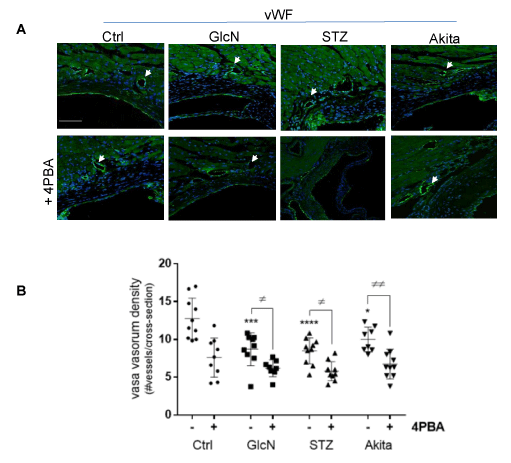
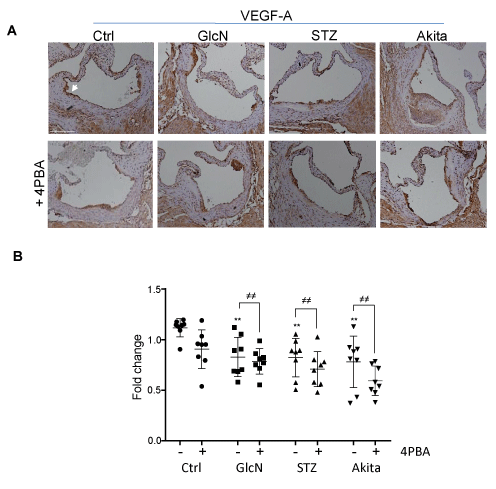
To determine if arterial hypoxia results from an impaired supply of oxygenated blood to the arterial wall, we quantified the vasa vasorum density at the aortic sinus and ascending aorta by immunostaining aortic cross sections with an antibody against the endothelial marker vWF and counting the number of positively stained microvessels per cross section, as previously described [15]. The vasa vasorum, which is almost exclusively localized to the adventitia, presented with a significantly reduced microvessel density in the glucosamine supplemented mice and the hyperglycemic mice, relative to controls (Figure 4). 4PBA treatment did not rescue the vasa vasorum density in any of the experimental groups. In fact, 4PBA administration further reduced vasa vasorum density in all groups compared to hyperglycemic mice without 4PBA treatment. To further investigate the effect on vasa vasorum density, aortic cross sections were immunostained with an antibody against VEGF-A (Figure 5). Results indicate that VEGF-A levels are significantly reduced in hyperglycemic and glucosamine supplemented mice, relative to controls. This significant reduction was not affected by 4PBA treatment. Furthermore, serial aortic cross sections were immunostained with an antibody against the lymph vessel marker LYVE-1 (Supplementary Figure 2) and lymph vessels were quantified by counting the number of positively stained vessels per cross section [25]. Interestingly, hyperglycemia, but not glucosamine supplementation, was associated with an increase in the density of lymphatic vessels within the arterial wall. This effect was not altered by 4PBA treatment.
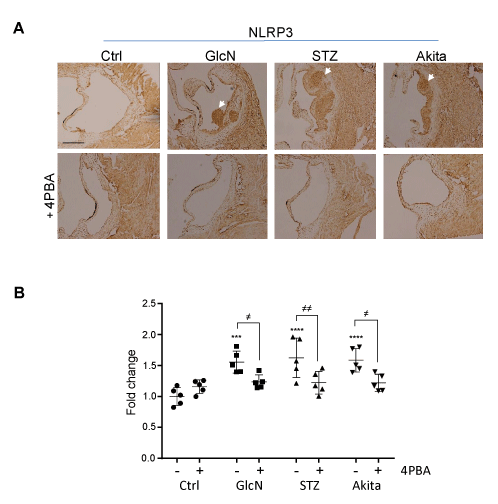
The activation of inflammatory cells, including macrophage foam cells, within the arterial wall can increase the demand for oxygen. To investigate if arterial hypoxia may be arising from the activation of inflammatory cells, serial aortic cross sections were immunostained with an antibody against the inflammasome protein, NLRP3 (Figure 6) [26]. Hyperglycemic and glucosamine supplemented mice showed a significantly elevated expression of NLRP3, relative to controls, which is consistent with arterial inflammation. Treatment with 4PBA significantly attenuated NLRP3 expression in each experimental group.
Discussion
In this study we used chemical (STZ) and genetic (Ins2Akita) approaches in ApoE-/- mice to investigate hyperglycemia-associated accelerated atherosclerosis. In these model systems, hyperglycemia is not associated with any significant changes in plasma lipid profiles. Therefore, the effects on atherosclerosis can be directly attributed to blood glucose levels. Our previous findings suggested that hyperglycemia promotes arterial hypoxia by impairing neovascularization of the vasa vasorum in the artery wall, thereby contributing to the development of atherosclerosis [15,27]. The mechanisms underlying this effect are not clear. However, hyperglycemia does appear to impair HIF-1α signaling as VEGF-A expression was suppressed despite the presence of lesional hypoxia and HIF-1α.
In this study, we supplemented the drinking water of hyperglycemic ApoE-/- mice with 4PBA, a chemical chaperone that reduces ER stress levels in cultured cells and in animal model systems [28]. This intervention has been shown to significantly attenuate atherosclerosis in glucosamine-supplemented and hyperglycemic (STZ-injected and Ins2+/Akita) ApoE-/- mice without altering blood glucose and insulin, or plasma lipid levels [23]. The aim of the study was to investigate the relationship between arterial hypoxia, vasa vasorum density, and vascular inflammation to shed light on the molecular mechanisms by which hyperglycemia promotes atherosclerosis.
We found that both glucosamine supplementation and hyperglycemia induced vascular ER stress and accelerated the development of atherosclerosis in the aortic sinus and ascending aorta of ApoE-/- mice, as expected. Accelerated atherosclerosis was associated with increased vascular hypoxia in all models. As previously observed in STZ-injected hyperglycemic mice, there was reduced VEGF-A expression and reduced vasa vasorum density in both hyperglycemic mouse models. VEGF-A expression and microvessel density was similarly affected in the glucosamine-supplemented mice, perhaps suggesting that ER stress and the unfolded protein response (UPR) pathways were involved in the impaired HIF-1α-VEGF signaling. This finding is consistent with a study that showed that UPR signaling through the PERK pathway inhibits HIF-1α-induced gene expression [29].
Treatment with 4PBA reduced ER stress levels and attenuated the development of atherosclerosis, consistent with previous observations and supporting the theory that ER stress actively promotes atherosclerosis [20,23]. In addition, 4PBA treatment was associated with a significant reduction in vascular hypoxia. Interestingly, the hyperglycemia-associated reductionn lesional VEGF-A and vasa vasorum neovascularization was not rescued by 4PBA. This finding may suggest that O2 supply via the vasa vasorum is not a significant factor in regulating arterial hypoxia and atherogenesis. It is also possible that 4PBA improves circulation quality through existing microvessels, thereby increasing oxygen concentration without altering microvessel quantity.
Examination of very early atherosclerosis in 10-week-old hyperglycemic mice revealed evidence of hypoxia in fatty streaks composed of very few macrophage foam cells, prior to any substantial thickening of the artery wall. This is consistent with hypoxia resulting from increased oxygen demand. The existence of crosstalk between inflammatory and hypoxia-response pathways is relatively well established [30,31]. Nuclear factor κB (NF-κB) has been shown to regulate the induction of HIF-1α and the expression of hypoxia-regulated genes both in vitro and in mouse model systems [32,33]. Alternatively, HIF-1α can regulate NF-κB and also potentiates the expression of NLRP3, an inflammasome protein that plays a role in the maturation and secretion of IL-1β [34-36]. The significance of this cross talk was demonstrated by Song et al. (2018), who showed that selective inhibition of endothelial NF-κB could attenuate atherosclerotic lesion development in a model of chronic intermittent hypoxia-induced atherosclerosis [37].
Glucosamine supplementation and hyperglycemia were associated with increased NLRP3 staining within the atherosclerotic lesion. Furthermore, treatment with 4PBA significantly attenuated NLRP3 expression, which corresponded to the reduction in atherosclerotic lesion size. Together these findings suggest that lesional inflammatory macrophage foam cells become hypoxic as a result of increased glycolytic metabolism (oxygen demand), which ultimately drives atherosclerosis [38].
This study further supports the existence of mechanistic links between ER stress and inflammatory pathways and a causative role for ER stress in the development of atherosclerosis. In addition, these results provide evidence for cross talk between ER stress and hypoxia response pathways. It has long been established that conditions of hypoxia can induce the unfolded protein response as a strategy to cope with the stress of low oxygen concentrations [39-41]. Herein, we provide the first evidence that reducing ER stress levels with a chemical chaperone can attenuate hypoxia, suggesting that the status of the ER feeds back on the regulation of HIF-1α. Further investigations will be required to better understand the interactions between these pathways and how they modulate the development and progression of atherosclerosis.
Author Contributions
LHZ, AB, VD, DVP and GHW conceived the study and designed the experiments. LHZ, AB, CA and YS performed the experiments. LHZ, AB, CA, VD, DVP and GHW analyzed and interpreted the data. LHZ, AB, DVP and GHW drafted the manuscript and all authors revised and approved the final version of the manuscript.
Availability of Data and Material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Funding
This work was supported by the Heart and Stroke Foundation of Canada (HSFC) (G- 17-0017029). AB is supported by an Ontario Graduate Fellowship. GHW is supported by a HSFC Ontario Mid-Career Investigator Award.
- Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008; 51: 1258-1265. DOI: 10.1016/j.jacc.2007.12.025
- Marsch E, Theelen TL, Demandt JA, Jeurissen M, van Gink M, Verjans R, et al. Reversal of hypoxia in murine atherosclerosis prevents necrotic coreexpansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014; 34: 2545-2553. DOI: 10.1161/ATVBAHA.114.304023
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000; 407: 249-257. DOI: 10.1038/35025220
- Sarah E. Corcoran, Luke AJ. O’Neill. HIF1α and metabolic reprogramming in inflammation. J Clin Invest. 2016; 126: 3699-3707. DOI: 10.1172/JCI84431
- Björnheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol. 1999; 19: 870-876. DOI:10.1161/01.ATV.19.4.870
- Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, et al. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010; 209: 381-386. DOI: 10.1016/j.atherosclerosis.2009.10.017
- Fang G, Song D, Ye X, Mao SZ, Liu G, Liu SF. Chronic intermittent hypoxia exposure induces atherosclerosis in ApoE knockout mice: role of NF-κB p50. Am J Pathol. 2012; 181: 1530-1539. DOI: 10.1016/j.ajpath.2012.07.024
- Dengler VL, Galbraith M, Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol. 2014; 49: 1-15. DOI: 10.3109/10409238.2013.838205
- Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest. 1995; 95: 1798-1807. DOI: 10.1172/JCI117858
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992; 12: 5447-5454. DOI: 10.1128/mcb.12.12.5447
- Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995; 182: 1683-1693. DOI: 10.1084/jem.182.6.1683.
- Liu D, Lei L, Desir M, Huang Y, Cleman J, Jiang W, et al. Smooth Muscle hypoxia-inducible factor 1α links intravascular pressure and atherosclerosis. Arterioscler Thromb Vasc Biol. 2016; 36: 442-445. DOI: 10.1161/ATVBAHA.115.306861
- Aarup A, Pedersen TX, Junker N, Christoffersen C, Bartels ED, Madsen M, et al. Hypoxia-inducible factor-1α expression in macrophages promotes development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016; 36: 1782-1790. DOI: 10.1161/ATVBAHA.116.307830
- Ben-Shoshan J, Afek A, Maysel-Auslender S, Barzelay A, Rubinstein A, Keren G, et al. HIF-1alpha overexpression and experimental murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2009; 29: 665-670. DOI: 10.1161/ATVBAHA.108.183319
- Veerman KJ, Venegas-Pino DE, Khan MI, Shi Y, Gerstein HC, Werstuck GH. Hyperglycaemia is associated with impaired vasa vasorum neovascularisation and accelerated atherosclerosis in apolipoprotein-E deficient mice. Atherosclerosis. 2013; 227: 250-258. DOI: 10.1016/j.atherosclerosis.2013.01.018
- Gerstein H, Nair V, Chaube R, Stoute H, Werstuck GH. Dysglycemia and the density of the coronary vasa vasorum. Diabetes Care. 2019; 42: 980-982. DOI: 10.2337/dc18-2483
- Werstuck GH, Khan MI, Femia G, Kim AJ, Tedesco V, Trigatti B, et al. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes. 2006; 55: 93-101. DOI: 10.2337/diabetes.55.01.06.db05-0633
- Venegas-Pino DE, Stoute HK, Wang PW, Singh-Pickersgill NA, Hong BY, Khan MI, et al, Werstuck GH. Gender specific hyperglycemia-induced accelerated atherosclerosis in ApoE-/- Ins2+/Akita mice. Am J Pathol. 2016; 186: 67-77. DOI: 10.1016/j.ajpath.2015.09.009
- Beriault DR, Sharma S, Shi Y, Khan MI, Werstuck GH. Glucosamine-supplementation promotes endoplasmic reticulum stress, hepatic steatosis and accelerated atherogenesis in apoE-deficient mice. Atherosclerosis. 2011; 219: 134-140. DOI: 10.1016/j.atherosclerosis.2011.07.108
- Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009; 15: 1383-1391. DOI: 10.1038/nm.2067
- Huang A, Young TL, Dang VT, Shi YY, McAlpine CS, Werstuck GH. 4-Phenylbutyrate and valproate treatment attenuate the progression of atherosclerosis and stabilize existing plaques. Atherosclerosis. 2017; 266: 103-112. DOI: 10.1016/j.atherosclerosis.2017.09.034
- Venegas-Pino DE, Banko N, Khan MI, Shi Y, Werstuck GH. quantitative analysis and characterization of atherosclerotic lesions in mice. J of Visualized Experiments. 2013; 82: 50933. DOI: 10.3791/50933
- Dang VT, Beriault DR, Deng A, Shi Y, Werstuck GH. Glucosamine-induced ER stress accelerates atherogenesis: A potential link between diabetes and cardiovascular disease. J Mol Gen Med. 2016; 9: 197. DOI: 10.4172/1747-0862.1000197
- Raleigh JA, Chou SC, Arteel GE, Horsman MR. Comparisons among pimonidazole binding, oxygen electrode measurements, and radiation response in C3H mouse tumors. Radiat Res. 1999; 151: 580-589. DOI: 10.2307/3580034
- Jang JY, Koh YJ, Lee SH, Lee J, Kim KH, Kim D, Koh GY, et al. Conditional ablation of LYVE-1+ cells unveils defensive roles of lymphatic vessels in intestine and lymph nodes. Blood. 2013; 122: 2151-2161. DOI: 10.1182/blood-2013-01-478941.
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010; 464: 1357-1361. DOI: 10.1038/nature08938
- Gerstein HC, Werstuck GH. Vasculopenia and the chronic consequences of diabetes. The Lancet Diabetes & Endocrinology. 2013; 1: 71-78. DOI: 10.1016/S2213-8587(13)70025-1
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006; 313: 1137-1140. DOI: 10.1126/science.1128294
- Ivanova IG, Park CV, Yemm AI, Kenneth NS. PERK/eIF2α signaling inhibits HIF-induced gene expression during the unfolded protein response via YB1-dependent regulation of HIF1α translation. Nucleic Acids Res. 2018; 46: 3878-3890. DOI: 10.1093/nar/gky127
- D'Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016; 283: 413-424. DOI: 10.1111/febs.13578
- Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008; 586: 4055-4059. DOI: 10.1113/jphysiol.2008.157669
- Belaiba RS, Bonello S, Zähringer C, Schmidt S, Hess J, Kietzmann T, et al. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007; 18: 4691-4697. DOI: 10.1091/mbc.E07-04-0391
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008; 453: 807-811. DOI: 10.1038/nature06905.
- Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002; 10: 417-426. DOI: 10.1016/S1097-2765(02)00599-3
- Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, et al. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A. 2017; 114: 4763-4768. DOI: 10.1073/pnas.1620458114
- Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005; 201: 105-115. DOI: 10.1084/jem.20040624
- Song D, Fang G, Mao SZ, Ye X, Liu G, Miller EJ, et al. Selective inhibition of endothelial NF-κB signaling attenuates chronic intermittent hypoxia-induced atherosclerosis in mice. Atherosclerosis. 2018; 270: 68-75. DOI: 10.1016/j.atherosclerosis.2018.01.027
- Tawakol A, Singh P, Mojena M, Pimentel-Santillana M, Emami H, MacNabb M, et al. HIF-1α and PFKFB3 mediate a tight relationshiP between pro-inflammatory activation and anaerobic metabolism in atherosclerotic macrophages. Arterioscler Thromb Vasc Biol. 2015; 35: 1463-1471. DOI: 10.1161/ATVBAHA.115.305551
- Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002; 22: 7405-7416. DOI: 10.1128/MCB.22.21.7405-7416.2002
- Blais JD, Filipenko V, Bi M, Harding HP, Ron D, Koumenis C, et al. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol. 2004; 24: 7469-7482. DOI: 10.1128/MCB.24.17.7469-7482.2004
- Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004; 64: 5943-5947. DOI: 10.1158/0008-5472.CAN-04-1606

Sign up for Article Alerts