Trends in Mechanical versus Bioprosthetic Valve Selection for Patients undergoing Surgical Aortic Valve Replacement
Melissa Manzuk, PA-C, MBA-HCM; Sara Turley, MBA*; Clifford J. Kavinsky, M.D., Ph.D.
Rush University Medical Center, Chicago, Illinois, USA
*Address for Correspondence: Sara Turley, Rush University Medical Center, 1725 W. Harrison Street, Suite 364 Professional Office Building, Chicago, IL 60612, USA, Tel: +1-312-563-2039; E-mail: Sara_Turley@rush.edu
Submitted: 21 August 2020; Approved: 08 September 2020; Published: 10 September 2020
Citation this article: Manzuk M, Turley S, Kavinsky CJ. Trends in Mechanical versus Bioprosthetic Valve Selection for Patients undergoing Surgical Aortic Valve Replacement. Int J Cardiovasc Dis Diagn. 2020;5(2): 022-025.
Copyright: © 2020 Manzuk M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: TAVR; SAVR; Valve; Bioprosthetic; Mechanical
Download Fulltext PDF
The landscape of aortic valve replacement has evolved rapidly and significantly in the past decade as Transcatheter Aortic Valve Replacement (TAVR) has proven to be as safe and effective as traditional open-heart surgery for low, intermediate and high-risk patients. Not only has TAVR volume increased substantially in the past few years, this newer technology seems to have influenced fundamental characteristics of how traditional Surgical Aortic Valve Replacement (SAVR) is performed, most notably the type of valve used. A retrospective review of data from almost one hundred hospital members of the Vizient Clinical Data Base across the United States examined the trend in type of surgical valve selected for patients undergoing SAVR. The data reveals a significant increase in the usage of bioprosthetic valves as compared to mechanical valves, with trends mimicking those of FDA approvals for expanded use of TAVR. Additionally, the emergence of the Heart Team, which is a cornerstone of TAVR programs, may have played a vital role in longitudinal care planning for this patient population. This supports the establishment and centralization of comprehensive valve centers, which encourage shared decision-making, and provides patients with safer outcomes and better longitudinal planning from providers.
Abbreviations
SAVR: Surgical Aortic Valve Replacement; TAVR: Transcatheter Aortic Valve Replacement; AVR: Aortic Valve Replacement; VIV: Valve-In-Valve
Introduction
Aortic valve replacement therapy has evolved substantially over the past decade as Transcatheter Aortic Valve Replacement (TAVR) has proven to be as safe and effective as traditional open-heart surgery for low, intermediate, and high-risk patients [1-3]. While Surgical Aortic Valve Replacement (SAVR) has historically been considered the gold standard for aortic valve replacement, the number of TAVR sites has more than quadrupled since the first FDA approval in 2012 with the number of annual TAVR procedures now surpassing SAVR procedures [4]. Perhaps one of the most prominent and emerging trends in AVR is the type of valve utilized, whether mechanical or bioprosthetic.
There are distinct differences between mechanical and bioprosthetic valves (Table 1). Mechanical valves can only be placed surgically, are highly durable and are expected to last for the entire span of the patient’s life. For decades, cardiologists and surgeons have acknowledged the superiority of a mechanical valve, pointing to its durability [5-7]. This is particularly important for younger patients as evidenced by the 2017 AHA/ACC Guidelines [8], which states that mechanical valves are reasonable for patients 50 years of age, and bioprosthetic valves are reasonable for those above 70 years of age or who cannot tolerate lifetime use of an anticoagulant, as well as the European Guidelines [9] which state that mechanical valves should be considered in those patients less than 65 years of age and bioprosthetic valves are reasonable for those aged 65 years and above. While historically considered the gold standard, mechanical valves also require lifetime use of an anticoagulant, which can cause issues later in a patient’s life if bleeding or comorbidities develop.
A bioprosthetic valve, on the other hand, can be used in either SAVR or TAVR procedures and does not require use of an anticoagulant, although the durability is limited [10]. Bioprosthetic valves have a lifespan of 10 to 15 years, so subsequent procedures may be necessary, particularly when used in younger patients. Additionally, bioprosthetic valves allow for a subsequent valve to be placed; a type of TAVR procedure known as a Valve-In-Valve (VIV).TAVR VIV have been shown to have good short term and long-term outcomes [11,12].
As TAVR programs and volumes continue to increase, key characteristics of these programs seem to be reshaping the SAVR landscape, specifically which type of surgical valve is implanted. Hospitals that perform TAVR are required to have the necessary infrastructure to provide SAVR. Conversely, as of 2019, only 64% of hospitals that perform SAVR also have a TAVR program [13]. TAVR programs have their own infrastructure requirements, most notably a Heart Team, which is a multidisciplinary clinical care team comprised of interventional cardiologists, cardiac surgeons and a nurse valve coordinator. Other specialists, including anesthesiologists, advanced practice providers and cardiac imagers, are often a part of this team.
Surgeons who participate in TAVR as part of the multidisciplinary heart team have become accustomed to discussing a broader range of therapeutic options and collaborating with structural interventional cardiologists to consider the staging of procedures. For example, a 58-year-old patient in need of an AVR could receive a mechanical heart valve that will last her entire life; however, she will also need to take an anticoagulant indefinitely. Alternatively, this same patient could receive a bioprosthetic valve and avoid having to take an anticoagulant, as well as the associated side effects. While this approach will likely require the patient to have the valve replaced in 10 to 15 years, it can be replaced as a planned TAVR VIV. This is only possible when a bioprosthetic valve (preferably greater than 21 mm) is used for the initial SAVR and when this longer-term planning is conducted in partnership with the surgeon, structural interventional cardiologist, and patient.
Surgeons without TAVR experience and Heart Team involvement may have placed a mechanical valve, or perhaps a smaller biologic valve (19-21mm), not fully understanding the long-term implications. Given the integral role that surgeons play in TAVR programs, we hypothesize that the growth and expansion of TAVR across the United States has influenced the valve selection preferences of surgeons performing SAVR, resulting in a substantial increase in usage of bioprosthetic valves.
Materials and Methods
To understand whether the emergence of TAVR has guided surgeon valve selection for SAVR, we conducted a ten-year retrospective study from 91 hospital members of the Vizient Clinical Data Base. Data from 2010 to 2019 was compiled and the percentage of bioprosthetic versus mechanical valves was compared [14]. This data was then compared to programmatic growth across the U.S., as well as milestone FDA approvals for TAVR over the same period of time.
Results
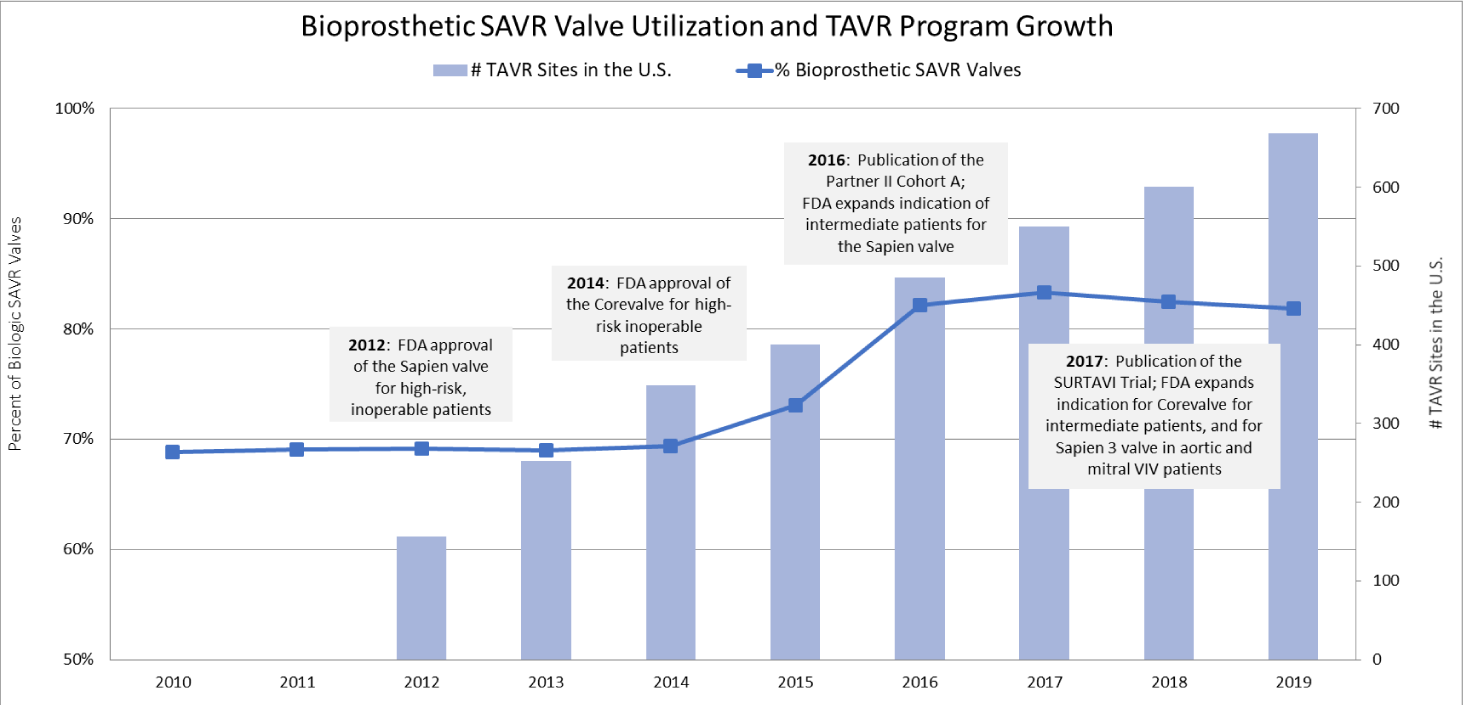
Analysis of the data revealed an overall statistically significant increase in bioprosthetic valve usage for SAVR from 2010 to 2019 with a p-value < 0.05 (Table 2) [10]. From 2010 to 2014, 69% of valves implanted were bioprosthetic. In 2015, this rate increased significantly to 76%, and in 2016, an additional increase occurred and both were statistically significant with a p-value < 0.05. From 2016 to 2019, the rate of bioprosthetic valve use remained steady between 82% and 83%. Figure 1 illustrates the relationship between the usage of bioprosthetic valves for SAVR, the number of TAVR program sites in the United States according to the TVT registry, and key milestones in TAVR FDA approvals.
Discussion
Bioprosthetic SAVR valves have been available and in use for decades without significant changes to the technology, durability or clinical indications. However, as the data demonstrates, there is an increasing trend in use of bioprosthetic valves for SAVR that coincide with expanded FDA approval for TAVR starting with high-risk patients in 2012, intermediate-risk in 2016, and low-risk in 2019.
The establishment of a Heart Team in TAVR programs has created an environment in which surgeons and structural interventional cardiologists work together to develop individualized care plans for patients being considered for TAVR. These teams have increased communication and provide a more comprehensive evaluation of the patient – considering multiple therapeutic options including whether to recommend surgery or transcatheter placement of the valve, and factors such as the type and size of valve. Surgeons and interventional cardiologists increase their knowledge base and familiarity of TAVR and VIV therapies, which may be leading to the increase in bioprosthetic valve usage. The patient is also provided multiple clinical perspectives leading to shared decision-making.
The data used in this study was limited to hospital members of the Vizient Clinical Data Base in the United States, and member hospitals are more likely to be academic medical centers. Further, an Academic Medical Center may be more likely to have a TAVR program. A more comprehensive evaluation of data from all medical centers that perform SAVR is needed in order to validate trends and generalize the information. Additionally, detailed patient-level data was not examined in this study to review factors such as patient age, annulus size, valve size, or whether the patient is able to tolerate an anticoagulant. These additional factors would affect clinical decision-making, and would therefore be expected to influence the study data. Utilizing a more detailed and specific data set would allow for better and more thorough analysis of the SAVR valve selection trends. We would expect to see that patients receiving a mechanical valve to be younger (<50) where a TAVR VIV would not get them the lifelong longevity needed, whereas 50-70 year old low-risk patients with small annuluses in the TAVR era would be receiving a bioprosthetic valve that would allow for TAVR VIV when the valve deteriorates. Lastly, no comparison group was utilized, as this was a retrospective study looking only at hospital members of the Vizient Clinical Data Base.
Conclusion
Trends in SAVR valve selection over the past ten years demonstrate a significant shift towards the usage of bioprosthetic valves. While additional analysis is needed to address the study limitations, the increased adoption of bioprosthetic valves coincides with the expansion of TAVR programs and the emergence of the multidisciplinary heart team.
Currently in the United States, more than one-third of the open-heart cardiac programs do not offer TAVR. If a patient presents to a SAVR-only hospital, his or her clinical options may be limited to SAVR, as no TAVR evaluation is required. Given the clinical benefits of bioprosthetic valves, data supports the use of a Heart Team for patients in need of aortic valve replacement. For patients, having a care team that offers a thorough assessment of options with decreased procedural morbidity and mortality [15] should yield better and more customized options with shared decision making that keeplong-term consideration in mind. In this environment, patients are presented with all options by a team of clinicians working together, and can therefore make a better, more informed decision. Further, this data may encourage the creation of comprehensive valve centers, which offer patients options for either TAVR or SAVR; thus eliminating SAVR-only programs.
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Susheel KK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016; 374: 1609-1620. DOI: 10.1056/NEJMoa1514616
- MackM J, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019; 380: 1695-1705. DOI: 10.1056/NEJMoa1814052
- Hawkey MC, Lauck SB, Perpetua EM, Fowler J, Schnell S, Martina SM, et al. Transcatheter aortic valve replacement program development: recommendations for best practice. Catheter Cardiovasc Interv. 2014; 84: 859-867. DOI: 10.1002/ccd.25529
- Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, et al. 2016 Annual report of the society of thoracic surgeons/american college of cardiology transcatheter valve therapy registry. J Am Coll Cardiol. 2017; 69: 1215-1230. DOI:10.1016/j.jacc.2016.11.033
- Goldstone AB, Chiu P, Baiocchi M, Lingala B, Patrick WL, Michael PF, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med. 2017; 377: 1847-1857. DOI: 10.1056/NEJMoa1613792
- Nelson J, Maul T, Wearden P, Najm HK, Baloglu O, Johnston D, et al. Aortic valve replacement in young and middle-aged adults: current and potential roles of tavr. Ann Thorac Surg. 2010; 4975: 31292-31293. DOI: 10.1016/j.athoracsur.2020.05.180
- Zhao DF, Seco M, Wu JJ, Edelman JB, Wilson MK, Vallely MP, et al. Mechanical versus bioprosthetic aortic valve replacement in middle-aged adults: A systematic review and meta-analysis. Ann Thorac Surg. 2016; 102: 315-327. DOI: 10.1016/j.athoracsur.2015.10.092
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 aha/acc guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017; 70: 252-289. DOI: 10.1016/j.jacc.2017.03.011
- Falk V, Baumgartner H, Bax JJ, De Bonis, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2017; 52: 616-664. DOI: 10.1093/ejcts/ezx324
- Bourguignon T, Lhommet P, El Khoury R, Candolfi P, Loardi C, Mirza A, et al. Very long-term outcomes of the carpentier-edwards perimount aortic valve in patients aged 50-65 years. Eur J Cardiothorac Surg. 2016; 49: 1462-1468. DOI: 10.1093/ejcts/ezv384
- de Freitas Campos Guimarães L, Urena M, Wijeysundera HC, Munoz-Garcia A, Serra V, Benitez LM, et al. Long-term outcomes after transcatheter aortic valve-in-valve replacement. Circ Cardiovasc Interv. 2018; 11: e007038. DOI: 10.1161/CIRCINTERVENTIONS.118.007038
- Mahmoud AN, Gad MM, Elgendy IY, Mahmoud AA, Taha Y, Elgendy AY, et al. Outcomes of valve-in-valve transcatheter aortic valve replacement in patients with failed bioprosthetic aortic valves: systematic review and meta-analysis of observational evidence. EuroIntervention. 2010. DOI: 10.4244/EIJ-D-19-00928
- Data from STS/ACC and TVT Registries. Sts.org.
- Data from the Vizient Clinical Data Base/Resource ManagerTM used by permission of Vizient, Inc. All rights reserved.
- He J, Zhang Z, Wang H, Cai L. The relation between volume and outcome of transcatheter and surgical aortic valve replacement: a systematic review and meta-analysis. Cardiovasc Ther. 2020, 2601340. DOI:10.1155/2020/2601340

Sign up for Article Alerts