The Cardioprotective Effects of Flavonoids
Marguerite M. Engler* and Mary B. Engler
Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA
*Address for Correspondence: Marguerite M. Engler, PhD Program, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA, Tel: +1-301-295-9544; E-mail: marguerite.engler@usuhs.edu
Submitted: 02 November 2020; Approved: 09 December 2020; Published: 10 December 2020
Citation this article: Engler MM, Engler MB. The Cardioprotective Effects of Flavonoids. Int J Cardiovasc Dis Diagn. 2020 Dec 25;5(2): 042-046.
Copyright: © 2020 Engler MM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Disclaimer: The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Keywords: Endothelial function; Polyphenols; Vascular
Download Fulltext PDF
Cardiovascular Disease (CVD) is the leading cause of death worldwide. Flavonoids derived from plants and fruit polyphenols offer cardioprotective benefits. Epidemiologic studies have demonstrated that flavonoid intake is inversely associated with risk of CVD. Flavonoids are phenolic compounds that possess antioxidant, anti-inflammatory, anti-thrombotic and vasodilatory properties that may enhance cardiovascular health. This review presents the main sources of flavonoids, their intake, metabolism, potential mechanisms of action and the evidence for an important preventive role of flavonoids in cardiovascular health.
Abbreviations
CVD: Cardiovascular Disease; LDL-C: Low-Density Lipoprotein Cholesterol; HDL-C: High-Density Lipoprotein Cholesterol; NO: Nitric Oxide; eNOS: Endothelial Nitric Oxide Synthase; FMD: Flow Mediated Dilation; NF-κβ: Nuclear Transcription Factor
Introduction
Cardiovascular Disease (CVD) remains the leading global cause of death [1]. It is predicted that by 2030, 44% of the population in the United States will have CVD [2]. Nutritional strategies with increasing intake of flavonoids may decrease the risk of CVD. Initial interest in the consumption of flavonoids was prompted by the “French Paradox” when cardiac death rates were found to be lower in populations with high saturated fat intake [3]. They also consumed wine with polyphenol resveratrol, which is considered a cardioprotective factor. Epidemiological evidence also suggests that the consumption of flavonoid-rich fruits, vegetables, wine, and tea is inversely associated with the risk of CVD [4-7]. In population-based studies, flavonoid subclasses including anthocyanins, flavanones, and flavonols, have also been associated with a reduction in the risk of hypertension, stroke, and CVD [8-11]. In this review, the evidence to support a cardioprotective role for flavonoids in cardiovascular health as well as their potential biological mechanisms will be presented.
Flavonoid Sources of Intake
Flavonoids are phenolic compounds with 15 carbons arranged in a 3-ring structure [12]. They comprise a ubiquitous and abundant group of polyphenols in the diet [13]. Rich sources of flavonoids include fruits, nuts, and vegetables, as well as, tea, cocoa and red wine [12]. The main dietary flavonoids are categorized in five subclasses including anthocyanins, flavanols, flavonols, flavones, and flavanones [14] (Table 1). It is estimated that the average flavonoid intake is between 20-200 mg daily with approximately 10-15 mg/day in anthocyanins [15,16]. Anthocyanin intake in Americans ages 51-70 years is on an average 4.2 mg/day and lower amounts of 2.6 mg/day are reported in those > 70 years old [17]. Compared to other flavonoids, anthocyanins have a higher antioxidant capacity [18]. Anthocyanins contribute to the food coloration of red, blue and purple hues found primarily in berries, red wine and vegetables (red cabbage, onions, beans) [12]. High anthocyanin intake can be incorporated in a typical diet by consuming 1-2 portions of berries or red grapes daily.
Metabolism
Following ingestion and passage into the stomach, flavonoids undergo phase I metabolism in the small intestine with only 5-10 % reportedly absorbed [19]. From uptake in the epithelial cells of the small intestine, flavonoids are then transformed via the phase II metabolic pathways in the gastrointestinal tract and liver and they are eliminated quickly after consumption [20]. There is also variation in the bioavailability of different flavonoids. Large structured flavonoids continue into the large intestine where they are degraded into smaller low molecular weight metabolites followed by their absorption. In the circulation, the metabolites are delivered to various tissues where they influence cellular processes [21]. A recent study suggests that flavonoids may improve health by modulating intestinal microorganisms. Blueberry anthocyanins reportedly have a prebiotic effect and the ability to alter the amount and composition of human intestinal microbiota [22]. Given the rapid metabolism and degradation of flavonoids, it may be beneficial to consume a variety of flavonoid-rich foods such as fruits, vegetables, cocoa or tea throughout the day to maintain continuous circulating concentrations of flavonoids and their positive effects. For example, improvement in flow-mediated dilation occurs two hours after consumption of 46 grams of dark chocolate and is associated with increased blood levels of epicatechin, the major flavonoid in chocolate [23].
Cardiovascular Effects of Flavonoids
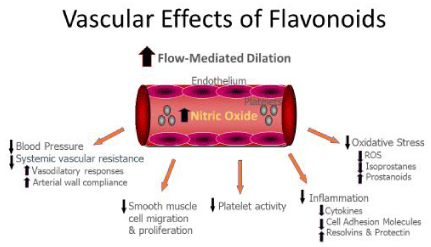
The positive cardiovascular effects of flavonoids are attributed to their interference in many pathophysiological processes including oxidative stress and inflammation that are associated with atherosclerosis [4] (Figure 1). Oxidative stress is one proposed mechanism for the oxidation of low-density lipoprotein cholesterol (LDL-C) and the subsequent onset of age-related CVD. It is characterized by a cellular increase in free radicals which are cytotoxic to the vascular endothelial cells. Flavonoids exert antioxidant actions [7,24] by scavenging free radicals and reducing oxidative stress; thereby limiting cellular injury [25]. Moreover, flavonoids stabilize cell membranes by decreasing membrane fluidity [26].
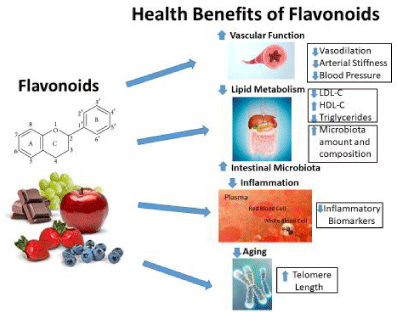
Flavonoids also provide beneficial effects by interfering with cholesterol metabolism. Dyslipidemic subjects with increased levels of circulating lipids (total cholesterol, LDL-C, triglycerides) are at high risk for CVD. Hepatic LDL receptors are impaired in CVD which causes LDL-C to circulate longer making the body more susceptible to oxidation in the vascular endothelium with progression to atherosclerosis. Supplementation with anthocyanins reportedly improves LDL-C and high density lipoprotein-cholesterol (HDL-C) concentrations and enhances cellular cholesterol efflux [27]. These benefits may be due to an inhibitory effect of anthocyanins on plasma cholesteryl ester transfer protein [27]. Moreover, healthy subjects who consumed 500 grams of strawberries daily for I month had similar improvements in lipid levels [28]. The subjects’s levels of total cholesterol, LDL-C and triglycerides were all decreased while HDL-C levels were not affected. These findings are also consistent with a meta-analysis of 45 studies on the consumption of berries and purified anthocyanin extracts and the association of cardiovascular risk factors [29]. Overall, a reduction in LDL-C, triglycerides, blood pressure (systolic and diastolic) and inflammatory biomarkers were reported with a significant increase in HDL-C levels [29]. It has been proposed that flavonoids modulate, regulate or inhibit cell signaling pathways [30]. This results in less activation and attenuated signaling networks which are downstream associated with inflammation and CVD.
Flavonoids also have anti-thrombotic and anti-inflammatory properties which preserve endothelial function. Platelets are implicated in the atherosclerotic process by their cellular effects and platelet activation. They interact with platelets, as well as, leukocytes and endothelial cells to form platelet-leukocyte aggregates. This in turn promotes leukocyte adhesion and migration into the vascular sub endothelial space which enhances inflammation and atherogenesis [31]. By inhibiting the arachidonic acid cascade and production of thromboxane A2 (TXA2), flavonoids have anti-aggregatory effects on platelets [32]. Anthocyanin extracts inhibit platelet aggregation and early thrombi formation [33]. Investigators have also shown that healthy subjects who consumed a flavanol-rich cocoa beverage (897mg cocoa flavanols) had reduced platelet activation two hours after consumption [34]. Moreover, platelet-related primary hemostasis was reduced in healthy adults following consumption of semi-sweet chocolate bits (25 grams) [35].
Inflammation is another mechanistic target for flavonoids. The inflammatory process plays a pivotal role in the progression and destabilization of atherosclerotic plaque that precedes clinical events [36]. Atherosclerosis progresses in the coronary arteries over time with damage to the endothelium and development of arterial plaques. The endothelial injury causes proliferation and migration of monocytes, leukocytes and vascular smooth muscle cells into the endothelium. Monocytes are transformed into macrophages and then lipid-filled foam cells in the vascular intima [37]. Chemokines and pro-inflammatory cytokines regulate these cellular processes. These pathophysiological processes in atherogenesis may be clinically silent until there is an acute rupture of vulnerable plaque with subsequent exposure of the lipid-rich core to arterial blood flow [38]. Platelets accumulate and the resulting thrombus occludes the coronary artery lumen limiting blood flow to the myocardium causing ischemia or an infarction. These events disrupt vascular homeostasis and endothelial cell function which affect the extent of myocardial injury.
Flavonoids exert their anti-inflammatory effects by modulating pro-inflammatory gene expression of enzymes including cyclooxygenase, lipoxygenase, nitric oxide synthase (eNOS) and cytokines [39]. Furthermore, flavonoids limit the expression of vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1) and E-selectin in the endothelium which recruits monocytes into the subendothelial layer. They also reduce production of inflammatory cytokines TNF-α, IL-6, and C-reactive protein [20] and decrease signaling through the nuclear transcription factor (NF-κβ) pathway which is important for inflammation [40].
Telomere length is also associated with CVD and biological aging. Telomeres are nucleotide sequences found at the end of chromosomes that stabilize the gene by allowing normal cell division [41]. After each cell division, telomere length decreases until they no longer function. A Mediterranean dietary intervention rich in flavonoids increased telomere length in the 5-year PREDIMED-NAVARRA trial [42]. In addition, various food groups, such as fruits and vegetables, as well as, individual flavonoid-rich nutrients including tea and grape seed are associated with longer telomere length [43]. The mechanisms for telomere lengthening may be related to increased enzymatic telomerase activity that adds telomeric repeats to the new DNA strands, enhanced DNA methylation or reduced oxidative stress [43]. These studies are promising and provide an added benefit of flavonoid-rich nutrients and components in CVD prevention.
Blood Pressure and Vascular Function
Blood pressure is an important indicator of cardiovascular health. A meta-analysis showed that lowering blood pressure significantly decreased the risk of CVD and death in different patient populations [44]. In an experimental study, a blood pressure lowering effect has been observed following flavonoid intake. Hypertensive rats fed a diet rich in blackcurrant oil for 7 weeks have a significant 30 mmHg reduction in blood pressure compared to controls [45]. Systolic and diastolic blood pressure is reduced by 4.4 and 3.9 mmHg, respectively, in healthy individuals consuming a flavanol-rich cocoa drink (900 mg) daily for 1 month [46]. Drinking black tea, another good source of catechins, is also effective and has been shown to lower systolic and diastolic blood pressure by approximately 2-3 mmHg in healthy and hypertensive subjects [47,48]. A meta-analysis of 99 randomized controlled trials demonstrated that intake of food products rich in anthocyanins such as berries, red grapes and wines, lowered both systolic and diastolic blood pressure in subjects independent of their health status [49].
Flavonoids exert direct effects on vascular tone. In vitro studies suggest that flavonoid subclasses can improve vascular function by increasing the bioavailability of nitric oxide (NO), a potent endothelial-derived vasodilator [50,51]. Blackcurrant fruit is a rich source of anthocyanins which has potent vasorelaxant activity in vessels from human and animal models [52,53]. Recent evidence also suggests that high anthocyanin intake reduces the risk of myocardial infarction by 32% [54] and is inversely associated with decreased arterial stiffness [55].
There is mounting evidence demonstrating the beneficial effects of flavonoids on endothelial function. The endothelium consists of a layer of endothelial cells that line the arterial wall. It provides an interface between the circulating blood and arterial wall. The endothelium produces and releases vasoactive molecules that constrict or dilate the artery. It regulates vascular homeostasis through changes in blood flow, arterial vasomotor tone and platelet activity [56]. Endothelial dysfunction is the earliest stage of atherosclerosis development [36] and correlates with future CVD events [57]. It is characterized by a reduction in NO which leads to a vasoconstrictive feature, pro-inflammatory and pro-thrombotic state in the endothelium [58,59]. Reduced NO also results in the loss of inhibition of NF-κβ which binds to promoter regions of genes that code for proinflammatory proteins such as cell adhesion molecules and cytokines [60]. NFκβ mediates inflammatory responses and if it’s activation is attenuated, the inflammatory state is altered. NO inhibits the synthesis and expression of the pro-inflammatory proteins that are involved in atherogenesis. Flavonoids may increase NO bioavailability by enhancing the expression and activity of eNOS [61]. This is evidenced by a study with flavonoid-rich red wine that produced increases in eNOS mRNA expression in endothelial cells [62].
Experimental studies have also demonstrated that wine extract rich in flavonoids increases NO production in the endothelium of isolated arteries and causes endothelium-dependent relaxation [63,64]. Moreover, we found daily consumption of dark chocolate for 2 weeks significantly improves endothelial function in healthy adults with concomitant increases in the flavanol, epicatechin in plasma [23]. Blackcurrant extract rich in polyphenolic compounds induces a NO- and endothelium-dependent vasorelaxation in rat thoracic aorta [52]. Another study demonstrated that supplementation with purified anthocyanins improves endothelial function in hypercholesterolemic individuals [65]. Collectively, these studies provide strong evidence of the important role of flavonoids in improving blood pressure and vascular function.
As described, flavonoids have a wide spectrum of positive effects on the human body. An overall summary of the health benefits of flavonoids is depicted in Figure 2.
Clinical Significance of Endothelial Function Assessed by Flow-Mediated Dilation
Flow-mediated dilation (FMD) of the brachial artery provides a non-invasive assessment of endothelial function. It is reflective of the bioavailability of NO. Attenuated FMD of the brachial artery is predictive of endothelial dysfunction in the coronary arteries [66] and occurs decades before the onset of symptomatic CVD [67]. FMD is reportedly decreased in the presence of atherosclerosis and risk factors [68]. In evaluating the FMD response following the consumption of various supplements, an increase in FMD indicates that there is enhanced activity and levels of eNOS in the endothelium producing NO [69]. In our previous investigations in hyperlipidemic children ages 9-20, we demonstrated a 2% increase in FMD after supplementation with ω-3 fatty acid, docosahexaenoic acid [70] and a 3.2% increase following antioxidant vitamins C and E for 6 weeks [71]. This is physiologically and clinically significant because it reflects the increase in blood flow from NO. A study in 12 hypercholesterolemic adults given oral berry anthocyanins had an increase of 2.7% in FMD two hours after the dose [65]. Another study in 14 adults reported a 3.7% increase in FMD after green tea consumption [72]. There is substantial evidence for the clinically relevant prognostic value of a 2% change in FMD. A meta-analysis suggests that for every 1% increase in FMD of the brachial artery, there is a 13% decrease in future risk of cardiovascular events [57]. Moreover, another meta-analysis was highly predictive of future CV events. They found a 1% increase in FMD was associated with a 9% (95% CI: 4%-13%) reduction in the future risk of CV events [73]. Based on these studies, a 2% increase in FMD would result in an18%-26% reduction in future risk of CV events. Therefore, by improving FMD, flavonoids may slow or inhibit the progression of CVD.
Clinical Considerations for Flavonoid use
Flavonoids are generally considered quite safe and well tolerated possibly due to their low bioavailability and rapid metabolism. However, adverse side effects could occur. Flavonoids have potent effects but they are not regulated like prescription or over-the-counter medications. The use of pharmaceutical grade or NSF international certified nutritional supplements is recommended to ensure the quality of products such as flavonoids offering health benefits. The efficacy and safety of supplement use has been charged to the Food and Drug Administration with the development of a surveillance system [74].
Careful consideration should be given to the potential risks of flavonoids such as allergic reactions or interactions with other supplements or medications. For example, anthocyanins may compete for drug-metabolizing enzymes and transporters [75]. Therefore, there may be an increased propensity for drug-supplement interactions leading to adverse effects. There is no evidence for adverse effects with the consumption of typical intake of dietary anthocyanins. However, high amounts of bilberry anthocyanins (>100 mg/day) should be cautioned in patients on Coumadin® (Warfarin) or other anti-platelet drugs [76].
Conclusion
Epidemiological and experimental evidence supports an important cardioprotective role for flavonoids. These phenolic compounds derived from plant, fruit and vegetable sources are ubiquitous. Flavonoids affect several atherogenic mechanisms linked to CVD including oxidative stress, inflammation, endothelial dysfunction and platelet aggregation. Flow-mediated dilation of the brachial artery provides a non-invasive assessment of endothelial function with flavonoid consumption that correlates with endothelial function in the coronary arteries. It is reflective of NO bioavailability and an increase in FMD also represents a reduction in future risk of CVD. This is a targeted area for future research that warrants well-controlled, randomized clinical trials on specific flavonoids to define optimal types and doses for cardiovascular health and CVD prevention.
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019 Mar 5;139(10):e56-e528. doi: 10.1161/CIR.0000000000000659. Erratum in: Circulation. 2020 Jan 14;141(2):e33. PMID: 30700139.
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017 Mar 7;135(10):e146-e603. doi: 10.1161/CIR.0000000000000485. Epub 2017 Jan 25. Erratum in: Circulation. 2017 Mar 7;135(10 ):e646. Erratum in: Circulation. 2017 Sep 5;136(10 ):e196. PMID: 28122885; PMCID: PMC5408160.
- St Leger AS, Cochrane AL, Moore F. Factors associated with cardiac mortality in developed countries with particular reference to the consumption of wine. Lancet. 1979 May 12;1(8124):1017-20. doi: 10.1016/s0140-6736(79)92765-x. PMID: 86728.
- Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001 Jun 19;134(12):1106-14. doi: 10.7326/0003-4819-134-12-200106190-00010. PMID: 11412050.
- Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol. 1997 Feb;26(1):1-13. doi: 10.1093/ije/26.1.1. PMID: 9126498.
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993 Oct 23;342(8878):1007-11. doi: 10.1016/0140-6736(93)92876-u. PMID: 8105262.
- Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995 Feb 27;155(4):381-6. Erratum in: Arch Intern Med 1995 Jun 12;155(11):1184. PMID: 7848021.
- Cassidy A, O'Reilly ÉJ, Kay C, Sampson L, Franz M, Forman JP, Curhan G, Rimm EB. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr. 2011 Feb;93(2):338-47. doi: 10.3945/ajcn.110.006783. Epub 2010 Nov 24. PMID: 21106916; PMCID: PMC3021426.
- Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR Jr. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007 Mar;85(3):895-909. doi: 10.1093/ajcn/85.3.895. PMID: 17344514.
- Hollman PC, Geelen A, Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. J Nutr. 2010 Mar;140(3):600-4. doi: 10.3945/jn.109.116632. Epub 2010 Jan 20. PMID: 20089788.
- McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012 Feb;95(2):454-64. doi: 10.3945/ajcn.111.016634. Epub 2012 Jan 4. PMID: 22218162; PMCID: PMC3260072.
- Corcoran MP, McKay DL, Blumberg JB. Flavonoid basics: chemistry, sources, mechanisms of action, and safety. J Nutr Gerontol Geriatr. 2012;31(3):176-89. doi: 10.1080/21551197.2012.698219. PMID: 22888837.
- Engler MB, Engler MM. The vasculoprotective effects of flavonoid-rich cocoa and chocolate. Nutr Res2004;24:695-706.
- Clark JL, Zahradka P, Taylor CG. Efficacy of flavonoids in the management of high blood pressure. Nutr Rev. 2015 Dec;73(12):799-822. doi: 10.1093/nutrit/nuv048. Epub 2015 Oct 21. PMID: 26491142.
- Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006 May 31;54(11):4069-75. doi: 10.1021/jf060300l. PMID: 16719536.
- Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. doi: 10.1093/database/bap024. Epub 2010 Jan 8. PMID: 20428313; PMCID: PMC2860900.
- Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. 2007 May;137(5):1244-52. doi: 10.1093/jn/137.5.1244. PMID: 17449588.
- Yang M, Koo SI, Song WO, Chun OK. Food matrix affecting anthocyanin bioavailability: review. Curr Med Chem. 2011;18(2):291-300. doi: 10.2174/092986711794088380. PMID: 21110799.
- Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013 Aug;24(8):1415-22. doi: 10.1016/j.jnutbio.2013.05.001. PMID: 23849454.
- Tangney CC, Rasmussen HE. Polyphenols, inflammation, and cardiovascular disease. Curr Atheroscler Rep. 2013 May;15(5):324. doi: 10.1007/s11883-013-0324-x. PMID: 23512608; PMCID: PMC3651847.
- Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol. 2014 Oct;88(10):1803-53. doi: 10.1007/s00204-014-1330-7. Epub 2014 Sep 3. PMID: 25182418.
- Zhou L, Xiec M, Yanga F, Liu J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. Food SciTechnol 2020;117:628101. doi: 10.1016/j.lwt.2019.108621
- Engler MB, Engler MM, Chen CY, Malloy MJ, Browne A, Chiu EY, Kwak HK, Milbury P, Paul SM, Blumberg J, Mietus-Snyder ML. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004 Jun;23(3):197-204. doi: 10.1080/07315724.2004.10719361. PMID: 15190043.
- Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996 Feb 24;312(7029):478-81. doi: 10.1136/bmj.312.7029.478. PMID: 8597679; PMCID: PMC2349921.
- Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000 Jul;63(7):1035-42. doi: 10.1021/np9904509. PMID: 10924197.
- Arora A, Byrem TM, Nair MG, Strasburg GM. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys. 2000 Jan 1;373(1):102-9. doi: 10.1006/abbi.1999.1525. PMID: 10620328.
- Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, Cao L, Ling W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009 Sep;90(3):485-92. doi: 10.3945/ajcn.2009.27814. Epub 2009 Jul 29. PMID: 19640950.
- Alvarez-Suarez JM, Giampieri F, Tulipani S, Casoli T, Di Stefano G, González-Paramás AM, Santos-Buelga C, Busco F, Quiles JL, Cordero MD, Bompadre S, Mezzetti B, Battino M. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J Nutr Biochem. 2014 Mar;25(3):289-94. doi: 10.1016/j.jnutbio.2013.11.002. Epub 2013 Nov 27. PMID: 24406274.
- Luís  , Domingues F , Pereira L . Association between berries intake and cardiovascular diseases risk factors: a systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Food Funct. 2018 Feb 21;9(2):740-757. doi: 10.1039/c7fo01551h. PMID: 29243749.
- Rasouli H, Farzael MH, Khodarahmi R. Polyphenols and their benefits: A review. Int J Food Prop. 2017;20:1700-1741. doi: 10.1080/10942912.2017.1354017
- Konic-Ristic A, Kroon PA, Glibetic M. Modulation of platelet function with dietary polyphenols. A promising strategy in the prevention of cardiovascular disease. Agro Food Ind Hi-Tech. 2015;26(2):15-19.
- Faggio C, Sureda A, Morabito S, Sanches-Silva A, Mocan A, Nabavi SF, Nabavi SM. Flavonoids and platelet aggregation: A brief review. Eur J Pharmacol. 2017 Jul 15;807:91-101. doi: 10.1016/j.ejphar.2017.04.009. Epub 2017 Apr 13. PMID: 28412372.
- Yang Y, Shi Z, Reheman A, Jin JW, Li C, Wang Y, Andrews MC, Chen P, Zhu G, Ling W, Ni H. Plant food delphinidin-3-glucoside significantly inhibits platelet activation and thrombosis: novel protective roles against cardiovascular diseases. PLoS One. 2012;7(5):e37323. doi: 10.1371/journal.pone.0037323. Epub 2012 May 18. PMID: 22624015; PMCID: PMC3356278.
- Pearson DA, Paglieroni TG, Rein D, Wun T, Schramm DD, Wang JF, Holt RR, Gosselin R, Schmitz HH, Keen CL. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res. 2002 May 15;106(4-5):191-7. doi: 10.1016/s0049-3848(02)00128-7. PMID: 12297125.
- Holt RR, Schramm DD, Keen CL, Lazarus SA, Schmitz HH. Chocolate consumption and platelet function. JAMA. 2002 May 1;287(17):2212-3. doi: 10.1001/jama.287.17.2212. PMID: 11980520.
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999 Jan 14;340(2):115-26. doi: 10.1056/NEJM199901143400207. PMID: 9887164.
- Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015 Jan 16;116(2):307-11. doi: 10.1161/CIRCRESAHA.116.301313. PMID: 25593275; PMCID: PMC4299915.
- Hellings WE, Peeters W, Moll FL, Pasterkamp G. From vulnerable plaque to vulnerable patient: the search for biomarkers of plaque destabilization. Trends Cardiovasc Med. 2007Jul;17(5):162-71. doi: 10.1016/j.tcm.2007.03.006. PMID: 17574124.
- Santangelo C, Varì R, Scazzocchio B, Di Benedetto R, Filesi C, Masella R. Polyphenols, intracellular signalling and inflammation. Ann Ist Super Sanita. 2007;43(4):394-405. PMID: 18209273.
- Magrone T, Jirillo E. Potential application of dietary polyphenols from red wine to attaining healthy ageing. Curr Top Med Chem. 2011;11(14):1780-96. doi: 10.2174/156802611796235116. PMID: 21506931.
- Boccardi V, Paolisso G, Mecocci P. Nutrition and lifestyle in healthy aging: the telomerase challenge. Aging (Albany NY). 2016 Jan;8(1):12-5. doi: 10.18632/aging.100886. PMID: 26826704; PMCID: PMC4761710.
- García-Calzón S, Gea A, Razquin C, Corella D, Lamuela-Raventós RM, Martínez JA, Martínez-González MA, Zalba G, Marti A. Longitudinal association of telomere length and obesity indices in an intervention study with a Mediterranean diet: the PREDIMED-NAVARRA trial. Int J Obes (Lond). 2014 Feb;38(2):177-82. doi: 10.1038/ijo.2013.68. Epub 2013 May 6. PMID: 23711776.
- Paul L. Diet, nutrition and telomere length. J Nutr Biochem. 2011 Oct;22(10):895-901. doi: 10.1016/j.jnutbio.2010.12.001. Epub 2011 Mar 22. PMID: 21429730.
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016 Mar 5;387(10022):957-967. doi: 10.1016/S0140-6736(15)01225-8. Epub 2015 Dec 24. PMID: 26724178.
- Engler MM. Comparative study of diets enriched with evening primrose, black currant, borage or fungal oils on blood pressure and pressor responses in spontaneously hypertensive rats. Prostaglandins Leukot Essent Fatty Acids. 1993 Oct;49(4):809-14. doi: 10.1016/0952-3278(93)90030-z. PMID: 8259378.
- Grassi D, Desideri G, Necozione S, di Giosia P, Barnabei R, Allegaert L, Bernaert H, Ferri C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J Hypertens. 2015 Feb;33(2):294-303. doi: 10.1097/HJH.0000000000000412. PMID: 25380152.
- Grassi D, Mulder TP, Draijer R, Desideri G, Molhuizen HO, Ferri C. Black tea consumption dose-dependently improves flow-mediated dilation in healthy males. J Hypertens. 2009 Apr;27(4):774-81. doi: 10.1097/HJH.0b013e328326066c. PMID: 19516176.
- Grassi D, Draijer R, Desideri G, Mulder T, Ferri C. Black tea lowers blood pressure and wave reflections in fasted and postprandial conditions in hypertensive patients: a randomised study. Nutrients. 2015 Feb 4;7(2):1037-51. doi: 10.3390/nu7021037. PMID: 25658240; PMCID: PMC4344573.
- García-Conesa MT, Chambers K, Combet E, Pinto P, Garcia-Aloy M, Andrés-Lacueva C, de Pascual-Teresa S, Mena P, Konic Ristic A, Hollands WJ, Kroon PA, Rodríguez-Mateos A, Istas G, Kontogiorgis CA, Rai DK, Gibney ER, Morand C, Espín JC, González-Sarrías A. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. Int J Mol Sci. 2018 Feb 28;19(3):694. doi: 10.3390/ijms19030694. PMID: 29495642; PMCID: PMC5877555.
- Karim M, McCormick K, Kappagoda CT. Effects of cocoa extracts on endothelium-dependent relaxation. J Nutr. 2000 Aug;130(8S Suppl):2105S-8S. doi: 10.1093/jn/130.8.2105S. PMID: 10917930.
- Schewe T, Steffen Y, Sies H. How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys. 2008 Aug 15;476(2):102-6. doi: 10.1016/j.abb.2008.03.004. Epub 2008 Mar 10. PMID: 18358827.
- Nakamura Y, Matsumoto H, Todoki K. Endothelium-dependent vasorelaxation induced by black currant concentrate in rat thoracic aorta. Jpn J Pharmacol. 2002 May;89(1):29-35. doi: 10.1254/jjp.89.29. PMID: 12083740.
- Siegel G, Ermilov E, Hammersen S, Elmen G. In vitro effect of blackcurrant anthocyanins on flow dependent dilatation of human intracerebral arteries and Alzheimer nanoplaque formation. Circulation. 2012;125:P356.
- Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 2013 Jan 15;127(2):188-96. doi: 10.1161/CIRCULATIONAHA.112.122408. PMID: 23319811; PMCID: PMC3762447.
- Jennings A, Welch AA, Fairweather-Tait SJ, Kay C, Minihane AM, Chowienczyk P, Jiang B, Cecelja M, Spector T, Macgregor A, Cassidy A. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am J Clin Nutr. 2012 Oct;96(4):781-8. doi: 10.3945/ajcn.112.042036. Epub 2012 Aug 22. PMID: 22914551.
- Vane JR, Anggård EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27-36. doi: 10.1056/NEJM199007053230106. PMID: 2113184.
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010 Aug;26(6):631-40. doi: 10.1007/s10554-010-9616-1. Epub 2010 Mar 26. PMID: 20339920.
- Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006 Jan;147 Suppl 1(Suppl 1):S193-201. doi: 10.1038/sj.bjp.0706458. PMID: 16402104; PMCID: PMC1760731.
- Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010 May;459(6):923-39. doi: 10.1007/s00424-010-0808-2. Epub 2010 Mar 21. PMID: 20306272.
- Cannon RO 3rd. Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin Chem. 1998 Aug;44(8 Pt 2):1809-19. Erratum in: Clin Chem 1998 Sep;44(9):2070. PMID: 9702990.
- Duarte J, Francisco V, Perez-Vizcaino F. Modulation of nitric oxide by flavonoids. Food Funct. 2014 Aug;5(8):1653-68. doi: 10.1039/c4fo00144c. PMID: 24901042.
- Wallerath T, Poleo D, Li H, Förstermann U. Red wine increases the expression of human endothelial nitric oxide synthase: a mechanism that may contribute to its beneficial cardiovascular effects. J Am Coll Cardiol. 2003 Feb 5;41(3):471-8. doi: 10.1016/s0735-1097(02)02826-7. PMID: 12575978.
- Fitzpatrick DF, Hirschfield SL, Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol. 1993 Aug;265(2 Pt 2):H774-8. doi: 10.1152/ajpheart.1993.265.2.H774. PMID: 8396352.
- Andriambeloson E, Kleschyov AL, Muller B, Beretz A, Stoclet JC, Andriantsitohaina R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br J Pharmacol. 1997 Mar;120(6):1053-8. doi: 10.1038/sj.bjp.0701011. PMID: 9134217; PMCID: PMC1564573.
- Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao Y, Mi M, Jin T, Ling W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin Chem. 2011 Nov;57(11):1524-33. doi: 10.1373/clinchem.2011.167361. Epub 2011 Sep 16. PMID: 21926181.
- Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998 Dec 15;82(12):1535-9, A7-8. doi: 10.1016/s0002-9149(98)00702-4. PMID: 9874063.
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992 Nov 7;340(8828):1111-5. doi: 10.1016/0140-6736(92)93147-f. PMID: 1359209.
- Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005 Jun;127(6):2254-63. doi: 10.1378/chest.127.6.2254. PMID: 15947345.
- Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension. 2014 Feb;63(2):376-82. doi: 10.1161/HYPERTENSIONAHA.113.02044. Epub 2013 Nov 25. PMID: 24277765.
- Engler MM, Engler MB, Malloy M, Chiu E, Besio D, Paul S, Stuehlinger M, Morrow J, Ridker P, Rifai N, Mietus-Snyder M. Docosahexaenoic acid restores endothelial function in children with hyperlipidemia: results from the EARLY study. Int J Clin Pharmacol Ther. 2004 Dec;42(12):672-9. doi: 10.5414/cpp42672. PMID: 15624283.
- Engler MM, Engler MB, Malloy MJ, Chiu EY, Schloetter MC, Paul SM, Stuehlinger M, Lin KY, Cooke JP, Morrow JD, Ridker PM, Rifai N, Miller E, Witztum JL, Mietus-Snyder M. Antioxidant vitamins C and E improve endothelial function in children with hyperlipidemia: Endothelial Assessment of Risk from Lipids in Youth (EARLY) Trial. Circulation. 2003 Sep 2;108(9):1059-63. doi: 10.1161/01.CIR.0000086345.09861.A0. Epub 2003 Aug 11. PMID: 12912807.
- Alexopoulos N, Vlachopoulos C, Aznaouridis K, Baou K, Vasiliadou C, Pietri P, Xaplanteris P, Stefanadi E, Stefanadis C. The acute effect of green tea consumption on endothelial function in healthy individuals. Eur J Cardiovasc Prev Rehabil. 2008 Jun;15(3):300-5. doi: 10.1097/HJR.0b013e3282f4832f. PMID: 18525384.
- Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011 Mar;57(3):363-9. doi: 10.1161/HYPERTENSIONAHA.110.167015. Epub 2011 Jan 24. PMID: 21263128.
- Cohen PA. Hazards of hindsight--monitoring the safety of nutritional supplements. N Engl J Med. 2014 Apr 3;370(14):1277-80. doi: 10.1056/NEJMp1315559. PMID: 24693886.
- Bártíková H, Skálová L, Dršata J, Boušová I. Interaction of anthocyanins with drug-metabolizing and antioxidant enzymes. Curr Med Chem. 2013;20(37):4665-79. doi: 10.2174/09298673113209990153. PMID: 23834170.
- Pulliero G, Montin S, Bettini V, Martino R, Mogno C, Castro GL. Ex vivo study of the inhibitory effects of vacciniummyrtillusanthocyanosides on human platelet aggregation. Fitoterapia 1989; 60: 69-76.

Sign up for Article Alerts