Primary Thyroid Lymphoma in a Patient with Hashimoto Disease?
Portmann-Baracco A1* and Rodriguez-Hurtado D2
1Internal Medicine, Cayetano Heredia University, Lima, Peru
2Cayetano Heredia University, Arzobispo Loayza Hospital, Lima, Peru
*Address for Correspondence: Portmann-Baracco A, Internal Medicine, Cayetano Heredia University, Lima, Peru, Tel: +51-98-94-05-840; ORCiD: https://orcid.org/0000-0003-3489-3751; E-mail: Arianna.portmann.b@upch.pe/ barianna_pb@hotmail.com
Submitted: 21 May 2019; Approved: 25 June 2019; Published: 27 June 2019
Citation this article: Portmann-Baracco A, Rodriguez-Hurtado D. Primary Thyroid Lymphoma in a Patient with Hashimoto Disease. Int J Case Rep Short Rev. 2019;5(6): 037-040.
Copyright: © 2019 Portmann-Baracco A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Thyroid lymphoma; Hashimoto thyroiditis; Fine needle aspiration biopsy
Download Fulltext PDF
Introduction: Primary Thyroid Lymphoma (PTL) is a rare disease with an incidence of 2 cases per million habitants per year and is associated with autoimmune thyroiditis. The diagnosis represents a challenge in the clinical practice because of its low incidence and nonspecific clinical presentation.
Case Presentation: A 65 year old female previously healthy was admitted complaining about a non-tender neck mass that started developing 2 years ago associated with dysphagia, odynophagia, cough and dyspnea. On examination, a large non-tender mass measuring approximately 6x5cm was detected on the left side of the neck. Laboratory exams showed a sub clinic hypothyroidism and pernicious anemia. Ultrasound and CT showed a heterogeneous mass with irregular borders which compressed adjacent structures. FNAC showed nonspecific thyroiditis (Bethesda II). Pathology with immunohistochemistry of incisional biopsy revealed Diffuse Large B Cell Lymphoma (DLBCL). The patient received R-CHOP chemotherapy and had a favorable evolution.
Discussion: Primary thyroid lymphoma is a disease rarely found in the clinical practice, is important to have a high index of suspicion to assure its diagnosis. It usually presents as a fast-growing neck mass producing compression symptoms and is highly associated with autoimmune thyroiditis. Ultrasound is the first diagnosis work-up but confirmation is obtained by tissue biopsy. Although FNAC has a limited effect on PTL, recent advances of immunophenotyping have increased its capacity to almost 80-100%. The most common type is B-cell derived non-Hodgkin’s lymphoma, mainly including DLBCL, MALT lymphoma or a mixed type. PTL is sensitive to chemotherapy; the treatment regimen and prognosis depend of the age of the patient, the subtype of lymphoma and the stage of disease.
Abbreviations
PTL: Primary Thyroid Lymphoma; DLBCL: Diffuse Large B-Cell Lymphoma; FNAC: Fine Needle Aspiration Cytology; MALT: Mucosa-Associated Lymphoid Tissue
Introduction
Primary Thyroid Lymphoma (PTL) is a rare disease with an incidence of 2 cases per million habitants per year. It represents less than 5% of all thyroid neoplasms and approximately 2% of all extra-nodal malignancies [1]. It occurs 3-4 times more frequently in women between 50-80 years old [2]. About 60-90% of all patients with this lymphoma present with autoimmune thyroiditis (Hashimoto disease) before or at the time of the diagnosis [3].
Most tumors are B-cell derived non-Hodgkin’s lymphoma, mainly including the Diffuse Large B-Cell Lymphoma (DLBCL), the Mucosa-Associated Lymphoid Tissue (MALT) or a mixed type [4]. The diagnosis of this disease represents a challenge in the clinical practice because of its low incidence and the nonspecific clinical presentation commonly found in other thyroid disorders.
Case Presentation
A 65 year old female previously healthy was admitted to the Arzobispo Loayza Hospital (HAL) complaining about a non-tender neck mass that started developing 2 years ago and had been increasing in size. A year previous to the hospital admission it was associated with dry cough and moderate dyspnea which then progressed to dyspnea at rest. 7-8 months later the patient presented odynophagia and dysphagia to solids and then to liquids, hoarseness and cough with hemoptysis. A month previous to the admission the patient went to another medical institution where a FNAC and a thyroid ultrasound were realized and an unknown treatment was given. Because of the exacerbation of all symptoms in spite of the treatment given, she decided to go to the emergency room. The patient was vitally stable and afebrile (HR: 61x’, RR: 20x’, BP: 110/70), she needed supplementary oxygen with a nasal cannula to maintain oxygen saturation over 97%. There was a large non-tender mass on the left side of the neck measuring approximately 6x5cm with a hard consistency, irregular shape, and low mobility to deglutition. Peripheral lymph node examination was normal. The rest of the exam was not contributory.
The thyroid function test showed a sub-clinic hypothyroidism (TSH 7.12 mIU/L, FT4: 1.15ng/dl; T3: 80.07ng/dl). The anti-thyroid peroxidase and anti-thyroglobulin antibodies were positive, indicating Hashimoto’s thyroiditis. The complete blood count showed a macrocytic anemia, normal platelets and leucocytes. Serum vitamin B-12 was decreased (81.09 ng/dl), anti-gastric parietal cell antibody and anti-intrinsic factor antibody were positive, indicating pernicious anemia.
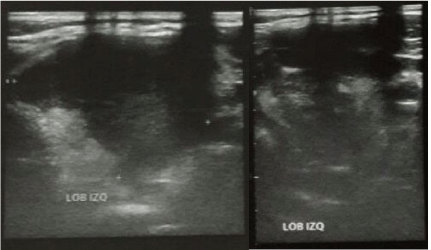
The first ultrasound of the thyroid gland done outside the hospital showed a large round-shaped mass on the left lobe of the thyroid gland with irregular borders and heterogeneous echogenicity with hypoechoic nodules. The mass markedly displaced the trachea to the right side. The second ultrasound (Figure 1), done in the hospital, showed a hypoechoic formation on the left lobule of the thyroid gland measuring 43x44mm. Nasolaryngoscopy showed left vocal cord paralysis, laryngeal displacement to the right side, normal motility of the right vocal cord and low right piriform sinus volume.
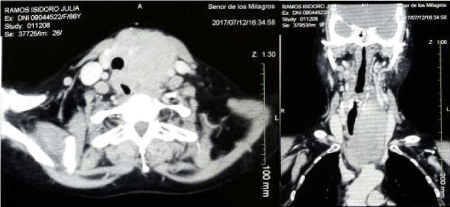
CT scanning of the neck and chest with contrast (Figure 2) revealed an isodense mass with heterogeneous enhancement and irregular borders in the left lobule of the thyroid gland. The lesion measured 5.7 x 7.8 x 11.1 cm and it reached the thyroid cartilage in its cranial portion and the middle and posterior mediastinum in its caudal portion at T4 vertebral level. The lesion compressed and infiltrated the left brachiocephalic vein in its middle portion and the internal jugular vein in its medial portion at C6-C7 level. At T2 level CT showed narrowing of the trachea (diameter of 1.3mm) and esophageal wall collapse. No lymph nodes were seen.
The first FNAC of the thyroid gland done outside the hospital showed round cells without cytoplasm with a lymphoid appearance, no follicular thyroid cells were found. Those characteristics were compatible with a reactive lymphoid tissue. A month later the second FNAC was done inside the hospital and it showed cytology compatible with nonspecific thyroiditis (Bethesda II) with abundant mature non-atypical lymphocytes and some homogeneous epithelial cells.
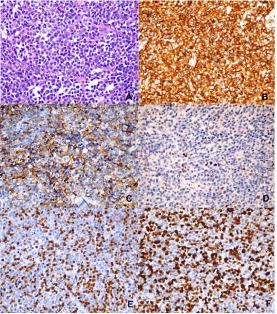
A week later the patient underwent an incisional biopsy of the cervical tumor which revealed a round cell tumor. Immunohistochemistry revealed CD20 (+), CD3 (-), Ki67 70%, BCL-2 (-), BCL-6 (-), CD10 (+), MUM-1 (+). Pathologist final diagnosis was Germinal Center B-Cell like (GCB) DLBCL (Figure 3).
Discussion
Primary thyroid lymphoma is a disease rarely found in the clinical practice, is important to have a high index of suspicion to assure its diagnosis. The annual incidence of this disease is of 2 cases per million habitants per year and it represents less than 5% of all thyroid neoplasms [1].
It usually presents as a fast-growing neck mass and produces compression symptoms like dysphagia, hoarseness and dyspnea. B symptoms such as fever, night sweats and weight loss are rarely present, approximately in 3-10% of all patients. 30% of patients have increased levels of TSH and half of those have sub-clinical hypothyroidism [5,6].
There is an important association between PTL and autoimmune thyroiditis. Approximately 0.6% of all patients with diagnosis of Hashimoto disease develop thyroid lymphoma. However the prevalence of autoimmune thyroiditis in patients with PTL is 80-90%. For this reason, in spite of the low rate of progression to thyroid lymphoma, physicians should have a high index of suspicion of this disease in those patients with Hashimoto disease, usually between the sixth and seventh decade of life, who developed a fast-growing neck mass [5]. Recently, the discovery of a clonal relationship between Hashimoto disease and PTL is helpful to understand the pathogenesis of the tumor. Similar clonal IgVH bands only present in a minority of patients with Hashimoto disease have been found in PTL which supports the hypothesis of clonal evolution of thyroid lymphoma from Hashimoto thyroiditis. Three nucleotide replacements in IgVH genes were found in Hashimoto thyroiditis sequence, each one leading to an amino acid change [7].
Ultrasonography is the initial diagnostic modality used in the workup of thyroid enlargement. Most patients with PTL present a heterogeneous hypoechoic parenchyma with intervening echogenic septa-like structures while others present less frequently features such as markedly hypoechoic masses or a mixed pattern [8]. Once the diagnosis has been confirmed, the next step is to make a staging of the disease, which can be done with a full-body CT scan. Also PET-SCAN has been implemented for the diagnosis and therapeutic following [9].
Diagnosis confirmation is obtained by tissue biopsy and FNAC is the initial diagnostic modality used to evaluate thyroid gland [10]. However, a study by Isik A, et al. found that the decision to make a FNAC depends of the opinion of the physician rather than the up-to-date guidelines about approaching thyroid nodules and perioperative thyroid surgery [11]. FNAC has a limited effect on PTL because cytological differentiation of thyroid lymphoma from lymphocytic thyroiditis and anaplastic carcinoma is difficult [12]. For this reason it usually requires a core needle or open biopsy to confirm the diagnosis and the subtype. Most tumors are B-cell derived non-Hodgkin’s lymphoma, mainly including the Diffuse Large B-Cell Lymphoma (DLBCL), the Mucosa-Associated Lymphoid Tissue (MALT) or a mixed type [4]. FNAC is used in the diagnosis of Hashimoto disease which results in single or multiple nodules. Microscopic evaluation of these nodules show typical cytomorphologic features such as diffuse mature and mixed lymphoid population, epithelioid histiocytes, metaplasic Hürtle cells and macrophages [13].
Sharma A, et al. [5] reported that 26.2% of the FNAC of patients with PTL were previously interpreted as benign, especially in the MALT subtype. Core biopsy showed a better sensibility (93%) than the FNAC (71%). The implementation of ancillary studies such as flow cytometry to the FNAC makes this technique more accurate to differentiate reactive tissues from malignant tissues. While suspicion of lymphoma can be based on morphology, flow patterns are essential for diagnosis and sub classification. Swart et al. found a sensitivity of 96.9% and a specificity of 86.7% by adding flow cytometry to FNAC [14]. Recent advances of immunophenotyping have increased the capacity of FNAC to detect PTL to almost 80-100% depending of the quality of the sample taken [3]. Although this technique is the first histologic test to diagnose PTL, sometimes an open incision biopsy will still be necessary [15].
The treatment of primary thyroid lymphoma is still controversial because of lack of studies with a high quality of evidence. PTL is sensitive to both chemotherapy and radiotherapy and the regimen depends of the lymphoma subtype. Because of its aggressive clinical course DLBCL regimen is multimodal including a combination of monoclonal antibodies (Rituximab) and chemotherapy (CHOP regimen). On the other side, a single-modality treatment such as surgery alone, radiotherapy alone or a combination of both may be used to treat MALT lymphoma [10]. Pyke et al. [16] showed no difference in remission or in survival rates between the combination of debulking surgery with external beam radiotherapy and surgical biopsy with radiotherapy. Therefore, a recent analysis of the National Cancer Database showed that surgery therapy such as lobectomy and total or subtotal thyroidectomy had a significant improvement in survival rates (HR 0.58) as well as beam radiation (HR 0.67), multi-agent (HR 0.4) and single agent chemotherapy (HR 0.43). Immunotherapy benefit was not found statistically significant [17].
The prognosis of PTL depends of the age of the patient, the subtype of lymphoma and the stage of disease. DLBCL subtype has a lower survival rate than that of MALT subtype. Patients have a life expectancy of 9 years after the diagnosis and mortality is usually associated with the lymphoma (44%), non-oncologic causes such as cardiovascular or cerebrovascular disease (35%) and additional non-thyroid malignancies (13%). The treatment of PTL such as chemotherapy, radiotherapy and surgery improves the prognosis of this disease. Five years after diagnosis, disease- specific survival was 86% for stage I, 81% for stage II, and 64% for stage III/IV [2]. For this reason, early diagnosis of this uncommon disease is important to have a better prognosis.
Result
During the hospitalization the patient developed oppressive chest pain radiating up into her neck. ECG showed no alteration, echocardiography showed type I diastolic dysfunction, mild enlargement of the left auricle and mild regurgitation of the mitral valve and tricuspid valve.
The patient was treated by the Oncology department with R-CHOP chemotherapy. The tumor was reduced and the symptoms such as dysphagia, odynophagia and dyspnea disappeared.
Conclusion
A case of a patient who came to the emergency room with a cervical mass that started developing and increasing 2 years ago associated with compression symptoms is presented. The patient is diagnosed with subclinic hypothyroidism and autoimmune thyroiditis and then an incisional biopsy was taken which revealed a Germinal Center B-Cell like (GCB) DLBCL. The patient received R-CHOP chemotherapy and had a favorable evolution.
Acknowledgement
We extend our thanks to Dr. Cesar Chian for providing the pathology and immunohistochemistry of thyroid gland tissue.
- Pedersen RK, Pedersen NT. Primary non-Hodgkin’s lymphoma of the thyroid gland: a population based study. Histopathology. 1996; 28: 25-32. https://bit.ly/2Fw4p9k
- Graff Baker A, Roman SA, Thomas DC, Udelsman R, Sosa JA. Prognosis of primary thyroid lymphoma: Demographic, clinical, and pathologic predictors of survival in 1,408 cases. Surgery. 2009; 146: 1105-1115. https://bit.ly/2YhEdac
- Stephanie Aleskow Stein, Leonard Wartofsky. Primary thyroid lymphoma: a clinical review. The Journal of Clinical Endocrinology & Metabolism. 2013; 98: 3131-3138. https://bit.ly/2LdqNIp
- Efstathios T. Pavlidis, Theodoros E. Pavlidis. A review of primary thyroid lymphoma: molecular factors, diagnosis and management. Journal of Investigative Surgery. 2017; 32: 137-142. https://bit.ly/2X1voVc
- Sharma A, Jasim S, Reading CC, Ristow KM, Villasboas Bisneto JC, Habermann TM, et al. Clinical presentation and diagnostic challenges of thyroid lymphoma: a cohort study. Thyroid. 2016; 26: 1061-1067. https://bit.ly/2Yb9snh
- Watanabe N, Noh JY, Narimatsu H, Takeuchi K, Yamaguchi T, Kameyama K, et al. Clinicopathological features of 171 cases of primary thyroid lymphoma: a long-term study involving 24553 patients with Hashimoto’s disease. Br J Haematol. 2011; 153: 236-243. https://bit.ly/2xizusB
- Moshynska OV, Saxena A. Clonal relationship between hashimoto thyroiditis and thyroid lymphoma.J Clin Pathol. 2008; 61: 438-444. https://bit.ly/2N9Zy49
- Nam M, Shin JH, Han BK, Ko EY, Ko ES, Hahn SY, et al. Thyroid lymphoma: correlation of radiologic and pathologic features. J Ultrasound Med.2012; 31: 589 -594. https://bit.ly/2ZL9QZM
- Stein SA, Wartofsky L. Primary thyroid lymphoma: a clinical review. J Clin Endocrinol Metab. 2013; 98: 3131-3138. https://bit.ly/2LbrOAR
- Walsh, Lowery, Evoy, McDermott EW, Prichard RS. Thyroid lymphoma: recent advances in diagnosis and optimal management strategies. Oncologist. 2013; 18: 994-1003. https://bit.ly/2xebCGM
- Isik A, Firat D, Yilmaz I, Peker K, Idiz O, Yilmaz B, et al. A survey of current approaches to thyroid nodules and thyroid operation. International Journal of Surgery. 2018.
- Sarinah B, Hisham AN. Primary lymphoma of the thyroid: diagnostic and therapeutic considerations. Asian J Surg. 2010; 33: 20-24. https://bit.ly/2J9n9wJ
- Paksoy N,Yazal K. Cervical lymphadenopathy associated with hashimoto's thyroiditis: an analysis of 22 cases by fine needle aspiration cytology. Acta Cytol.2009; 53: 491-496. https://bit.ly/2Ydp6ye
- Swart GJ, Wright C, Brundyn K, Mansvelt E, du Plessis M, ten Oever D, et al. Fine needle aspiration biopsy and flow cytometry in the diagnosis of lymphoma. Transfus Apher Sci. 2007; 37: 71-79. https://bit.ly/2KCOkTO
- Mack LA, Pasieka JL. An Evidence-based approach to the treatment of thyroid lymphoma. World J Surg. 2007; 31: 978-986. https://bit.ly/2X6Z1o4
- Pyke CM, Grant CS, HabermannTM, Kurtin PJ, van Heerden JA, Bergstralh EJ, et al. Non-Hodgkin’s lymphoma of the thyroid: Is more than biopsy necessary? World J Surg. 1992; 16: 604-609. https://bit.ly/2xeuKo8
- Vardell Noble V, Ermann DA, Griffin EK, Silberstein PT. Primary thyroid lymphoma: An analysis of the national cancer database. Cureus. 2019; 11: 4088. https://bit.ly/2Yb6Zch

Sign up for Article Alerts