A Case of Sudden Cardiac Arrest in a Fabry Disease Patient with Kidney Transplantation?
Emine Disbudak1*, Nedim Cekmen1, Ceren Gunt1 and Muzaffer Atlı2
1Guven Hospital, Department of Anaesthesiology and Reanimation, Simsek Sokak No: 29, Kavaklıdere, Ankara, Turkey
2Guven Hospital, Department of General Surgery, Simsek Sokak No: 29, Kavaklıdere, Ankara, Turkey
*Address for Correspondence: Emine Disbudak, Guven Hospital, Department of Anaesthesiology and Reanimation, Simsek Sokak No: 29, Kavaklıdere, Ankara, Turkey, Fax: +903-124-572-880; Tel: +905-323-962-597; E-mail: emine.uzundere@hotmail.com
Submitted: 30 June 2019; Approved: 24 July 2019; Published: 02 September 2019
Citation this article: Disbudak E, Cekmen N, Gunt C, Atlı M. A Case of Sudden Cardiac Arrest in a Fabry Disease Patient with Kidney Transplantation. Int J Case Rep Short Rev. 2019;5(9): 051-054.
Copyright: © 2019 Disbudak E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Fabry disease; Kidney transplantation; Cardiac arrest
Download Fulltext PDF
Fabry Disease (FD), also known as Anderson-Fabry disease, is an inherited X-linked disorder characterized by the absence (in men) or deficiency (in women) in α-galactosidase A, activity that causes a progressive accumulation of glycosphingolipids within lysosomes of cells in all the major organ systems and progressive organ damage that first manifests in childhood or early adulthood. End Stage Renal Disease (ESRD) is a major cause of morbidity and premature mortality in FD. We present a male patient with FD who was transplanted with kidney from a living donor and had a sudden cardiac arrest on the 4th day after operation. We suggest detailed preoperative examination including coronary angiography, echocardiography for these patients and also a multidisciplinary care is required for perioperative management of FD patients.
Introduction
Fabry Disease (FD) is an X-linked recessive lysosomal storage disorder associated with a deficiency of α-galactosidase A resulting in accumulation of glycosphingolipids in the vascular endothelium which leads to potentially fatal renal, cardiac and cerebrovascular conditions [1]. Accumulation of its major substrate, Globotriaosylceramide (Gb3) which is mainly responsible for organ damage and its deacylated derivative, Globotriaosylsphingosine (Lyso-Gb3) in the fluids and cellular lysosomes throughout the body deteriorate organ functions, most frequently of the kidneys and heart [2].
The clinical features of Fabry disease include acroparaesthesia, angiokeratomas, hypohidrosis, corneal and lenticular opacities, and major end-organ disease (with involvement of the kidneys, heart and brain) [3]. Renal pathology is one of the hallmarks of FD and is the most frequent cause of death, usually when patients are aged 30-50 years [4]. Because Fabry nephropathy does not recur in the allograft and transplanted Fabry patients appear to have better overall outcomes than those maintained on dialysis, kidney transplantation should be recommended as a first choice in Renal Replacement Therapy (RRT) for Fabry disease [5].
We present a male patient with FD who was transplanted with kidney from a living donor and had a sudden cardiac arrest on the 4th day after operation.
Case Report
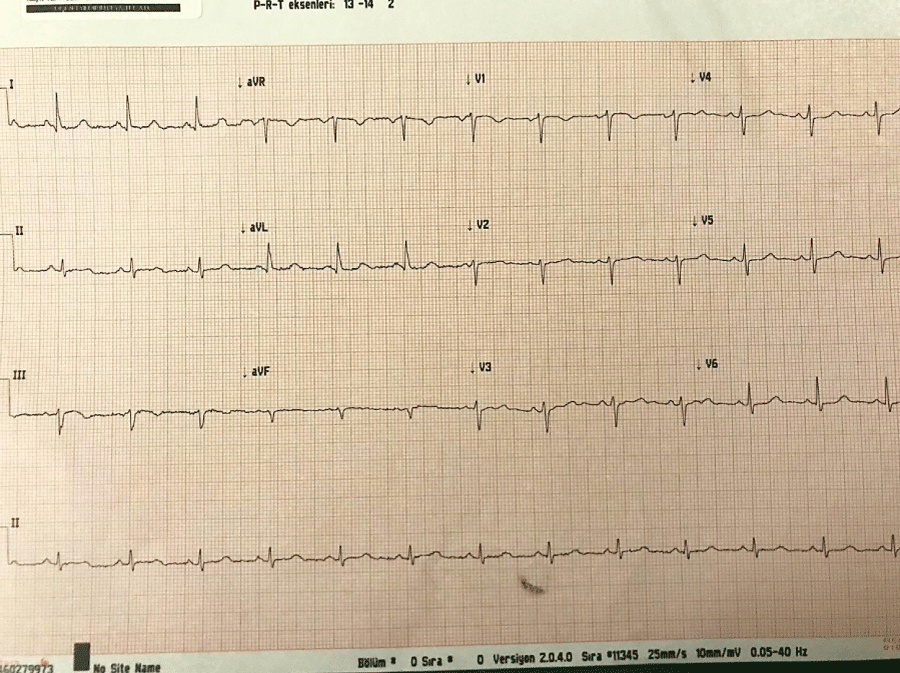
A 44-year-old male with FD had been on hemodialysis for 7 years. He had undergone enzyme replacement therapy with α-galactosidase A (0,2 mg/kg every two weeks) for nine months. His weight was 49 kg and height 166 cm (5.35 inches). According to the American Society of Anesthesiologists (ASA) physical status classification he was a ASA III patient. He had a history hyperlipidemia, coronary artery disease, arrhythmia (Ventricular Extrasystoles-VES), heart failure and ankylosing spondylitis. There was sinus rhythm on electrocardiogram (Figure 1). A screening pretransplant echocardiography showed left ventricular hypertrophy with ejection fraction of 60%, dilated left atrium, mitral valve regurgitation, and normal right ventricular and right atrial size with normal pulmonary artery pressure. Results of preoperative coronary angiography were left anterior descending artery with irregular borders and 50% stenosis at D2 branch, right coronary artery with irregular borders and plaque at ostium of circumflex artery. Medical therapy which were atorvastatin 20 mg/day, metoprolol 25 mg/day and acetylsalicylic acid 100 mg/day began for coronary artery disease. The patient had normal laboratory values except hemoglobin- hematocrit (10.8 g/dL-36.2 %), BUN (57 mg/dL), urea (123 mg/dL) and creatinine (5.01 mg/dL). He was on hemodialysis for 7 years and his estimated glomerular filtration rate was 13 mL/min (according to the Cockcroft gault formula).
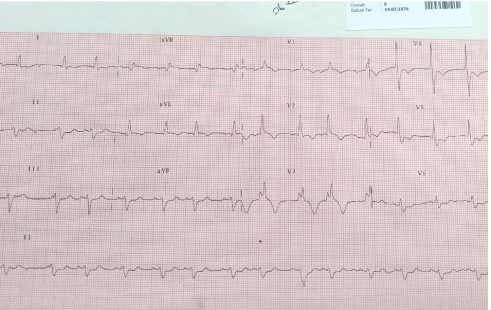
On the physical examination for the patient whose mallampathy score was evaluated to be III, macroglossia and limited neck flexion were observed, and a video laryngoscope was prepared in case of a difficult intubation. The patient was taken into operation hall and anaesthesia induction was started after monitorization. A hemodialysis catheter which was placed before operation day was used as an intravenous (I.V.) route, and lidocaine 40 mg, propofol 100 mg, and fentanyl 100 μg was administered and the patient was ventilated with a mask. After that, muscular relaxation was obtained with 0.5 mg/kg rocuronium. The patient was being intubated and localization of tube was confirmed by auscultation and capnography and fixed. Maintenance of anaesthesia was continued with propofol 4mg/kg/hour and remifentanil 0,15 mcg/kg/hour infusions and 0.15 mg/kg rocuronium I.V. as required. End-tidal CO2 and temperature monitorizations were made on the patient. Peripheral vascular route was opened with 16 G angiographic catheter, Central Venous Pressure (CVP) monitorization and left radial artery cannulation were performed and arterial blood pressure monitorization was made, and hourly urine output was monitored with a urinary catheter placed. The patient was hemodynamically stable maintaining a mean arterial pressure of 60-65 mmHg. After uncomplicated surgery, extubation was performed in operative room and awakening was uneventful. Analgesia was granted with intravenous tramadol. He was handed over to the clinic without problem after followed in postanaesthesia care unit for 1 hour during postoperative period. On the 4th day after operation, he had sudden cardiac arrest with asystole as the determined rhythm. Cardiopulmonary Resuscitation (CPR) was started immediately. Adrenaline was given 4 times at 1 mg doses intravenously every 3 minutes. The rhythm which was ventricular tachycardia returned at the 7th minute after CPR. Defibrillation was applied 4 times as 200 joules and amiodarone was given at 300 mg as a bolus dose in 10 minutes, 900 mg as a maintenance dose in 18 hours intravenously. The patient was stable hemodynamically at the 27th minute after cardiac arrest. He was admitted to the Intensive Care Unit (ICU) in an orotracheal intubated state. There was right bundle branch block and ST segment depression in the anterolateral leads on electrocardiogram (Figure 2). A transthoracic echocardiography was applied on post-cardiac arrest state. It showed left ventricular hypertrophy with ejection fraction of 50%, mitral valve regurgitation, hypokinetic interventricular septum, normal right ventricular and right atrial size with normal pulmonary artery pressure. His medical therapy was ordered as enoxaparin sodium 0,6 mL/day, acetylsalicylic acid 100 mg/day, metoprolol 50 mg/day, atorvastatin 20 mg/day and also immunosuppressive therapy. The patient was extubated on the 24th hour of his intubation, and he was discharged from the ICU on the 3th day. He was discharged to home without problem, and was followed up in the clinic of general surgery.
Discussion
Fabry disease is a rare lysosomal storage disorder. The disease first described simultaneously but separately in 1898 by dermatologists from Germany, Johannes Fabry and the United Kingdom, William Anderson [6]. The incidence has been estimated to be 1:117.000 live births and 1:50.000 males [7]. Birth prevalence of the female Fabry carriers were only studied in some reports, and were therefore not known exactly. In 1947, the observation of abnormal vacuoles in blood vessels throughout the body at post mortem examination in 2 patients who had died from renal insufficiency led to the recognition of this disease being a generalized storage disorder [8].
Accumulation of the glycosphingolipid Globotriaosylceramide (Gb3) results and causes cellular dysfunction not only in several organ systems, mainly skin, kidney, heart, lung, and brain, but also in the gastrointestinal tract and cornea. The injured endothelial cells of small and large vessels and vascular smooth muscle cells cause dysfunction of the heart and brain leading to early onset of hypertension, concentric left ventricular hypertrophy without obstruction, and coronary heart disease, as well as vertebrobasilar artery signs and symptoms, excessive daytime sleepiness, and stroke [6]. The major causes of death include cardiac death, stroke, or the consequences of ESRD [9-10]. ESRD occurs in the majority of male patients and in a significant proportion of females who require dialysis or transplantation. Importantly, 12 % of ESRD patients with FD were female in both registries [5].
The first kidney transplant in a FD patient was conducted in 1967 in Basel, Switzerland. Sorbello et al. reported two patients with FD who underwent a deceased donor kidney transplantation, by focusing on the anaesthesiologic implications for this rare disease [4]. They demonstrated that a standard anaesthesiological protocol could be applied in these patients, but careful perioperative management is mandatory. Kruger et al. presented two female patients with FD who required general anesthesia twice for gynecological and trauma surgery, respectively, and discuss their perioperative management [6]. They reported that compared with equivalent patients without FD, they had to deal with early-onset arterial hypertension, hyperalgesia, cardiomyopathy, and renal dysfunction. They also concluded that patients with mild progression of the disease require only a few adjustments to keep them stable with a beneficial outcome.
In this report, we present a male patient with FD who had arrhythmia and ankylosing spondylitis. The patients with FD have rheumatologic findings such as joint pain, acroparaesthesia, Raynaud phenomenon. However, ankylosing spondylitis is not common finding of FD in the literature. In our case we planned difficult airway algorithm because of ankylosing spondylitis, mallampathy score III, macroglossia and limited neck flexion, but intubation did not force us as we expected. FD has serious cardiovascular manifestations like conduction system abnormalities, arterial hypertension, myocardial infarction, congestive heart failure, arrhythmia which can result in sudden death [11]. Our patient also had a cardiac arrest on the 4th day after operation which was managed successfully. He had a history of hyperlipidemia, coronary artery disease, arrhythmia and heart failure preoperatively. Although CAG was done and treatment was started according to the results of CAG before operation, surgical stress may have caused cardiac depression additionally. He didn’t have any electrolyte abnormalities. We didn’t observe any non-sustained ventricular tachycardia during monitoring. He was hemodynamically stable before cardiac arrest, so we have linked cardiac arrest to pre-existing cardiomyopathy. We informed the patient about his cardiac status and advised him to be examined by cardiologist after discharge.
Conclusion
Patient diagnosed with FD challenge anesthesiologist due to cardiac, renal and cerebral disfunctions, especially cardiac ones. Preoperative examination should made carefully including coronary angiography and echocardiography. According to our experience, we recommend advanced hemodynamic monitoring during surgery. Careful airway examination should be further performed and alternative strategies for airway management should be planned by considering a difficult intubation. A nephroprotective and cardioprotective strategies and a particular care to the associated end-stage organ disease may significantly improve the long-term outcome of patients with FD. A multidisciplinary care is required for perioperative management of these patients.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Authors’ Contributions
All of the authors contributed to the medical management of the patient and preparation of the manuscript. All of the authors have read and approved the content of the manuscript.
- Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, et al. Fabry disease, an under-recognized multisystemic disorder: Expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Int Med.2003; 138: 338-346. https://bit.ly/2LCRpni
- Desnick RJ, Ioannou YA, Eng CM. α-galactosidase a deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular basis of inherited diseases. 7th ed. New York: McGraw-Hill; 2001; 150: 3733-3774.
- Pastores GM, Thadhani R. Advances in the management of Anderson-Fabry disease: enzyme replacement therapy. Expert Opin Biol Ther. 2002; 2: 325-333. https://bit.ly/2ZbpYEe
- Sorbello M, Veroux M, Cutuli M, Morello G, Paratore A, Sidoti MT, et al. Anaesthesiologic protocol for kidney transplantation in two patients with Fabry Disease: a case series. Cases J. 2008; 1: 321. https://bit.ly/2Y3RKpy
- Mignani R, Feriozzi S, Schaefer RM, Breunig F, Oliveira JP, Ruggenenti P, et al. Dialysis and transplantation in Fabry disease: indications for enzyme replacement therapy. Clin J Am Soc Nephrol. 2010; 5: 379-385. https://bit.ly/2Zc8MP0
- Kruger S, Nowak A, Muller TC. General anesthesia and Fabry Disease: A Case Report. A A Case Rep. 2017; 8: 247-249. https://bit.ly/30QPVct
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA.1999; 281: 249-254. https://bit.ly/2OeY895
- Pompen A, Ruiter M, Wyers H. Angiokeratoma corporis diffusum (universale) Fabry, as a sign of an unknown internal disease: two autopsy reports. Acta Med Scand. 1947; 128: 234-255. https://bit.ly/2YhBkcA
- Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry registry. Genet Med. 2009; 11: 790-796. https://bit.ly/30Z5Aqp
- Mehta A, Clarke JT, Giugliani R, Elliott P, Linhart A, Beck M, et al. Natural course of Fabry disease: Changing pattern of causes of death in FOS-Fabry Outcome Survey. J Med Genet. 2009; 46: 548-552. https://bit.ly/2JMcyJm
- Martins AM, D'Almeida V, Kyosen SO, Takata ET, Delgado AG, Gonçalves AM, et al. Guidelines to diagnosis and monitoring of Fabry disease and review of treatment experiences. J Pediatr. 2009; 155: 19-31. https://bit.ly/2SyPpgt

Sign up for Article Alerts