Arthroscopic Diagnosis and Management of Pigmented Villonodular Synovitis and Pseudogout of the Temporomandibular Joint?
Mohamed A Hakim1*, Jeffrey Taylor2, Kristen Yant2, David Y Ahn3 and Joseph P McCain1
1Department of Oral and Maxillofacial Surgery, Massachusetts General Hospital and Harvard School of Dental Medicine, Boston, MA, USA
2DMD Candidate, Harvard School of Dental Medicine, Boston, MA, USA
3Oral and Maxillofacial Surgery Attending, David Grant USAF Medical Center, United States Air Force, Fairfield, CA, USA
*Address for Correspondence: Mohamed A Hakim, Department of Oral and Maxillofacial Surgery, Massachusetts General Hospital and Harvard School of Dental Medicine 55 Fruit Street, Warren 1201, Boston, MA, 02114 USA, Tel: +164-646-74757; E-mail: mhakim@mgh.harvard.edu
Submitted: 28 November 2019; Approved: 10 January 2020; Published: 11 January 2020
Citation this article: Hakim MA, Taylor J, Yant K, Ahn DY, McCain JP. Arthroscopic Diagnosis and Management of Pigmented Villonodular Synovitis and Pseudogout of the Temporomandibular Joint. Sci J Res Dentistry. 2020;4(1): 001-006.
Copyright: © 2020 Hakim MA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: TMJ Arthroscopy; Temporomandibular Joint; Pigmented Villonodular Synovitis; PVNS; Pseudogout; Calcium Pyrophosphate Deposition Disease; CPPD; Arthroscopic Synovectomy; Minimally Invasive
Download Fulltext PDF
Traditionally, obtaining tissue diagnosis from the Temporomandibular Joint (TMJ) has required invasive open techniques. In this case-series, the authors demonstrate a minimally invasive technique using arthroscopy to diagnose and treat Pigmented Villonodular Synovitis (PVNS) and pseudogout of the TMJ, followed by a review of the literature.
Introduction
Obtaining tissue diagnosis from the temporomandibular joint requires biopsy which is frequently done with open techniques [1]. Fine Needle Aspiration (FNA) has been described as a minimally invasive alternative, but the diagnostic accuracy is questionable [2]. The purpose of this case-series is to demonstrate the utility of arthroscopic synovial biopsy in the diagnosis of Pigmented Villonodular Synovitis (PVNS) and Calcium Pyrophosphate Deposition Disease (CPPD, formerly known as pseudogout), as well as provide a literature review.
Patients and Methods
Three patients underwent double puncture arthroscopy after completing an unsuccessful course of conservative therapy (NSAIDs, mouth guard, and diet modification). The areas of significant pathology during the diagnostic arthroscopy were biopsied. Then thermal cautery, laser fibers (0.5-0.6-micron), shavers and curettes were used to perform synovectomy on the diseased synovium and debridement of the articular surfaces. Specimens were sent for histological examination. All patients were planned for long-term follow-up.
Case 1
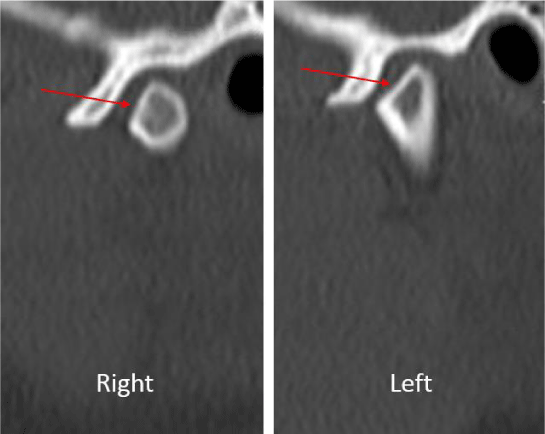
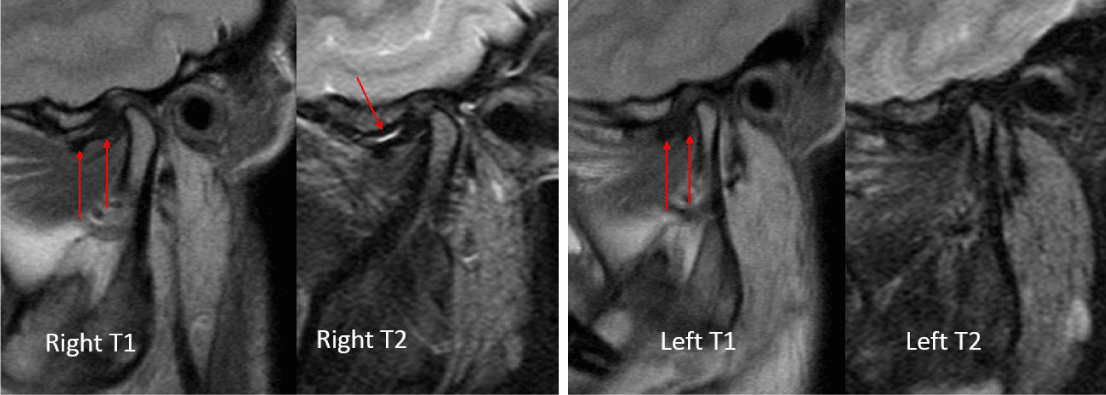
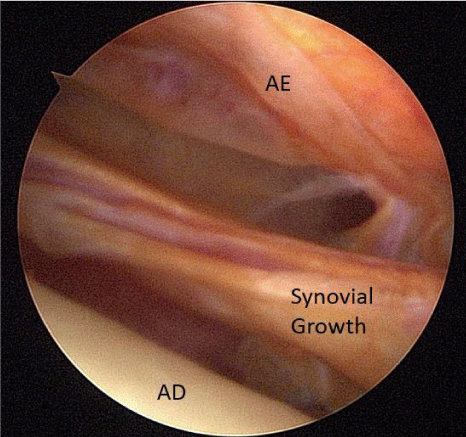
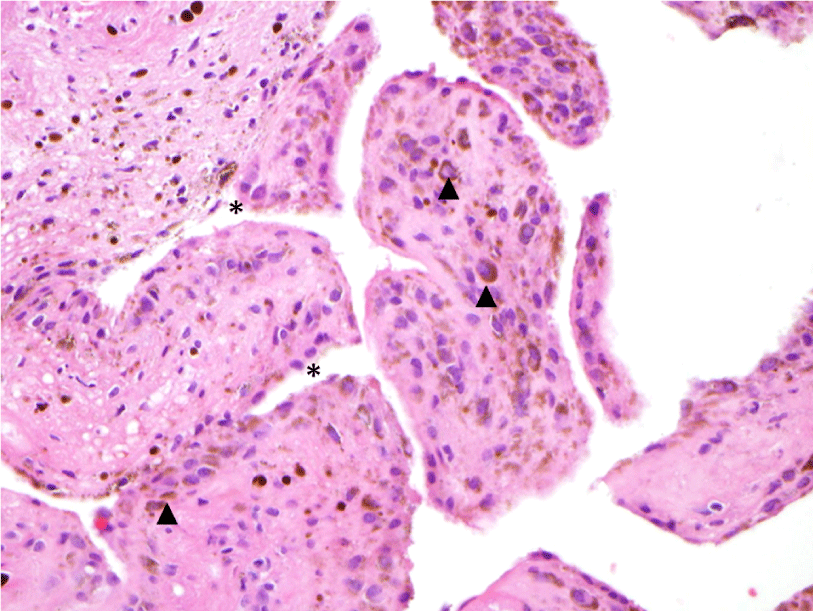
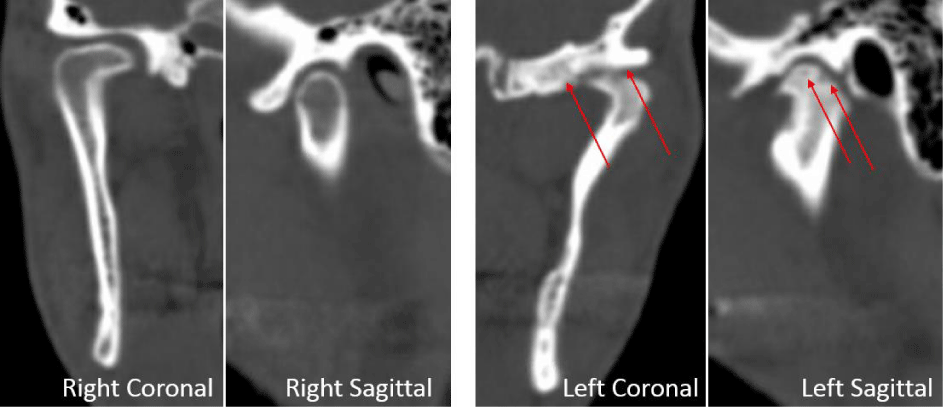
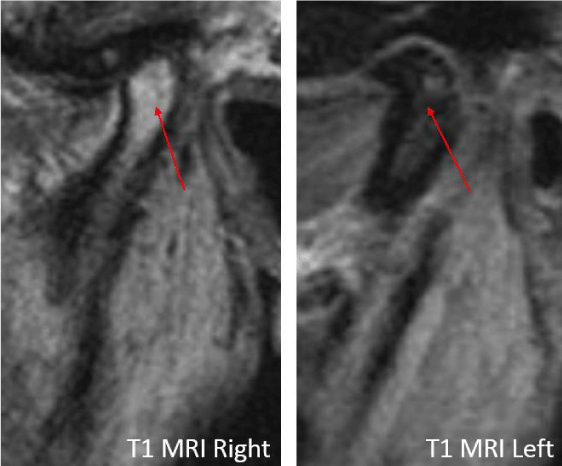
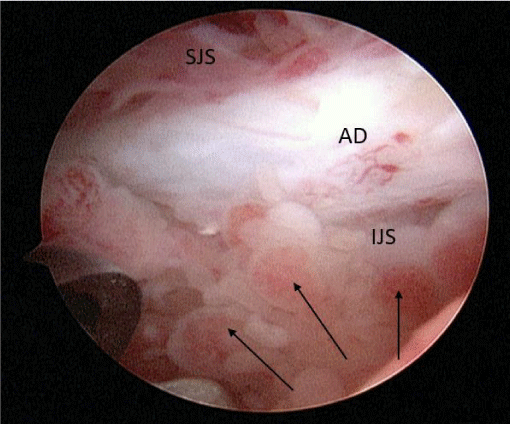
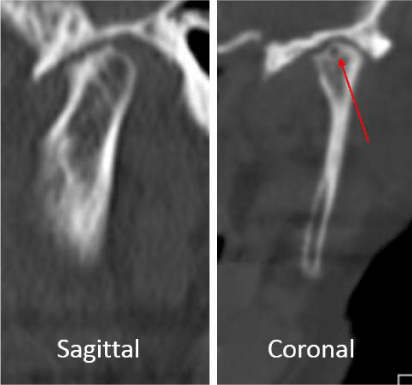
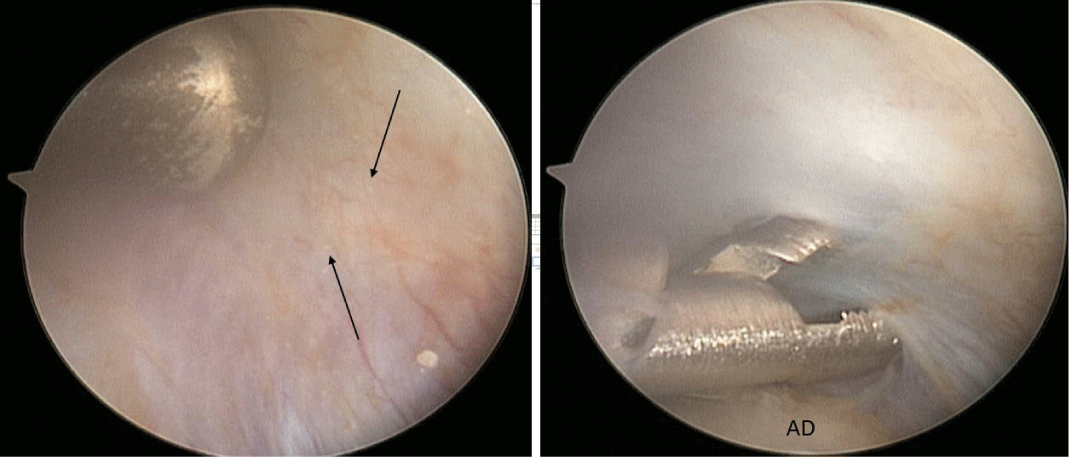
A 24-year-old female presented with a two-month history of right TMJ pain and locking. Examination revealed a 33mm mouth-opening with deviation to the right, bilateral mandibular condyles and right masseter tenderness to palpation, intact cranial nerves and no joint loading. There was no facial or preauricular swelling. The CT scan showed left greater than right condylar flattening with intact cortical outline and no osteolysis (Figure 1). The MRI showed bilaterally displaced discs with the left being deformed (Figure 2). The patient then underwent bilateral arthroscopy. Diagnostic arthroscopy on the left revealed synovial growth with striking reddish-orange discoloration. The disc was seemingly spared from the disease process (Figure 3,4). Synovectomy and debridement were completed. Histologic examination revealed PVNS (Figure 5). On her 10-month follow-up she reported significant improvement in pain and functional-limitation scores. She had a painless, symmetric 35mm mouth-opening with no joint tenderness or loading.
Case 2
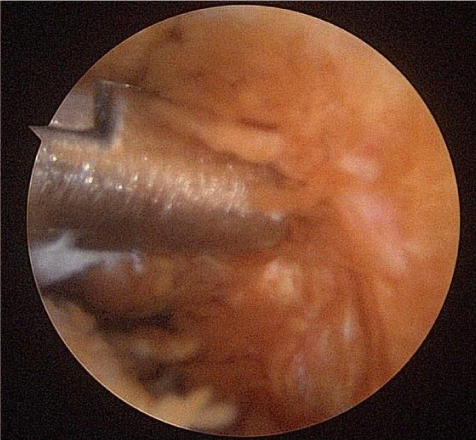
A 53-year-old male presented with a complicated musculoskeletal history consisting of head and neck dystonia and suprahyoid musculature spasms resulting in painful recurrent mandibular subluxation. Examination revealed a mouth-opening of 60 mm with ongoing subluxation due to muscle spasms. The CT scan of the left side suggested severe degenerative changes with multiple rounded erosive foci and a hazy periosteal reaction at the skull base (Figure 6). The T1 weighted MRI showed low signal intensity in the condylar bone marrow on the left. The disc was not visualized and there was no evidence of intra-articular mass (Figure 7). Diagnostic arthroscopy of the left showed a large central perforation of the disc with remarkable nodular synovitis. Synovial biopsy, synovectomy and debridement were performed (Figure 8). Histologic examination showed PVNS (Figure 9). Additional surgical treatment for the dislocation and the degenerative changes of the TMJ was deferred until further control of the muscular dystonia was achieved.
Case 3
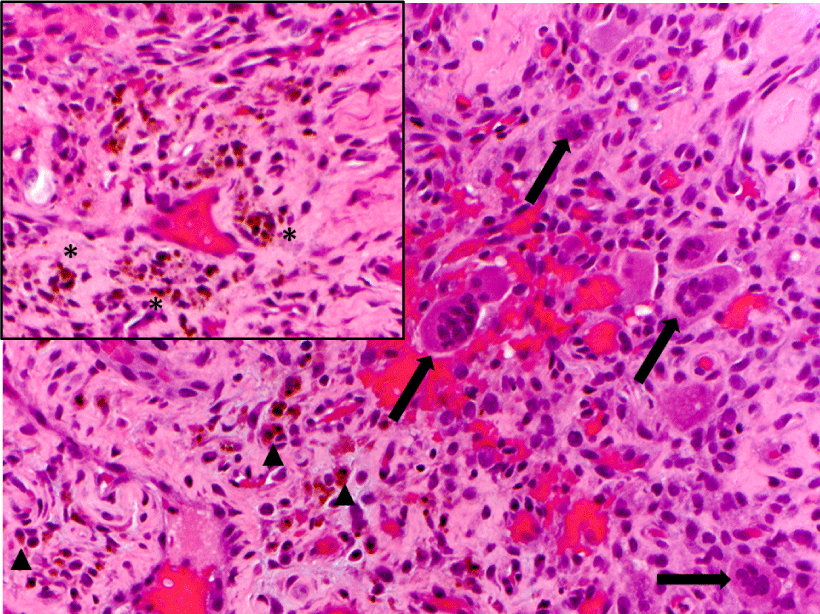
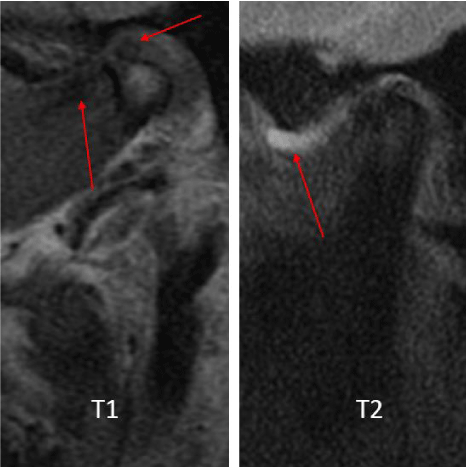
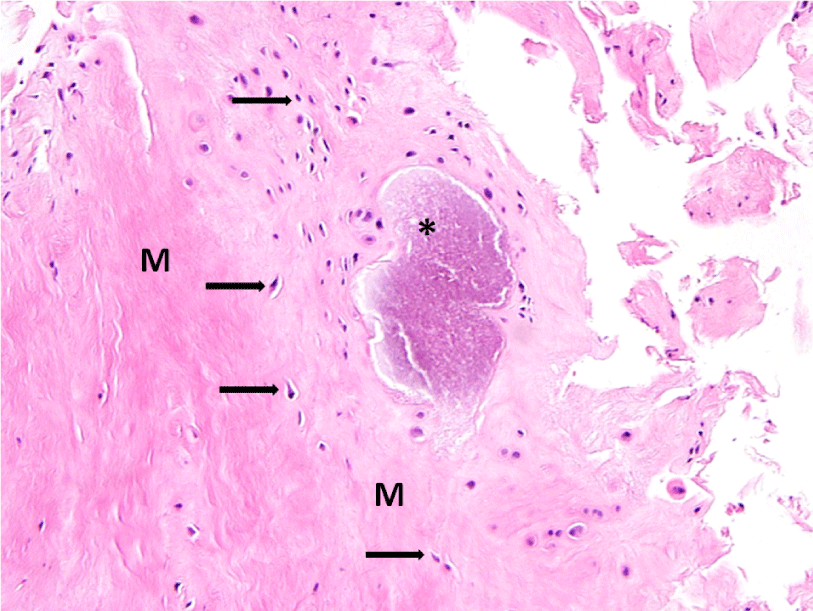
A 72-year-old female presented with left-sided jaw pain. Examination revealed tenderness over the left masseter, a mouth-opening of 35 mm and bilateral joint noises. Her left side had direct and indirect loading. The CT scan showed degenerative changes of the left condyle with a 2x2 mm subchondral cyst (Figure 10). The MRI revealed a degenerative left condyle with a deformed disc. The T2 weighted imaging showed a superior joint space effusion (Figure 11). Diagnostic arthroscopy showed retrodiscal synovitis, chondromalacia and a perforated disc. There were crystal-like structures within the synovium antero-medially. Synovial biopsy, debridement and partial synovectomy were performed (Figure 12). Histologic examination showed proliferative synovitis with calcium pyrophosphate deposition (Figure 13). The patient was referred for rheumatological management of CPPD but no treatment was initiated due to symptom resolution. On her 6-month follow-up her pain had resolved; she was maintaining a regular diet and her mouth-opening had become 45 mm with no joint loading.
Discussion
The purpose of this case-series is to demonstrate the utility of minimally invasive TMJ arthroscopy in obtaining a tissue diagnosis and management of PVNS and CPPD, which traditionally required open approaches. Arthroscopic synovial biopsy of the TMJ was described in the 1990’s and primarily focused on correlating arthroscopic evidence of synovitis with histologic examination [3]. There are few published cases on arthroscopic management of PVNS and none on CPPD [1,4].
Pigmented villonodular synovitis
PVNS is a rare entity characterized by localized or diffuse monoarticular neoplastic proliferation of the synovium and is considered a subtype of tenosynovial giant cell tumors. The knee is the most common site (75%), followed by the hip (15%). The etiology remains unclear but trauma and overexpression of colony-stimulating factor 1 (CSF-1) are thought to be contributing factors [5]. It is typically benign but a malignant variant has been reported [6]. To the authors’ knowledge, 62 cases of PVNS in the TMJ have been reported in the English literature. Of those cases, 31 were male, 30 were female and 1 was non-reported. Fifty-nine showed boney invasion and 48 intracranial invasion. Most of the cases reported have been diagnosed and treated with open surgery while a few were managed arthroscopically [4]. Fine needle aspiration was useful in the diagnosis of one while it was inconclusive in another [2]. The commonly reported clinical picture of PVNS-TMJ is preauricular pain and swelling. Vertigo and headaches are usually associated with intracranial extension. Computed tomography findings include osteolysis and osteosclerosis of the condyle and temporal bone, while MRI may show synovial hyperplasia and reduced signal intensity [5,7]. There is no consensus for treatment of PVNS-TMJ but there have been reports of arthroscopic and open synovectomy, and aggressive resection with prosthetic reconstruction. Temporal craniotomy has been performed when the tumor invaded the skull base [4,8]. In the knee, open or arthroscopic synovectomy is the first line of treatment for localized cases with comparable recurrence rates (24.6% and 26.7% respectively) but other studies showed a wider range (4.7 to 60%). Total joint arthroplasty is typically reserved for severe degenerative changes [5,9,10]. Intra-articular (radiation synovectomy) and external beam radiation have been described as adjuncts to reduce recurrence rates but the long-term effects of radiation are yet to be studied [11,12]. Adjunctive drugs are being studied which include: CSF-1 inhibitors for advanced diffuse cases and TNF-alpha inhibitor in refractory cases with overexpression of TNF-alpha [5,13].
Both cases of PVNS presented in this series had no preauricular swelling or radiographic evidence of mass formation. Case 1 was considered to have articular disorder while Case 2 was thought to have advanced degenerative arthritis. Case 1 did not have radiographic evidence of osteolysis or change in signal intensity on MRI. It is likely that this patient had an early stage of the disease that was diagnosed before osteolysis, extra-articular extension or radiographic evidence. Case 2 had osteolysis of the condyle and osteosclerosis of the skull base, which raised concerns for an infiltrative process (like PVNS), yet the MRI was inconclusive. These two examples suggest that the reported characteristic imaging of PVNS-TMJ fail to identify early disease process. Cai. et al. previously highlighted the delay in diagnosis of PVNS using clinical and radiographic examination [4]. The average duration of joint pain before PVNS-TMJ is not clear in the literature. Early arthroscopy can offer a minimally invasive intervention to aid in the early diagnosis of PVNS before intracranial extension occurs, thus changing the number of localized and diffuse cases reported. Fine needle aspiration is another minimally invasive method for diagnosis, but it is only 79% sensitive and 72% specific, and often requires biopsy to confirm the diagnosis [14]. Additionally, arthroscopy provides the ability to perform synovectomy as first line treatment for localized lesions. Similar treatment was documented in a series of four patients with one having a 44-month follow-up with no recurrence [4]. More data on the long-term outcome of this procedure is needed.
Calcium pyrophosphate deposition disease (formerly known as pseudogout)
Calcium Pyrophosphate Deposition (CPPD) disease, according to the European League Against Rheumatism, was agreed upon as an umbrella term that includes acute Calcium Pyrophosphate (CPP) crystal arthritis, osteoarthritis with CPPD and chronic CPP crystal inflammatory arthritis. It demonstrates higher predilection for fibrocartilage than hyaline cartilage which explains how this can occur in the TMJ. The most common sites are the triangular ligament of the wrist and the meniscus of the knee [15]. To the authors’ knowledge there have been 56 cases of CPPD-TMJ previously reported, none of which were managed arthroscopically. Forty-nine were diagnosed via biopsy at the time of open surgery and seven were diagnosed via FNA [1,16]. Laviv and Keith described diagnostic arthroscopy on a case of CPPD-TMJ, but the final diagnosis was with open biopsy [17]. Chondrocalcinosis is defined as cartilage calcification, which is most commonly due to CPPD and detected by imaging or histological examination [18].
The mean age of CPPD-TMJ is 58 years old [19]. There is a female predilection (male-to-female ratio of 1:1.9) [20]. Patients often present with symptoms that include: intermittent acute arthritic attacks, joint effusion, preauricular swelling, and decreased joint mobility. It can present with otalgia and unilateral conductive hearing loss [21]. There are two presentations of CPPD-TMJ. The first type involves acute inflammatory attacks that cause painful preauricular swelling of the TMJ. The second is called tophaceous CPPD which can mimic a tumor (eg. synovial chondrosarcoma). The first published case of tophaceous CPPD-TMJ was described by Pritzker. et al. in 1976 as a pseudotumor [22]. The majority of cases reported have been the symptomatic tophaceous type. Of these, only ten presented with extra-articular extension and one extended to the skull-base; however, none showed intracranial invasion [23].
Radiographic description of CPPD can include: a radiopaque mass with articular erosion, a dense mass between the condyle and the coronoid process with a hypertrophic condyle, degenerative changes in the mandibular condyle with a mass which appear to be like calcifications and chondrocalcinosis [24,25]. For the type that may show degenerative changes with no mass associated, the differential can include osteoarthritis and primary arthritis [26]. For the tophaceous (tumoral) type, which can be more evident radiographically, the differential diagnosis includes synovial chondromatosis and other benign or malignant neoplasms such as osteochondroma and chondrosarcoma [1,5]. In those instances, diagnostic arthroscopy with synovial biopsy is invaluable to establish diagnosis and plan for additional treatment if needed. When crystal deposition is suspected during arthroscopy the biopsy specimen should be sent in saline rather than formalin, to reduce the risk of crystal dissolution. Patients at risk for CPPD include those with hyperparathyroidism, osteoarthritis and those on loop diuretics [27]. Medical management is aimed towards treating joint inflammation. NSAIDs, corticosteroids, colchicine and methotrexate are commonly used [28]. Three out of the 56 CPPD-TMJ cases reported a recurrence.
Conclusion
Advanced TMJ arthroscopy with synovial biopsy is helpful in obtaining tissue diagnosis of CPPD and PVNS. Additionally, arthroscopic synovectomy and debridement can be used as first line of treatment. Larger studies with long-term follow-up are needed to evaluate the therapeutic efficacy of the procedure.
Acknowledgement
This work was funded in part by Fellowship grants from DePuy Synthes (Raynham, MA, USA) and KLS Martin (Jacksonville, FL, USA).
- Meng J, Guo C, Luo H, Chen S, Ma X. A case of destructive calcium pyrophosphate dihydrate crystal deposition disease of the temporomandibular joint: A diagnostic challenge. Int J Oral Maxillofac Surg. 2011; 40: 1431-1437. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21676588
- Pianosi K, Rigby M, Hart R, Trites J, Taylor SM. Pigmented villonodular synovitis of the temporomandibular joint: a unique presentation. Plast Reconstr Surg Glob Open. 2016; 4: 674. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27200236
- Suzuki T, Segami N, Sato J, Nojima T. Accuracy of histologic grading of synovial inflammation in temporomandibular joints with internal derangement using Gynther's system. J Oral Maxillofac Surg. 2001; 59: 498-501. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11326369
- Cai XY, Yang C, Chen MJ, Jiang B, Yun B, Fang B. Arthroscopic management of intra-articular pigmented villonodular synovitis of temporomandibular joint. Int J Oral Maxillofac Surg. 2011; 40: 150-154. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20961736
- Pigmented villonodular synovitis. 2019. https://bit.ly/36QEHIF
- Yoon HJ, Cho YA, Lee JI, Hong SP, Hong SD. Malignant pigmented villonodular synovitis of the temporomandibular joint with lung metastasis: A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 111: 30-36. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21444225
- Le WJ, Li MH, Yu Q, Shi HM. Pigmented villonodular synovitis of the temporomandibular joint: CT imaging findings. Clin Imaging. 2014; 38: 6-10. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24100118
- Vellutini EAS, Alonso N, Arap SS, Godoy LFS, Souza E Souza RA, Mattedi RL, et al. Functional reconstruction of temporomandibular joint after resection of pigmented villonodular synovitis with extension to infratemporal fossa and skull base: A case report. Surg J (N Y). 2016; 2: 78-82. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28824995
- Rodriguez Merchan EC. Open versus arthroscopic synovectomy for pigmented villonodular synovitis of the knee. J Orthop Surg (Hong Kong). 2014; 22: 406-408. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25550027
- Georgiannos D, Boutsiadis A, Agathangelidis F, Papastergiou S, Karataglis D, Bisbinas I. Arthroscopically-assisted mini open partial synovectomy for the treatment of localized pigmented villonodular synovitis of the knee. A retrospective comparative study with long-term follow up. Int Orthop. 2017; 41: 925-930. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27866235
- Nassar WA, Bassiony AA, Elghazaly HA. Treatment of diffuse pigmented villonodular synovitis of the knee with combined surgical and radiosynovectomy. HSS J. 2009; 5: 19-23. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19096892
- Horoschak M, Tran PT, Bachireddy P, West RB, Mohler D, Beaulieu CF, et al. External beam radiation therapy enhances local control in pigmented villonodular synovitis. Int J Radiat Oncol Biol Phys. 2009; 75: 183-187. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19211195
- Kroot EA, Kraan MC, Smeets TJ, Maas M, Tak PP, Wouters JM. Tumour necrosis factor α blockade in treatment resistant pigmented villonodular synovitis. Ann Rheum Dis. 2005; 64: 497-499. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15297283/
- Kasraeian S, Allison DC, Ahlmann ER, Fedenko AN, Menendez LR. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin Orthop Relat Res. 2010; 468: 2992-3002. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20512437
- Chuong R, Piper MA. Bilateral pseudogout of the temporomandibular joint: report of case and review of literature. J Oral Maxillofac Surg. 1995; 53: 691-694. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7776053
- Good AE, Upton LG. Acute temporomandibular arthritis in a patient with bruxism and calcium pyrophosphate deposition disease. Arthritis Rheum. 1982: 25: 353-355. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6279120
- Laviv A, Sadow PM, Keith DA. Pseudogout in the temporomandibular joint with imaging, arthroscopic, operative, and pathologic findings. Report of an unusual case. J Oral Maxillofac Surg. 2015; 73: 1106-1112. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25843817
- Zhang W, Doherty M, Bardin T, Barskova V, Guerne PA, Jansen TL, et al. European league against rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011; 70: 563-570. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21216817
- Reynolds JL, Matthew IR, Chalmers A. Tophaceous calcium pyrophosphate dihydrate deposition disease of the temporomandibular joint. J Rheumatol. 2008; 35: 717-721. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18398950
- Ascani G, Pieramici T, Filosa A, Balercia P, Messi M, Rubini C. Pseudogout of the temporomandibular joint: a case report. J Oral Maxillofac Surg. 2008; 66: 386-388. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18201629
- Sklenicka S, Dierks EJ, Jarmin J, Miles C. Pseudogout of the temporomandibular joint: An uncommon cause of temporomandibular joint pain and swelling. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 111: 709-714. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21167760
- Pritzker KP, Phillips H, Luk SC, Koven IH, Kiss A, Houpt JB. Pseudotumor of temporomandibular joint: Destructive calcium pyrophosphate dihydrate arthropathy. J Rheumatol. 1976; 3: 70-81. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1271391
- Kudoh K, Kudoh T, Tsuru K, Miyamoto Y. A case of tophaceous pseudogout of the temporomandibular joint extending to the base of the skull. Int J Oral Maxillofac Surg. 2017; 46: 355-359. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27641810
- Kamatani Y, Tagawa T, Hirano Y, Nomura J, Murata M. Destructive calcium pyrophosphate dihydrate temporomandibular arthropathy (pseudogout). Int J Oral Maxillofac Surg. 1987: 16: 749-752. https://bit.ly/2TaQIEM
- Abdelsayed RA, Said Al Naief N, Salguerio M, Holmes J, El Mofty SK. Tophaceous pseudogout of the temporomandibular joint: A series of 3 cases [Abstract]. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014; 117: 369-375. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24528794
- Nakagawa Y, Ishii H, Shimoda S, Ishibashi K. Pseudogout of the temporomandibular joint. A case report. Int J Oral Maxillofac Surg. 1999: 28: 26-28. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10065644
- Rho YH, Zhu Y, Zhang Y, Reginato AM, Choi HK. Risk factors for pseudogout in the general population. Rheumatology (Oxford). 2012; 51: 2070-2074. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22886340
- Macmullan P, McCarthy G. Treatment and management of pseudogout: insights for the clinician. Ther Adv Musculoskelet Dis. 2012; 4: 121-131. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22870500

Sign up for Article Alerts