Periodontal Status of Type 2 Diabetic Patients Attending UNRWA Health Centers in Gaza Governorates?
Emad Alqedra1* and Yousef I Aljeesh2
1Department of Health, UNRWA Gaza field, Palestine
2Faculty of Nursing Islamic University Gaza, Palestine
*Address for Correspondence: Alqedra Emad, Department of Health, UNRWA Gaza field, Palestine, ORCID ID: https://orcid.org/0000-0003-0786-2909; Tel: +972-599-686-372; Fax: 009-708-264-4800; E-mail: I.ElQedra@unrwa.org / Emad103020@gmail.com
Submitted: 28 December 2019; Approved: 01 February 2020; Published: 04 February 2020
Citation this article: Alqedra E, Aljeesh YI. Periodontal Status of Type 2 Diabetic Patients Attending UNRWA Health Centers in Gaza Governorates. Sci J Res Dentistry. 2020;4(1): 015-022.
Copyright: © 2020 Alqedra E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Background: Diabetes affects millions of people each year, it is one of the leading causes of mortality and morbidity worldwide. Periodontal disease has recently been recognized as the “sixth complication” of diabetes mellitus, the relationship between diabetes and periodontal disease is actually bi-directional. Generally, poor oral hygiene, a long history of diabetes, greater age, and poor metabolic control are associated with more severe periodontal disease.
Method: The study is an analytical cross-sectional study, 406 patients with type 2 diabetes mellitus selected through systematic random sampling from 5 UNRWA health centers. The World Health Organization’s basic methods tools were used to collect data and assess oral health.
Results: Showed 36.3% of participants never brush their teeth, only 16.5% brush their teeth twice or more a day. Only 16.4% of participants have no gingival bleeding, the mean number of teeth showing no gingival bleeding is (9.79), showing gingival bleeding (9.91), and not present for bleeding test (9.14). While 2.4% have no periodontal pockets, the mean number of teeth showing absence of pocket (7.15), showing pocket 4-5 mm (7.84), showing pocket 6 mm or more (4.96) and not present for pocket measurement (9.13). Gingival bleeding was statistically significant associated with gender, and frequency of teeth brushing, but there was no statistically significant association between gingival bleeding and periodontal pocket, and sociodemographic, Glycated Hemoglobin (HbA1c) and diabetic duration.
Conclusion: Type 2 diabetes mellitus patients already had chronic periodontitis worsen by diabetes. Oral and periodontal health should be promoted as integral components of diabetes management
Introduction
The mouth is a “Gate” of the body, reveals signs of general health disorders. However oral conditions have an impact on overall health and disease. Periodontal disease has been associated with a number of systemic conditions. Major chronic diseases-for instance, cancer, diabetes mellitus and heart disease-share common risk factors with oral disease; so it is obvious that oral health is a basic component of health and must be considered and included in the provision of healthcare and the design of community programs [1].
On Gaza strip, with over 2 million population, more than 70% are refugees, and 90% of them served by UNRWA health centers, about 40000 diabetic patients are followed by 22 health centers at Gaza field according to UNRWA health report 2015, with a prevalence of 15.1% among served population over 40 years old [2].
Many studies have revealed that periodontal infection and DM have a two-way relationship [3,4]. Loe stated that periodontal disease is the sixth most common complication of DM [5], whereas Lalla and Lamster reported that DM is the strongest risk factor for periodontal infection compared to the other systemic conditions such as hypertension [3].
Study objective
The aim of this study is to know the periodontal status of type 2 diabetic patients attending UNRWA health centers in Gaza Governorates.
Methodology
An analytical, cross-sectional design to assess the oral health of 381 type 2 diabetic patients from five UNRWA health centers were examined and interviewed. The World Health Organization’s (WHO) basic methods 5th Edition were used [6]. A representative sample had taken from five health centers according to systematic random sampling from type 2 diabetic patients attending UNRWA primary health care centers (39448 type 2 DM) with active DM file during 2017.
Results and Discussion
Socio-demographic characteristics
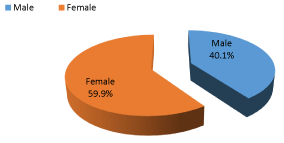
The total number of study participants was 406 type 2 DM patients. Among them; 59.9% were female and 40.1% were male. The mean age for participants was 54.6 years with a Standard Deviation (SD) 8.02, 24.6% of participants were of age group less than 50 years old, while 23.4% were of age group from 51-55 years old, 25.4% were of the age group 56-60 years, and 26.6% were of the age group more than 60 years which was the highest percentage among all group. This distribution was consistent with UNRWA field disease control report which showed that 26% of patients were more than 60 years [7], another report showing that 43.3% of all type 2 DM patients are more than 55 years old [8]. The discrepancies in percentages are attributed to the difference in the age group where UNRWA field disease control reports for all patients while the age group of this study is limited from 35-65 years only.
(Figure 1) showing that females represent 59.9% of study participants, UNRWA field disease control report showed that females percentage among DM type 2 is 51%, 61% among diabetes and hypertension and 60% among all NCD patients [7]. Gender differences arise from socio-cultural processes, such as different behaviors of women and men, exposition to specific influences of the environment, different forms of nutrition, life styles or stress, or attitudes towards treatments and prevention. Moreover, women show more dramatic changes in hormones and body due to reproductive factors during lifetime [9].
(Table 1) showing that approximately 90% of participants have formal schooling, only 9.1% have no formal schooling, 14.5% less than primary school, 14.0% primary school completed, 22.2% preparatory school completed, 19.0% secondary school completed, and 21.1% college/university completed and above.
The researcher noted that despite the majority of participants are less than 60 years old (73.4%), only 19.7% of participants were working and 80.3% were not working, moreover the mean of monthly income of participants was 959.55 NIS.
When the researcher categorized the participants according to deep poverty line: The poverty line and deep poverty line for the reference household (two adults and three children) stood at 2,290 New Israeli Shekels (NIS) and 1,832 NIS respectively [10], the result was 12.8% of participants above deep poverty line and 87.2% under deep poverty line many of them their monthly income was zero and eight participants refused to declare their monthly income. Most of participants were refugees living in poor and crowded refugee camps. This explains why the majority of them were not working and don’t have sustainable sources of income, also this is in line with current conditions in the Gaza Strip due to the siege, unemployment and low wages [11].
The socio-demographic distribution of study participants is almost identical to the official statistics of Field Disease Control UNRWA, some differences emerged as a result of the inclusion criteria of the study; where age is limited from 35-65 years old.
Diabetes mellitus related characteristics
According to the annual report of UNRWA health department 2016: The number of patients with NCDs is increasing consistently by approximately 5.0% per year [12]. This is quite obvious when researcher note that the number of DM patients is almost doubled last 10 years, where participants had DM type 2 since less than 5 years were 33.0%, and those who had DM type 2 since 5-9 years were 26.8%, while 22.2% of them from 10-14 years, and 18.0% 15 years and more.
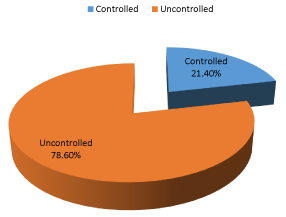
The HbA1c test is an important blood test that gives a good indication of how well your diabetes is being controlled. Depending UNRWA categorization of participants according to their HbA1c, participants were divided into two major groups; controlled DM equal or less than 7% and uncontrolled more than 7%. The results showed that 21.4% of participants were controlled while 78.6% were uncontrolled (Figure 2). This result is almost running with UNRWA reports where the percentage of controlled DM participants was 30 % in 2016, and 27% in 2017 and they are targeting 30 % in 2018 [8], the difference between the result of the study and UNRWA reports is attributed to limited age group of the study.
A cross-sectional study of 369 patients with Type 2 Diabetes Mellitus (T2DM) from four Ministry of Health centers in 2016 showed the mean of HbA1c was 8.97 and one fifth of patients had good glycemic control (HbA1c < 7%) [13], the result is consistent with our study findings.
Frequency of teeth cleaning
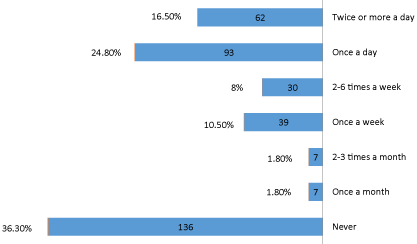
Regarding the frequency of teeth cleaning, (Figure 3) showing more than one third of participants (36.3%) never clean their teeth while only 16.5% of participants used to clean their teeth twice or more a day (minimum required) and 24.8% once a day, rest of participants varying from 2-6 times a week (8.0%), to 2-3 times a month (1.8%), or once a month (1.8%). Generally, the patients need two thorough brushings a day. Studies have shown that to a achieve gingival health, the interval between tooth cleaning session should be not less than 12 hours but not greater than 48 hours [14].
The distribution of participants according to their frequency of teeth cleaning, confirms the lack of awareness for oral health maintenance, lack of knowledge about oral complications of DM and absence of appropriate health education.
Periodontal status
People with diabetes are more likely to have periodontal disease than people without diabetes. In fact, periodontal disease has often considered a complication of diabetes. (Table 3) showing that only 16.4% of participants have no gingival bleeding and 9 participants representing 2.4% have no periodontal pockets. Moreover, the mean number of teeth showing absence of bleeding was 9.97 while the mean number of teeth showing presence of gingival bleeding was 9.91 and mean number of teeth not present for bleeding test was 9.14. In addition to that, the mean number of teeth showing absence of pocket 7.15, mean number of teeth showing pocket of 4-5 mm 7.84, mean number of teeth showing pocket of 6 mm or more 4.96 and the mean number of teeth not present for pocket measurement 9.13. These results, although frustrating, are in line with global studies, one of these studies indicated that the prevalence of periodontal disease in diabetic patients was 86.8% among fifteen hundred patients with diabetes mellitus were examined [15]. A study reported the prevalence of periodontitis to be 39% in individuals aged 19 years and older, while in patients above 35 years of age [16], while another study reported the prevalence of periodontitis to be 87% [17], but study of Bacic, et al. reported the prevalence to be 50% [18].
Relationship between periodontal status and socio-demographic characteristics
(Tables 5,6) showed no statically significant association between periodontal status (gingival bleeding and periodontal pockets) with all socio-demographic characteristics of participants except gingival bleeding was statistically significant with gender (p = 0.023), where female participants showing no bleeding (44) higher than male participants showing no bleeding (17), the main reason behind this result could be increased number of teeth not present for gingival examination or pockets measurement, moreover the early onset of chronic periodontitis among most of the participants. The results are in disagreement with most available studies.
Regarding age of participants, despite there is no statistically significant association between age and both gingival bleeding and periodontal pickets, a quick look to both mentioned tables showing that only 2.4% of participants showing no gingival bleeding and 0.5% absence of periodontal pockets among participants more than 60 years old, while participants less than 50 years old, 5.1% showing no gingival bleeding and 0.5% showing no periodontal pockets.
The increased severity of periodontal disease and bone loss with age is probably related to the length of time, where the periodontal tissues have been exposed to bacterial plaque and is considered to reflect individual’s cumulative oral history [19]. Several studies show that the prevalence and severity of periodontal disease increase with age [20-22]. A study demonstrated that the mean annual rate of bone loss among the initially 70-year-old subjects was 0.28 mm compared to 0.07 on the 25-year-old individuals [23].
Numerous studies reported higher periodontal destruction among males compared to the female population [20], this inconsistent with this study, where males participants with no gingival bleeding were 17 while female participants were 48, moreover males participants showing no periodontal pocket were only 3 but females participants 6. The reasons for these gender differences are not clear, but they are thought to be related to the ignorance of oral hygiene, which is usually observed among males [24,25]. However, the relationship observed between gender and periodontal pockets is not statistically significant but statistically significant with gingival bleeding.
(Tables 5,6) showed clearly that among all educational level the number of participants showing gingival bleeding and periodontal pockets is greater than number of participants showing no gingival bleeding and absence of periodontal pockets. However, the observed relationship between educational level and the disease is not apparent and is not considered as statistically significant. Thus, educational level may be a demographic factor, which may interfere with the effects of other factors. Periodontal disease has a reciprocal relationship with educational level. The higher the educational level, the lower the periodontal diseases [26].When education levels were compared to periodontal status in a study, the results showed a positive association between higher education levels and better periodontal status [27]. This is in accordance with another study which identified education level also a strong indicator of periodontal status [28].
(Tables 5,6) showed that unemployed participants showing no bleeding (47) more than employed participants showing no bleeding (14). And unemployed participants showing absence of periodontal pockets were 9 and no employed participants showing absence of periodontal pockets. Again the relationship observed between employment status and the disease is not apparent and is not considered as strong, statistically significant, and consistent. Thus, employment status may be a socio-economic factor, which may interfere with the effects of other factors.
Among participants under deep poverty line, 72.1% of participants showed gingival bleeding and 13.1% showed no gingival bleeding while 83.1% of them showed periodontal pockets and 2.1% showed absence of pockets and regarding participants above deep poverty line 9.4% showed gingival bleeding and 3.2% showing no gingival bleeding while 12.3% of participants above deep poverty line showed periodontal pockets and only 0.3% showed no periodontal pocket.
This result is not consistent with many studies, when the socioeconomic status was compared to the periodontal status by Rupasree Gundala and Vijiay K Chava, the study showed a positive association between higher socioeconomic groups and better periodontal status [28]. According to another study, the gingival condition is clearly related to lower SES, but the relationship between socioeconomic status and periodontitis is less direct. It can be certain that gingival health is better among individuals with higher education and with more secure income. SES is a modifiable factor and it can be examined in multivariate models for the disease [20]. The possible relationship between periodontal disease and socioeconomic status was found in several studies [26,29-31]. The researcher believes that the reason behind such gaps because socioeconomic factors are related to many other factors mainly the oral health awareness.
Relationship between periodontal status and diabetic characteristics
Contrary to expectations, there was neither a clear relationship nor statistically significant association between periodontal status (gingival bleeding and periodontal pockets) and diabetic duration, and control status of DM as showed by (tables 7,8). Contrary to supposed to be, the number of participants showing no gingival bleeding among participant with diabetic duration less than 5 years, from 5 to 9 years, from 10-14 years and 15 years and above were 11,13,26, respectively. While number of participants showing no gingival bleeding among the controlled group were 18 participants and the uncontrolled group were 43 participants. Moreover, participants showing no periodontal pockets among participants with diabetic duration less than 5 years, from 5 to 9 years, from 10-14 years and 15 years and above were 2, 4, 1 and 2 respectively. While the number of participants showing no periodontal pockets among the controlled group were 4 participants and the uncontrolled group were 5 participants.
The researcher believes that the differences in the numbers of patients between diabetic duration categories and the differences in the missed teeth (Number of teeth not present for gingival examination or pockets measurement) behind these results and moreover, improvement of the HbA1c level will prevent further progress of already chronic periodontal diseases rather than eliminating the condition. The results are in disagreement with most available studies. One of these studies had conducted by Cerda, et al. and another study conducted by Firatli, et al. they concluded that the duration of diabetes was a significant factor for the severity of periodontal disease [32,33], while another study stated that the diabetic status was significantly and strongly related to both prevalence and severity of periodontal disease [34]. The severity of periodontal disease was more prevalent in diabetics who had the disease for > 5 years, according to Faulconbridge, et al. Patients are having poor glycemic level had more severe periodontitis as compared to patients having a fair glycemic level [35], a study had also demonstrated that as age of the diabetic increases, the prevalence and severity of periodontal disease increases, poorer the control and longer the duration of diabetes, the greater will be the prevalence and severity of periodontal disease [15]. Collagen is the predominant component of gingival connective tissue accounting for approximately 60% of connective tissue volume and 90% of the organic matrix of alveolar bone. Oliver and Tervonen had stated that the properties of human collagen are changed during aging and with the metabolic abnormalities of diabetes mellitus. Thus, altered collagen metabolism in diabetics would be expected to contribute to the progression of periodontal disease [36]. Periodontitis also progresses more rapidly in poorly controlled diabetics [37], and early age of onset of the disease is seen as a risk factor for more severe diseases [38].
Relationship between dental and periodontal status, and frequency of tooth cleaning
A statistically significant association between gingival bleeding and frequency of teeth cleaning or brushing (p = 0.000), while there was no significant association between periodontal pockets and frequency of teeth-brushing, (table 9) showed that 42 of 61 participants showed no gingival bleeding used to brush their teeth on daily basis ( either once or twice or more). The relationship is inverse, the decrease in the frequency of teeth brushing, increase teeth showing bleeding, this relationship is unclear in the (table 9) because of big differences in the number of participants of each category. (Table 10) showed increased number of participants without periodontal pockets with increasing the frequency of teeth cleaning and brushing where 4 of 6 participants who have no periodontal pockets used to brush their teeth on daily basis, the relationship is strong but not a statistically significant because most of the participants showed chronic periodontitis. Plaque-induced gingivitis is the most common oral disease in dentate persons and the most common type of periodontal disease. Gingivitis is implicated as a precursor of periodontitis, so preventing gingivitis may indirectly prevent periodontitis and loss of tooth support. The principal method used to prevent gingivitis is the regular removal of plaque from all tooth surfaces via tooth brushing. The American Dental Association (ADA) recommends that brushing is performed twice a day [39].
Conclusion
Type 2 diabetes mellitus patients already had chronic periodontitis worsen by diabetes. Oral and periodontal health should be promoted as integral components of diabetes management.
- Dental Health Foundation . Links between oral health and general health. 2017. https://bit.ly/37LrOQi
- United Nations relief and works agency for Palestine refugees in the near east. Health department annual report. 2015-2016. https://bit.ly/37LAc2b
- Lalla E, Lamster IB. Assessment and management of patients with diabetes mellitus in the dental office. Dent Clin North Am. 2012; 56: 819-829. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23017553
- Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001; 6: 99-112. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11887478
- Löe, H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993; 16: 329-334. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8422804
- World Health Organization. Oral Health Surveys- Basic Methods. 5th edition. Geneva. 2013. https://bit.ly/37MIB5r
- Saleh I. Annual report on special care for non-communicable disease. Gaza: Field Disease Control. Health department. 2018 a. https://bit.ly/390ZRV8
- Saleh I. Management health information system - non-communicable disease care. Field Disease Control. Health Department. 2018 b. https://bit.ly/2UebDqX
- Kautzky Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016; 37: 278-316. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27159875
- Palestinian Central Bureau of Statistics. PCBS: The International Population Day- 11/7/2014. https://bit.ly/37OZkoR
- Obaid H, Eljedi A. Risk factors for the development of diabetic foot ulcers in Gaza Strip: A case-control study. Int J Diabetes Res. 2015; 4: 1-6. https://bit.ly/2tnCvK8
- United Nations Relief and Works Agency for Palestine Refugees in the Near East 2017. Annual Health Report 2016. Amman: UNRWA health department.
- Radwan M, Elsous A, Al Sharif H, Abu Mustafa A. Glycemic control among primary care patients with type 2 diabetes mellitus in the Gaza Strip, Palestine. Ther Adv Endocrinol Metab. 2018; 9: 3-14. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29344335/
- Marya CM. A textbook of Public Health Dentistry. Japee Brothers Medical publishers: New Delhi India. 2001.
- Rajhans NS, Kohad RM, Chaudhari VG, Mhaske NH. A clinical study of the relationship between diabetes mellitus and periodontal disease. J Indian Soc Periodontol. 2011; 15: 388-392. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22368365/
- Cianciola LJ, Park BH, Bruck E, Mosovich L, Genco RJ. Prevalence of periodontal disease in insulin-dependent mellitus (juvenile diabetes). J Am Dent Assoc. 1982; 104: 653-660. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7042797
- Rylander H, Ramberg P, Blohme G, Lindhe J. Prevalence of perio-dontal disease in young diabetics. J Clin Periodontol. 1987; 14: 38-43. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3468127
- BacicM, Plancak D, Granic M. CPITN assessment of periodontal disease in diabetic patients. J Periodontol. 1988; 59: 816-822. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3225728
- Loe H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol. 1986; 13: 431-445. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3487557
- AlJehani YA. Risk factors of periodontal disease: Review of the literature. Int J Dent. 2014; 2014: 182513. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24963294/
- Gengo RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996; 67: 1041-1049. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8910821
- Axelsson P, Lindhe J. Effect of controlled oral hygiene procedures on caries and periodontal disease in adults. Results after 6 years. J Clin Periodontol. 1981; 8: 239-348. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6947990
- Papapanou PN, Wennstrom JL. Radiographic and clinical assessments of destructive periodontal disease. J Clin Periodontol. 1989; 16: 609-612. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2794096
- Slade GD, Spencer AJ. Periodontal attachment loss among adults aged 60+ in South Australia. Community Dent Oral Epidemiol. 1995; 23: 237-242. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7587146
- Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States,1988-1994. J Periodontol. 1999; 70: 30-43. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10052768
- Beck JD, Koch GG, Rozier RG, Tudor GE. Prevalence and risk indicators for periodontal attachment loss in a population of old community-dwelling blacks and whites. J Periodontol. 1990; 61: 521-528. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2391631
- Gundala R, Chava VK. Effect of lifestyle, education and socioeconomic status on periodontal health. Contemp Clin Dent. 2010; 1: 23-26. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22114373
- Richard P GT, Chava V. Influence of lifestyle, gender and socioeconomic status as determinants of dental health behavior, periodontal status awareness. JPFA. 2000; 14: 21-5.
- Gilbert GH. Racial and socioeconomic disparities in health from population‐based research to practice‐based research: the example of oral health. J Dent Educ. 2005; 69: 1003-1014. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16141086
- Susin C, Oppermann RV, Haugejorden O, Albandar JM. Tooth loss and associated risk indicators in an adult urban population from south Brazil. Acta Odontol Scand. 2005; 63: 85-93. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16134547
- Locker D, Leake JL. Periodontal attachment loss in independently living older adults in Ontario, Canada. J Public Health Dent. 1993; 53: 6-11. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8474051
- Cerda J, Vazquez de la Torre C, Malacara JM, Nava LE. Periodontal disease in non-insulin dependent diabetes mellitus (NIDDM): The effect of age and time since diagnosis. J Periodontol. 1994; 65: 991-995. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7853135
- Firatli E, Yilmaz O, Onan U. The relationship between clinical attachment loss and the duration of insulin-dependent diabetes mellitus (IDDM) in children and adolescents. J Clin Periodontol. 1996; 23: 362-366. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8739168
- Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin dependent diabetes mellitus. J Periodontol. 1991; 62: 123-131. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2027060
- Faulconbridge AR, Bradshaw WC, Jenkins PA, Baum JD. The dental status of a group of diabetic children. Br Dent J. 1981; 151: 253-255. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6945112
- Oliver RC, Tervonen T. Diabetes a risk factor for periodontitis in adults? J Periodontol. 1994; 65: 530-538. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8046569
- Seppala B, Seppala M, Ainamo J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol. 1993; 20: 161-165. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8450080
- Thorstensson H, Hugoson A. Periodontal disease experience in adult long-duration insulin-dependent diabetics. J Clin Periodontol. 1993; 20: 352-358. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8501275
- Pinto TM, de Freitas GC, Dutra DA, Kantorski KZ, Moreira CH. Frequency of mechanical removal of plaque as it relates to gingival inflammation: A randomized clinical trial. J Clin Periodontol. 2013; 40: 948-954. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23909568

Sign up for Article Alerts