Association between Unexplained Recurrent Miscarriage and Insulin Resistance
Tarek Tamara1, Abdellatif Elkholy1, Nashwa Elsaed1, Mohamed Abdellatif1*, Nermine Essam1 and Mohamed Selem2
1Department of Obstetrics and Gynecology, Ain Shams University, Cairo, Egypt
2Research Fellow, Ain Shams University Maternity Hospital, Cairo, Egypt
*Address for Correspondence: Mohamed Abdellatif, Lecture of Obstetrics and Gynaecology, Ain Shams University Maternity Hospital, Abbasiya, Cairo, Egypt, Tel: 010-029-231-46; E-mail: mlatif_obi@yahoo.com
Submitted: 15 December 2018; Approved: 01 January 2018; Published: 02 January 2018
Citation this article: Tamara T, Elkholy A, Elsaed N, Abdellatif M, Essam N, et al. Association between Unexplained Recurrent Miscarriage and Insulin Resistance. Int J Reprod Med Gynecol. 2018;4(1): 001-005.
Copyright: © 2018 Tamara T, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Recurrent Pregnancy Loss; Insulin Resistance
Download Fulltext PDF
Methods: The current case-control study was conducted at Ain Shams University Maternity Hospital. The study included two groups of women: group A, including pregnant women with a history of unexplained recurrent miscarriage; and group B, including control pregnant women with no prior miscarriage. Women included in either group were at their first trimester of pregnancy (6-13 weeks of gestation). For all included women, 3-hour oral glucose test was performed. Serum insulin levels were measured at the same times. Markers of insulin resistance, including HOMA-IR, HOMA-B, AUCG and AUCI were calculated.
Results: There were no significant differences between women of both groups regarding HOMA-IR and HOMA-B. The mean values of AUCG and AUCI were, however, significantly higher in women of group A when compared to group B.
Introduction
Recurrent early miscarriage was traditionally described as three or more clinically diagnosed consecutive pregnancy losses prior to the 20th gestational week [1]. Since similar etiologic factors have been identified between two or three pregnancy losses has been detected in recent years, investigation of the couple for the etiology is currently sought for, after two consecutive pregnancy losses [2]. The incidence of two or three subsequent miscarriages is 2% and 0.3-1%, respectively [3]. The list of etiologies for recurrent miscarriage includes a number of chromosomal, anatomical, endocrine, infectious, immunologic factors. Nevertheless, the underlying cause is not infrequently identifiable in most of cases [4]. Glycemic control and insulin sensitivity are of the most important factors in reproductive pathophysiology. Impaired glucose tolerance, diabetes mellitus and Insulin Resistance (IR) have been long known to be lined to adverse reproductive outcomes, including infertility, miscarriages, and adverse pregnancy outcomes [5]. Several studies have shown a biochemical and clinical association between miscarriage and both poor glycemic control and IR [6]. The aim of the current study is to evaluate association between recurrent early miscarriages and IR in early pregnant women.
Methods
The current case-control study was conducted at Ain Shams University Maternity Hospital during the period between December 2013 and June 2014. The study protocol was in agreement to the Helsinki declaration of Ethical Medical Research [last updated in South Korea, 2013] and had been approved by the Ethical Research Committee of Obstetrics and Gynecology Department at Ain Shams University. All participating women had to sign informed written consent after thorough explanation of the purpose and procedure of the study. Any participating woman had the right to withdraw from the study without being adversely affected regarding the medical service she should have received.
The study included two groups of women: group A, including pregnant women with a history of unexplained recurrent miscarriage; and group B, including control pregnant women with no prior miscarriage. Women included in either group were at their first trimester of pregnancy (6-13 weeks of gestation). Women with history of gestational or pregestational diabetes mellitus, women on medications that might affect glucose metabolism (e.g. metformin), those who were obese (Body Mass Index (BMI) ≥ 30 kg/m2) or had Polycystic Ovarian Syndrome (PCOS) were not included in the study. Unexplained recurrent miscarriage was defined as two or more failed clinical pregnancies (as documented by ultrasonography or histopathological examination) with no detectable underlying (endocrine, anatomical, chromosomal or immune) cause [7].
All included women were asked to go on a normal diet for 3 days prior to Oral Glucose Tolerance Testing (OGTT). A fast for 8–10 h was required prior to sampling. A venous blood sample was drawn on the following morning from each woman to determine the concentrations of Fasting Glucose (FG) and Fasting Insulin (FI). Women were then asked to drink a mixture of 75 g of pure glucose in 250 ml of water; venous blood samples were drawn after 1, 2, and 3 hours to determine the concentrations of glucose and insulin [8]. Glucose concentration was determined using the hexokinase endpoint method; while insulin concentration was determined using the immunoluminescence method. The Immulite2000 Immunoassay Analyzer® [Siemens Healthineers®, Erlangen, Germany] was used along with the necessary reagents. The homeostasis model assessment of insulin resistance index (HOMA-IR) for each subject was calculated as follows: [FI (U/ ml) × FG mmol/l)]/22.5. The larger the HOMA-IR, the more severe the degree of Insulin Resistance (IR). HOMA-B, which represents the endocrine function of insulin, is calculated as 20× FI / (FG-3.5). The Area under the Curve of Glucose (AUCG) is equal to half of the FG plus 1-hour glucose, 2-hour glucose, and half of the 3-hour glucose. The Area under the Curve of Insulin (AUCI) is also computed in this manner for insulin. The ratio AUCI/AUCG represents the rate of AUCI to AUCG; and the higher the rate, the more severe the degree of IR [9].
Sample size justification
Sample size was calculated, setting the type-1 error (α) at 0.05 and the power (1-β) at 0.80. Data from a previous study [10], showed that mean values of HOMA-IR were 4.2 ± 6.3 and 1.6 ± 1.6 in the recurrent miscarriage and control groups, respectively. Calculation according to these values to find such a difference produced a minimal sample size of 37 cases in each group. Assuming a drop-out ratio of 10%, the sample size will be 40 women in each group.
Statistical methods
Statistical analysis was performed using SPSS for Windows version 20.0. Difference between two groups was analyzed using independent student’s t-test as well as the mean difference and its 95% confidence interval (95% CI). Receiver Operator Characteristics (ROC) curves were constructed for estimating the association between unexplained recurrent miscarriage and measured markers of IR. Significance of association was presented in terms of Area under the Curve (AUC) and its 95% CI. Validity of the association was presented in terms of sensitivity and specificity and their 95% CIs. Significance level was set at 0.05.
Results
Forty women were included as group A [RPL group], along with 40 women as group B [control group]. The mean age of included women was 30.4 ± 4.3 years (range: 22-39 years). The mean gestational age at recruitment was 7.3 ± 0.6 weeks (range: 6-10 weeks). There were no significant differences between women of both groups regarding the age, BMI and gestational age (Table 1).
The mean levels of fasting blood glucose and fasting serum insulin were comparable in both groups. The mean values of 1-hour, 2-hour and 3-hour postprandial levels of blood glucose and serum insulin were, however, significantly higher in women of group A when compared to group B (Table 2).
There were no significant differences between women of both groups regarding HOMA-IR and HOMA-B. The mean values of AUCG and AUCI were, however, significantly higher in women of group A. The AUCI/AUCG ratio was slightly higher in women of group A; this latter difference was not statistically significant.
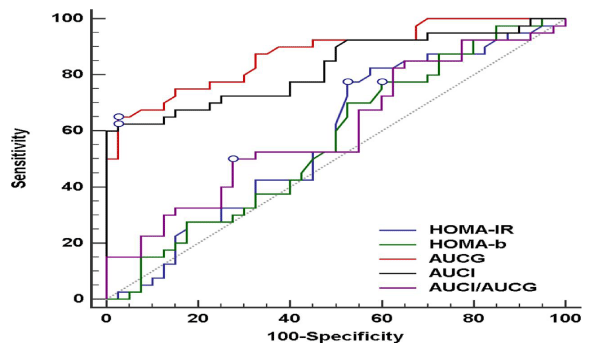
ROC curves for estimating the association between unexplained recurrent miscarriage and measured markers of IR showed that AUCG and AUCI were the only markers significantly associated with unexplained recurrent miscarriage (Table 3, Figure 1). The difference between area under the curves for both AUCI and AUCG, and other markers of IR was statistically significant. The difference between the two markers (AUCI and AUCG) themselves was, however, not significant (Table 4).
Discussion
The current study showed significantly higher postprandial blood levels of glucose and insulin, as well as, significantly higher AUCG and AUCI among women with recurrent miscarriage when compared to their age- and BMI-matched controls. The fasting levels of blood glucose and insulin, along with HOMA-IR and HOMA-B were, however, comparable in both groups of women. This can be explained by the observation that IR of both the liver and the peripheral tissues (e.g. muscle and fat) tend to exhibit a ‘separation’ phenomenon. In the liver, the IR phenomenon is mainly manifested as elevated fasting blood glucose, while in peripheral tissues IR manifests as elevated post-prandial blood glucose after glucose loading. HOMA-IR estimates an individual’s overall insulin sensitivity via the insulin sensitivity of the liver, which mainly reflects the degree of IR in the fasting state [11].
Women with recurrent miscarriage included in the current study showed elevated postprandial blood glucose levels, indicating that IR of the peripheral tissues is more pronounced than that of the liver. Included women also showed a deferred peak of blood glucose and insulin. As such, an evaluation in IR using HOMA-IR may actually underestimate the degree of IR of an individual. Meanwhile, there is no universal consensus about the most accurate method of measuring IR. IR is generally difficult to define and measure in epidemiological studies. The glucose clamp technique, which is considered the gold standard direct in vivo test of insulin sensitivity, is laborious and expensive. All practical tests assessing IR (including HOMA-IR, glucose/insulin ratio, and other tests) are indirect measures [12-13].
The association between IR and ‘otherwise’ unexplained recurrent miscarriage is well known and well observed in several previous studies. Celik, et al. compared 64 pregnant women with recurrent prior pregnancy loss to 64 pregnant controls, and found significantly higher mean values of fasting blood glucose, fasting serum insulin, and HOMA-IR in the recurrent pregnancy loss group [10].
In a larger study conducted on 621 pregnant women (of them 161 women had a prior history of recurrent spontaneous miscarriage), Hong, et al. found a significantly higher fasting plasma glucose, fasting plasma insulin, and HOMA-IR among women with recurrent miscarriage when compared to their controls. The authors of this study also showed that serum hCG and serum progesterone concentrations were negatively correlated to HOMA-IR and positively correlated to fasting glucose-to-insulin ratio [14].
In a large systematic review and meta-analysis conducted by Li, et al. 7 studies between 1996 and 2012 were included, with a total of 467 women with recurrent miscarriage and 413 control women. The authors found no significant difference between both groups regarding the fasting glucose, and a significantly higher fasting insulin level as well as a significantly higher proportion of women with HOMA-IR > 4.5 and glucose-to-insulin ratio < 4.5 among women with recurrent miscarriage [6].
In addition to this biochemical association between recurrent miscarriage and IR, a clinical evidence of association has been also shown. Metformin (a long-known treatment of IR) was shown to significantly improve pregnancy outcome in women with previous miscarriage in a number of clinical trials [15-17].
The mechanism underlying the association between IR, or effect of metformin, and the risk for miscarriage remains unclear. Two possible mechanisms have been postulated by studies involving patients with PCOS [18]. Jakubowicz, et al. found that hyperinsulinemia led to reduced concentrations of Insulin-Like Growth Factor Binding Protein-1 (IGFBP-1) and glycodelin in early stage of pregnancy, thereby increasing the likelihood for miscarriage. Glycodelin may play a role in inhibiting the endometrial immune response of the embryo and IGFBP-1 appears to facilitate adhesion processes at the fetal-maternal interface [19]. Insulin, however, can negatively regulate the concentrations of glycodelin and IGFBP-1, increasing risk for miscarriage [20]. Hyperinsulinemia may increase the level of plasminogen activator inhibitor-1 and induce villous thrombosis, thereby reducing the blood supply to the placenta and leading to trophoblastic hypoplasia, resulting in miscarriage [21].
- Mehmet O, Mehmet S, Hatice S, Muhammet S, Ali O, Mehmet F, et al. Evaluation of etiologic causes of recurrent pregnancy loss. J Turk Soc Obstet Gynecol. 2013; 10: 67- 71. https://goo.gl/yYxPfs
- Jaslow CR, Carney JL and Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent miscarriage. Fertil Steril. 2010; 93: 1234-1243. https://goo.gl/zFoWrV
- Fox-Lee Laura and Schust DJ. Recurrent pregnancy loss: In Berek JS ed, Berek & Novak Gynecology, 14th ed. Philadelphia : Lippincott Williams &Wilkins, 2007; 1277- 322.
- Maryam K, Bouzari Z, Basirat Z, Kashifard M and Zadeh MZ. The comparison of insulin resistance frequency in patients with recurrent early pregnancy loss to normal individuals. BMC. 2012; 5: 133. https://goo.gl/in5RHP
- Tian L, Shen H, Lu Q, Norman RJ and Wang J. Insulin resistance increases the risk of spontaneous abortion after assisted reproduction technology treatment. Clin Endocrinol Metab. 2007; 92: 1430-1433. https://goo.gl/bwLejG
- Li ZL, Xiang HF, Cheng LH, Cao YX,Wei ZL, Liu C and Hu JJ. Association between recurrent miscarriages and insulin resistance: a meta-analysis. Reprod Med Center. 2012; 47: 915-919. https://goo.gl/dsT9sr
- American Society for Reproductive Medicine "ASRM". Definitions of infertility and recurrent miscarriage. Fertil Steril; 2008; 90: 60. https://goo.gl/HwgPav
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007; 30: 42-47 . https://goo.gl/9xKM95
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF and Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412- 419. https://goo.gl/Xs7xAY
- Celik N, Evsen MS, Sak ME, Soydinc E and Gul T. Evaluation of the relationship between insulin resistance and recurrent pregnancy loss. Ginekol Pol. 2011; 82: 272-275. https://goo.gl/acZkZd
- Matsuda M and DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999; 22: 1462-1470. https://goo.gl/VE6AGa
- DeFronzo RA, Tobin JD and Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979; 237: 214-223. https://goo.gl/Mq9LW2
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000; 23: 57-63. https://goo.gl/CtZrcY
- Hong Y, Xie QX, Chen CY, Yang C, Li YZ and Chen DM. Insulin resistance in first-trimester pregnant women with pre-pregnant glucose tolerance and history of recurrent spontaneous abortion. Biol Regul Homeost Agents. 2013; 27: 225-231. https://goo.gl/BSDGiB
- Jacubowicz D, Iuorno M, Jacubowicz S, Roberts K and Nestler J. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. Clin Endocrinol Metab. 2002; 87: 524-529. https://goo.gl/aNjjrH
- Khattab S, Mohsen IA, Foutouh IA, Ramadan A, Moaz M and Al Inany H. Metformin reduces abortion in pregnant women with polycystic ovary syndrome. Gynecol Endocrinol. 2006; 22: 680-684. https://goo.gl/6fWhn4
- Zolghadri J, Tavana Z, Kazerooni T, Soveid M and Taghieh M. Relationship between abnormal glucose tolerance test and history of previous recurrent miscarriages, and beneficial effect of metformin in these patients: a prospective clinical study. Fertil Steril. 2008; 90: 727-730. https://goo.gl/p9Fjng
- Porter TF and Scott JR. Evidence-based care of recurrent miscarriage. Clin Obstet Gynecol. 2005; 19: 85-101. https://goo.gl/4TXeqe
- Jakubowicz D J, Essah PA, Seppala M, Jakubowicz S, Baillargeon JP, Koistinen R, et al. Reduced serum glycodelin and insulin-like growth factor-binding protein-1 in women with polycystic ovary syndrome during first trimester of pregnancy. Clinic Endocrinol Metabol. 2004; 89: 833-839. https://goo.gl/4eNujU
- Stephenson M and Kutteh W. Evaluation and management of recurrent early pregnancy loss. Clin Obstet Gynecol. 2007; 50: 132-145. https://goo.gl/aFRhBe
- Glueck CJ, Sieve L and Zhu B. Plasminogen activator inhibitor activity, 4G5G polymorphism of the plasminogen activator inhibitor-1 gene and first-trimester miscarriage in women with polycystic ovary syndrome. Metabolism. 2006; 55: 345-352. https://goo.gl/D4nN9a

Sign up for Article Alerts