Effectiveness of Bovine Lactoferrin versus Ferrous Fumarate in the Management of Iron Deficiency Anemia in Pregnancy: Randomized Clinical Trial
Hossam M Hemeda, Ayman Abd El Kader Mohamed, Bassem Aly Islam, Amany Hasan A Eweis*
Obstetrics and Gynecology Department, Ain Shams University
*Address for Correspondence: Amany Hasan A Eweis, Ain Shams Maternity Hospital, Faculty of Medicine, Ain Shams University, Ramses Street, Abbassia, Cairo, Egypt, Tel: +009-743-399-8645; E-mail: profobgyn81@hotmail.com
Submitted: 29 January 2018; Approved: 12 February 2018; Published: 13 February 2018
Citation this article: Hemeda HM, El Kader Mohamed AA, Islam BA, A Eweis AH. Effectiveness of Bovine Lactoferrin versus Ferrous Fumarate in the Management of Iron Deficiency Anemia in Pregnancy: Randomized Clinical Trial. Int J Reprod Med Gynecol. 2018;4(1): 006-011.
Copyright: © 2018 A Eweis AH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Recurrent Pregnancy Loss; Insulin Resistance
Download Fulltext PDF
Objective: The aim of this research study is to compare ferrous fumarate and bovine lactoferrin as regard treatment of anemia in a pregnant patient with iron deficiency anemia.
Study design: A prospective open label randomized clinical trial which was conducted at Ain Shams University Maternity Hospital outpatient clinic in the period from 15 February 2016 to 15 August 2016. The study included 146 pregnant women suffering from iron deficiency anemia and divided in two groups.
Results: Comparison between research groups (ferrous fumarate vs lactoferrin) showed that both Hb after 1 month, Hb after 2 months, serum ferritin after 1month and serum ferritin after 2 months were statistically significantly higher among group B in comparison to group A (p < 0.05).
Conclusion: Comparison between research groups (ferrous fumarate vs lactoferrin) showed that both Hb after 1 month, Hb after 2 months, serum ferritin after 1month and serum ferritin after 2 months were statistically significantly higher among group B in comparison to group A (p < 0.05).
Introduction
The World Health Organization (WHO) define anemia in gestation as a Hemoglobin (Hb) level of < 11 g/dl. Iron Deficiency Anemia (IDA) is the dominant and most widespread type of anemia in gestation [1]. Management of iron deficiency anemia with ingestion of iron medications will allow the hemoglobin levels to increase in a slow pattern, around after 1-2 weeks of therapy, will eventually increase roughly 2 g/dL [2]. Lactoferrin represents an attractive and promising alternative to oral ferrous sulfate administration. In pregnant women, oral administration of bovine lactoferrin, 30% iron saturated, significantly improved hematological parameters, including number of red blood cells, hemoglobin, total serum iron, serum ferritin concentrations compared to those observed in pregnant women treated with ferrous sulfate [3]. Lactoferrin (formerly known as lactotransferrin) is a glycoprotein, and a member of a transferrin family, thus belonging to those proteins capable of binding and transferring iron [4]. Lactoferrin is a naturally existing iron-binding multifunctional protein; it is present at high concentrations in human milk and in the milk of other mammals. It is also present in other body fluids such as tears, saliva, bile, pancreatic juice, genital and nasal secretions as well as in circulating neutrophils. Therefore, oral administration of bovine lactoferrin as an iron-supplying molecule is an appealing therapeutic strategy [5]. The molecular composition of lactoferrin is composed of a single polypeptide chain which is folded into two lobes (N and C lobes). Both lobes are connected by a α-helical residue, making Lactoferrin a structurally flexible molecule in character. Actoferrin could sustain binding of iron in variable pH array [6]. Specific receptors mediate and influence the physiological action, by directly altering the cell membrane, by competitive mode of absorption for the iron ions or via its enzymatic action. Its molecular and physiological behavior and features are augmented by its capability of sustaining the iron binding feature at low pH [7]. Data and results of research studies display and reveal that bovine lactoferrin has considerably less gastrointestinal side effects than ferrous sulfate [8]. The rise in RBC cellular mass is associated by a rise in maternal physiological requirements and demand of iron by an additional 500 mg during gestation and additional 300 mg transferred to the developing fetus and 200 mg that are necessary for physiologically normal daily iron loss, making total iron demands in total gestational period is about 1 g [9].
Methods
A prospective non-blinded randomized clinical trial which was performed at Ain Shams University Maternity Hospital outpatient clinic from the period from 15 February 2016 to 15 August 2016. The research included 146 pregnant women diagnosed with iron deficiency anemia and grouped in two research study categories. Inclusion criteria were the following: age 20-35 years, Gestational age > 14 weeks. Hb 7-11 g/dl (mild and moderate anemia) [10]. Serum ferritin < 20 mcg/L. Exclusion criteria anemia due to any other etiologies such as chronic blood loss, hemolytic anemia and thalassemia, familial history of thalassemia or sickle cell anemia, cases with Hb level less than 7%. Clinical and/or laboratory proof of hepatic, renal, hematologic, cardiovascular abnormalities, history of acid-peptic disorders, esophagitis, hiatal hernia, or malabsorption syndrome in which a history of involuntary weight loss, chronic diarrhea for more than 4 weeks and steatorrhea may be present [11]. Clinical history of any other medical disorder or hypersensitivity to iron preparations. All cases recruited for the research were subjected to full medical history ,physical examination, Complete blood picture ,serum ferritin. Abdomino-pelvic ultrasound to evaluate the fetal viability, the gestational age and to exclude multifetal gestation, and fetal congenital malformations. Investigations performed for clinical follow up of cases were implemented at week 4 and week 8. Cases recruited in the research study are categorized into two groups, each group used one of the two drugs. According to computer generated randomization list each case have joined one of the two study research groups at the start of the research. Group 1, involved 73 pregnant women ingested bovine lactoferrin (Mamy vital sachets, Dulex lab co., Egypt. A multivitamins that contain 200 mg lactoferrin, 30% iron saturated) for two consecutive months. Group 2, involved 73 pregnant women ingested ferrous fumarate capsules (Haema-Caps, AMOUN pharmaceutical co., Egypt. A multivitamin containing 350 mg iron equivalent to 115 mg elemental iron.) For two consecutive months. Follow up of study subjects at weeks and 8 weeks after the initial evaluation. At each visit the study subjects involved were questioned about the appearance of any complications in relation to the mode of management e.g. epigastric pain, constipation, black stool, nausea, vomiting or gastric distress to evaluate the tolerance of the drugs of interest. CBC and serum ferritin were performed at each visit in addition to routine antenatal work up.
Statistical Methods
The required sample size has been calculated using the IBM© SamplePower© Software (IBM© Corp., Armonk, NY, USA). Statistical presentation and analysis of the present study was conducted, using the mean, standard Deviation, Student t-test [Unpaired], paired t-test, and chi-square tests by SPSS V. 20. Significance level: Non Significant > 0.05, Significant < 0.05*, High Significant < 0.001*.
Statistical analysis
Data was tabled and statistically analyzed using SPSS vs. 15. Parametric data was expressed as minimum, maximum, mean and SD. Comparison between two groups was done using unpaired t-test (t). Comparison between serial measurements was done using One-Way ANOVA test (F), and if significant post-hoc test was done. Non parametric data was expressed as number and percentage. Comparison between two groups was done using Chi-square (X2). Two tailed p value > 0.05 was considered insignificant and ≤ 0.05 was considered significant.
Results
Table 1 shows that group A patients’ age ranged from 20 to 35 years old with a mean of 27.8 ± 4.04. Gestational age ranged from 15 to 31 weeks with a mean of 23.47 ± 4.33. Among the studied patients 24 (32.9) were primi-gravida and 49 (67.1) were multi-gravida. Group B patients’ age ranged from 20 to 35 years old with a mean of 28.6 ± 3.96. Gestational age ranged from 15 to 30 weeks with a mean of 24.65 ± 4.16. Among the studied patients 20 (27.4) were primi-gravida and 53 (72.6) were multi-gravida. There were insignificant differences between both groups as regards age, gestational age and parity (p > 0.05).
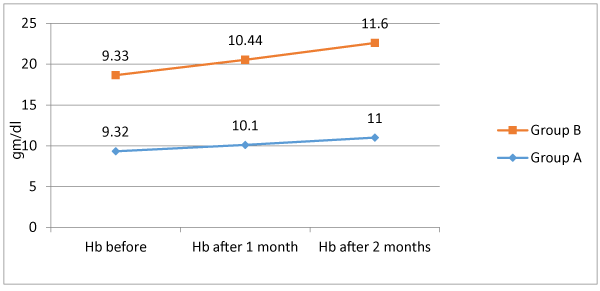
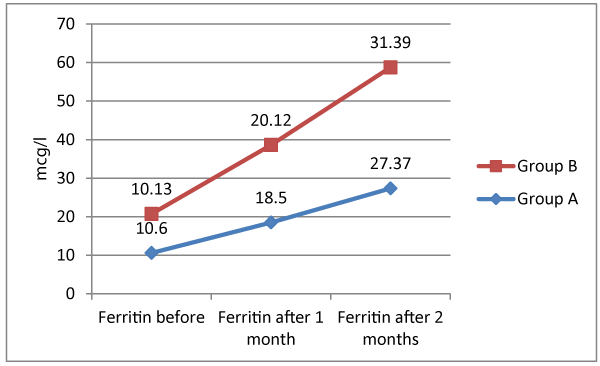
Table 2 shows group A; level of Hb before ranged from 8.4 to 10 gm/dl with a mean of 9.32 ± 0.47, Hb after 1 month ranged from 9 to 11.2 gm/dl with a mean of 10.1±0.49, and Hb after 2 months ranged from 10 to 12.2 with a mean of 11 ± 0.49. There were significant differences between serial Hb measurement; being significantly increased (p < 0.05) Serum level of ferritin before ranged from 9 to 11.9 with a mean of 10.6 ± 0.76, serum ferritin after 1 month ranged from 15.4 to 21.3 with a mean of 18.5 ± 1.43, and serum ferritin after 2 months ranged from 21.9 to 32.7 with mean of 27.37 ± 1.96. There were significant differences between serial serum ferritin; being significantly increased (p < 0.05). As regards group B level of Hb before ranged from 8.3 to 10 gm/dl with a mean of 9.33 ± 0.47, Hb after 1 month ranged from 9.5 to 11.2 gm/dl with a mean of 10.44 ± 0.46, and Hb after 2 months ranged from 10.8 to 12.4 with a mean of 11.6 ± 0.43. There were significant differences between serial Hb measurement; being significantly increased (p < 0.05) Serum level of ferritin before ranged from 9 to 11.9 with a mean of 10.13 ± 0.95, serum ferritin after 1 month ranged from 15.5 to 29.4 with a mean of 20.12 ± 2.37, and serum ferritin after 2 months ranged from 27.4 to 36.6 with mean of 31.39 ± 2.17. There were significant differences between serial serum ferritin; being significantly increased (p < 0.05). There were insignificant difference between group A and B regarding Hb before (p > 0.05). Serum ferritin before of group A was significantly higher in comparison to that of group B (p < 0.05). Comparison between both groups showed that both Hb after 1 month, Hb after 2 months, serum ferritin after 1month and serum ferritin after 2 months were significantly higher among group B in comparison to group A (p < 0.05).
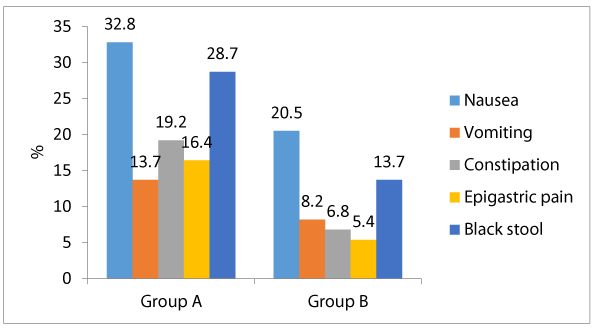
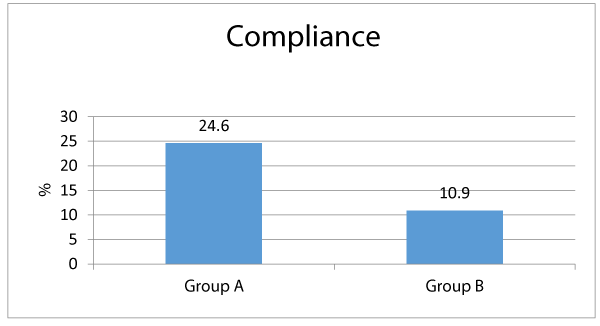
Table 3 shows that among group A, the most frequent side effects of ferrous were nausea (32.8%), black stool (28.7%), constipation (19.2%), epigastric pain (16.4%) and vomiting (13.7%). Meanwhile among group B; nausea (20.5%) then black stool 13.7%), vomiting (8.2%), constipation (6.8%) and epigastric pain (5.4%). There were significant differences between both groups as regards the frequency of each of constipation, epigastric pain and black stool; being significantly associated among group A who received ferrous (p < 0.05) Meanwhile there were insignificant differences between both groups as regards the frequency of nausea and vomiting (p > 0.05) Regards the patients’ compliance and desire to stop drug intake; 24.6% of group A patients desired to stop the drug intake, meanwhile only 10.9% of group B patients had desire to stop the drug intake, and that difference was statistically significant (p < 0.05).
Discussion
Anemia is one of the major and significant clinical diseases that influence obstetric management pathways and clinical scenarios (Breymann et al. 2010). The main goal of this research study was to compare and contrast between ferrous fumarate and bovine lactoferrin as regard management of anemia in pregnant cases diagnosed with iron deficiency anemia. This randomized controlled clinical trial was performed at Ain Shams University Maternity Hospital outpatient clinic during the time period from 15 February 2016 to 15 August 2016. A whole number of 146 cases were involved in the research study categorized into two groups; Group A involved 73 patients who received ferrous fumarate capsules (Haema Caps) for two months, and Group B included 73 patients ingested bovine Lactoferrin (Mamy vital sachets) for two months.
As regards the demographic data, there were insignificant differences statistically between group A and B regarding age (27.8 ± 4.04 vs. 28.6 ± 3.96 respectively), gestational (23.47 ± 4.33 vs. 24.65 ± 4.16, respectively) and parity (24 primi-gravida & 49 multi-gravida vs. 20 primi-gravida and 53 multi-gravida respectively). Our research data findings are in harmony with a group of researchers [12]. WHO performed their research study on two categories, category A received bovine lactoferin (n = 49) and category B received ferrous sulfate (n = 48). The two research categories did not show statistically significant difference as regard to age (27.3 ± 2.7 vs. 26.0 ± 5.4 years, mean ± SD) and parity (2.0 ± 1.0 vs. 1.5 ± 1.0). Amongst group A, serial Hb levels revealed significant physiological improvement after 1 month and 2 months in relationship with Hb before inducing treatment, and in relationship with each other (10.1 ± 0.49, 11 ± 0.49 vs. 10.1 ± 0.49). the findings and indices obtained from our research study were similar with Paesano et al. (2009) displaying that Hb level revealed statistically significant clinical improvement in values obtained after 30 days in contrast and comparison to that before inducing treatment (11.9 vs. 10.9, p < 0.05) among the group of cases who ingested ferrous sulphate. Also our indices are in harmony with results obtained from a research group previously conducted a similar study [13]. WHO concluded that mean Hb significantly raised after 30 days of therapy induction of ferrous sulfate in contrast and comparison to its serum level before start of therapy (11.5 ± 0.6 vs. 10.1 ± 0.5, p < 0.05). Amongst group A, sequential serum ferritin levels displayed and revealed statistically significant changes denoting improvement after 1 month and 2 months in contrast with serum ferritin measured before starting management mode (18.5 ± 1.43, 27.37 ± 1.96 vs. 10.6 ± 0.76). Our research study results obtained were in contradiction to findings and results obtained by the research group conducting similar methodology [14]. displaying that serum ferritin level revealed statistically significant reduction after 30 days in comparison to the serum levels before starting treatment (3 vs. 5, p < 0.05) among the group of cases who ingested ferrous sulphate. On the other hand our data obtained is in harmony and agreement with results and findings obtained by another group of researchers [15], uncovering the fact that that mean serum ferritin statistically significantly raised after 30 days of ingestion of ferrous sulfate in comparison to its serum level before starting treatment (12.6 vs. 10.7, p < 0.05).
Among group B, serial Hb levels showed significant improvement after 1 month and 2 months in comparison with Hb before treatment, and in comparison with each other (10.44 ± 0.46, 11.6 ± 0.43 vs. 9.33 ± 0.47). Our results were concordant with another previously performed research [16], found that Hb level showed significant improvement after 30 days in comparison to that before treatment (11.5 vs. 12.6, p < 0.05) among the group of patients who received bovine lactoferrin. In addition another group of researchers [17], found that mean Hb significantly increased after 30 days of administration of bovine lactoferrin in comparison to its level before treatment (11.2 ± 0.5 vs. 10.1 ± 0.5, p < 0.05).
Among group B, serial ferritin levels showed significant improvement after 1 month and 2 months in comparison with ferritin before treatment (20.12 ± 2.37, 31.39 ± 2.17 vs. 10.13 ± 0.95). Our results were concordant with another research group study conducting similar methodology [18], found that serum ferritin level showed significant increase after 30 days in comparison to that before treatment (27 vs. 12, p < 0.05) among the group of patients who received ferrous sulphate. Also the same research group [18], found that mean serum ferritin significantly increased after 30 days of administration of bovine lactoferrin in comparison to its level before treatment (12.4 vs. 10.5, p < 0.05).
There was insignificant difference between group A and B as regards Hb level before treatment (9.32 ± 0.47 vs. 9.33 ± 0.47, p < 0.05). Meanwhile Hb level after 1 month and 2 months were significantly higher among group B in comparison to group A (10.44 ± 0.46 vs. 10.1 ± 0.49 and 11.6 ± 0.43 vs. 11 ± 0.49, respectively). Our results agree with a previously conducted research [18], found that delta mean value of Hb among patients receiving Bovine lactoferrin was significantly higher than that of patients receiving Ferrous sulfate (1.5 vs. 0.9).
There was significant difference between group A and B as regards serum ferritin; being higher among group A (10.6 ± 0.76 vs. 10.13 ± 0.95, p < 0.05). Meanwhile serum ferritin after 1 month and 2 months were significantly higher among group B in comparison to group A (20.12 ± 2.37 vs. 18.5 ± 1.43 and 31.39 ± 2.17 vs. 27.37 ± 1.96, respectively, p < 0.05). Our results are in harmony with a previously conducted research with similar methodology and approach [17], displaying that delta mean value of ferritin among patients receiving Bovine lactoferrin was significantly higher than that of patients receiving Ferrus sulfate (54.2 vs. 8).
Concerning the clinically recorded side effects, constipation, epigastric pain were significantly linked with ferrous (group A) by comparison to group B (lactoferrin) (19.2% vs. 6.8%, 16.4% vs. 5.4%, and 28.7% vs. 13.7% respectively, p < 0.05). Additionally, there were statistically insignificant differences between both study research categories concerning occurrence of nausea and vomiting (32.8% vs. 20.5% and 13.7% vs. 8.2%, correspondingly, p > 0.05). Our research study findings partially agree with a similarly performed research study [17] ,revealing that among 98 patients who received Ferrous sulfate; 95% had stomach pain, cramps, and constipation and 2% had diarrhea, meanwhile among 107 patients received bovine lactoferrin none had side effects. Concerning the cases compliance to mode of therapy, more cases among group A demanded to discontinue the drug in contrast and comparison to group B (24.6% vs. 10.9%, p < 0.05). And this can be logically concluded and explained due to less side effects linked with ingestion of bovine Lactoferrin by comparing the gastro intestinal side effects with Ferrous sulfate. Many research groups conducted many clinical research trials on bovine Lf (bLf) oral administration, they displayed and concluded in a clear manner that the consumption of this natural compound is medically safe and efficient in treating pregnant women having iron deficiency disorders including iron deficiency anemia [15,16,17,18].
Conclusion
Both ferrous fumarate and bovine Lactoferrin are efficient in management of iron deficiency anemia in gestation. In comparison to ferrous fumarate, bovine Lactoferrin is more valuable in management of iron deficiency anemia in gestation. Management of iron deficiency anemia in gestation with bovine Lactoferrin displayed an excellent safety profile and fair case compliance. It is recommended to conduct future research involving and considering ethnic and racial differences and to monitor variability of response to therapy according to genetic backgrounds that differ between ethnic groups in addition different malabsorption disorders with pregnancy should be taken into consideration to analyse effectiveness of iron oral therapy different molecular structures including amino acid chelated iron with larger sample size and more different arms of study for future implementation in clinical guidelines and to improve evidence based practice.
- De Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia. 1993-2005: https://goo.gl/sG4iRG
- Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization. 2011. https://goo.gl/kNXZBZ
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000; 72: 257-264. https://goo.gl/t32csJ
- Miller EM. The reproductive ecology of iron in women. Am J Phys Anthropol. 2016; 159: 172-195. https://goo.gl/WMLUya
- Pavord S, Myers B, Robinson S, Allard S, Strong J, Oppenheimer C. British Committee for Standards in Haematology. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 2012; 156: 588-600. https://goo.gl/wzSYnU
- Di Renzo GC, Spano F, Giardina I, Brillo E, Clerici G, Roura LC. Iron deficiency anemia in pregnancy. Womens Health (Lond) 2015; 11: 891-900. https://goo.gl/1ZXJTr
- WHO. Guideline: Daily iron and folic acid supplementation in pregnant women. Geneva, World Health Organization. 2012. https://goo.gl/2N6n49
- Paesano R, Pacifici E, Benedetti S, Berlutti F, Frioni A, Polimeni A, et al. Safety and efficacy of lactoferrin versus ferrous sulphate in curing iron deficiency and iron deficiency anaemia in hereditary thrombophilia pregnant women: an interventional study. Biometals. 2014; 27: 999-1006. https://goo.gl/7YKs3J
- Rezk M, Kandil M, Dawood R, Shaheen AE, Allam A. Oral lactoferrin versus ferrous sulphate and ferrous fumerate for the treatment of iron deficiency anemia during pregnancy. J Adv Nutr Hum Metab. 2015; 2: 740. https://goo.gl/FNnGzm
- Mehedintu C, Ionescu OM, Ionescu S, Cirstoiu MM, Dumitrascu MC, Bratila E, et al. Iron deficiency and iron-deficiency anaemia in pregnant women corrected by oral bovine lactoferrin administration. Farmacia. 2015; 63: 922-926. https://goo.gl/5LLKqt
- Giunta G, Giuffrida L, Mangano K, Fagone P, Cianci A. Influence of lactoferrin in preventing preterm delivery: a pilot study. Mol Med Rep. 2012; 5: 162-166. https://goo.gl/CjGTMx
- Paesano R, Berlutti F, Pietropaoli M, Pantanella F, Pacifici E, Goolsbee W, et al. Lactoferrin efficacy versus ferrous sulfate in curing iron deficiency and iron deficiency anemia in pregnant women. Biometals. 2010; 23: 411-417. https://goo.gl/ghNdEf
- Rezk M, Dawood R, Abo-Elnasr M, Al Halaby A, Marawan H. Lactoferrin versus ferrous sulphate for the treatment of iron deficiency anemia during pregnancy: a randomized clinical trial. J Matern Fetal Neonatal Med. 2016; 29: 1387-1390. https://goo.gl/uusS1s
- Paesano R, Natalizi T, Berlutti F, Valenti P. Body iron delocalization: the serious drawback in iron disorders in both developing and developed countries. Pathog Glob Health. 2012; 106: 200-216. https://goo.gl/UqNtrH
- Cao C, O'Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev. 2013; 71: 35-51. https://goo.gl/933ima
- Langer AL, Ginzburg YZ. Role of hepcidin-ferroportin axis in the pathophysiology, diagnosis, and treatment in anemia of chronic inflammation. Hemodial Int. 2017; 21: 37-46. https://goo.gl/6ZFejW
- Sebastiani G, Wilkinson N, Pantopoulos K. Pharmacological Targeting of the Hepcidin/Ferroportin Axis. Front Pharmacol. 2016; 7: 160. https://goo.gl/N5Yiaf
- Cutone A, Frioni A, Berlutti F, Valenti P, Musci G, Bonaccorsi di Patti MC. Lactoferrin prevents LPS-induced decrease of the iron exporter ferroportin in human monocytes/macrophages. Biometals. 2014; 27: 807-813. https://goo.gl/8NQoeA

Sign up for Article Alerts