Can Schistocytes Percentage in the Peripheral Blood Smear Predict Cases of Thrombotic Thrombocytopenic Purpura in Women Provisionally Diagnosed as HELLP Syndrome?
Ayman M. Abdelkader, Ahmed H. Naguib, Mohammed A. Faris, Nermine Elserwi, Mohamed Abdel Fattah El Senity, Rasha Abd El Rahman El Gamal and Shimaa B. Mohammed
Obstetrics and Gynecology, Ain Shams University, Egypt and International clinical fellow at Frimley Park Hospital, UK
*Address for Correspondence: Ayman M. Abdelkader, Lecturer of Obstetrics and Gynecology Ain Shams University and International clinical fellow at Frimley Park Hospital, 172 gilbert road, Frimley, Surrey, UK, post code: GU167RE, Tel: +44- 077-149-848-34; E-mail: aymanabdelkader18@yahoo.com
Submitted: 26 June 2018; Approved: 26 July 2018; Published: 30 July 2018
Citation this article: Abdelkader AM, Naguib AH, Faris MA, Elserwi N, El Senity MAF, et al. Can Schistocytes Percentage in the Peripheral Blood Smear Predict Cases of Thrombotic Thrombocytopenic Purpura in Women Provisionally Diagnosed as HELLP Syndrome? Int J Reprod Med Gynecol. 2018;4(2): 034-040.
Copyright: © 2018 Abdelkader AM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: HELLP syndrome; Thrombotic Microangiopathy syndrome; Schistocytes; TTP/HUS
Download Fulltext PDF
The syndrome of Hemolysis, Elevated Liver Enzymes, Low Platelets (HELLP syndrome) is a serious and unclear disease. But, most patients will show evidence of improvement from the disease progress within 48 hours after delivery. However, Thrombotic Thrombocytopenic Purpura (TTP) which mimic the same clinical and biochemical findings of HELLP syndrome will lead to a worsening clinical picture. Schistocytes present in the peripheral blood film in almost all women with Thrombotic Microangiopathy Syndrome (TMA) including both HELLP syndrome and TTP. However, the schistocytes percentage is significantly higher in case of TTP. Nevertheless, until now there is no agreed protocol on how to early differentiate between both conditions in order to initiate the proper treatment which will reduce the maternal morbidity and mortality.
Synopsis: Schistocytes percentage in cases provisionally diagnosed as HELLP Syndrome might predict and red flag cases with TTP for early multidisciplinary team management.
Material and methods: The study was carried out at Ain Shams University Maternity Hospital. All patient with a provisional diagnosis of HELLP syndrome admitted in 2015 were recruited and tested for the schistocytes percentage just before delivery and then subsequently divided into two groups according to whether the condition improved or not after 48 hours of delivery.
Results: The current study was carried out at Ain Shams University Maternity Hospital on (250) pregnant women with singleton pregnancies who were admitted to the Ain shams maternity Hospital presented with preeclampsia 34-37 weeks gestation, during a period from July 2017 to March 2018.
Introduction
HELLP syndrome was first described by Louis Weinstein; he described a syndrome consisting of hemolysis, elevated liver enzymes and low platelets. Whether is it a severe form of preeclampsia or is it a separate disorder with overlapping features; this remains an un-solved question, this confusion mainly raised as 15-20% of cases with HELLP syndrome do not have hypertension or proteinuria [1]. In addition to its prevelance in certain ethnic group as it is more associated with Caucasian multiparous women above the age of 25 years [2]. The pathogenesis remains unclear, as in pre-eclampsia, abnormal placentation might be the initiating signal, but unlike pre-eclampsia the pathology is directed mainly to the liver and the coagulation system [3,4].
Either or both Hypertension and proteinuria may not present in cases with severe HELLP syndrome. However, their presence increases the liability of diagnosis in approximately 85 percent of cases [2]. Typically, HELLP develops in the third trimester between 28 and 36 weeks of gestation, but second trimester HELLP or even postpartum onset is not uncommon [5].
Serious morbidities develop initially or shortly thereafter; These morbidities include disseminated intravascular coagulation, placental abruption, acute kidney injury or failure, pulmonary edema, sub-capsular or intra-parenchymal liver hematoma, and retinal detachment. Bleeding disorders secondary to thrombocytopenia is an uncommon complication [5]. Serious maternal and foetal morbidities correlates with the severity of maternal symptoms and laboratory derangement [6]. However, the majority of patients will show evidence of resolution of the disease process within 48 hours after delivery [7].
The presence of diagnostic criteria is mandatory for both clinical researches and the prediction of morbidity and mortality. For example; Tennessee classification, which requires the presence of microangiopathic hemolytic anemia with characteristic schistocytes or other signs suggestive of hemolysis [5]. Differential diagnosis of HELLP syndrome include various number of clinical conditions aggravated or rose during pregnancy such as; immune thrombocytopenia, lupus flare, antiphospholipid syndrome and other Thrombotic Microangiopathies (TMAs) as: Hemolytic-uremic syndrome, thrombotic thrombocytopenic purpura [8]. TMAs comprise a group of distinct disorders which mimic the pathophysiology of HELLP syndrome, as TMAs also characterized by microangiopathic hemolytic anemia, thrombocytopenia, and micro vascular thrombosis [9]. The micro-vascular thrombosis will eventually lead to consumptive thrombocytopenia and creates a shear stress in the small vessels as well; The shear stress consequently leads to fragmentation of the erythrocytes and the presence of schistocytes in the peripheral blood [10,11]. Nevertheless, pregnancy itself can be a precipitating factor for TTP/HUS, accounting for 8%-18% of cases of TMAs [12].
The diagnostic criteria for TMAs including Thrombotic Thrombocytopenic Purpura and Hemolytic Uremic Syndrome (TTP/HUS) includes hemolytic anemia, thrombocytopenia, neurologic and/or renal abnormalities [13]. And should be considered in all pregnant women with severe thrombocytopenia, severe anemia and elevated lactate dehydrogenase enzyme [14]. Differentiating it from severe preeclampsia is crucial for therapeutic and prognostic reasons. However, the clinical and pathophysiological features are so similar that establishing the correct diagnosis is often challenging. Moreover, both conditions tend to overlap [15]. Highly specific diagnostic tool for TTP/HUS such as serum levels of ADAMTS13 is a difficult and un-available investigation except for highly specialized centers. That is why the presence of schistocytes in the peripheral blood smear is considered now the morphologic hallmark of the disease [15]. The quantification assessment of peripheral serum schistocytes in TTP in comparison to other differential diagnosis of TMAs, in which schistocytes percentage represented an average of nearly 8 percent of RBCs in the patients with TTP/HUS [15]; this percentage was significantly higher than all other differential diagnosis of TMAs, including SPET/HELLP syndrome, as the percentages fluctuate around 0.5 percent [16].
The current study is a trial to pick up cases of TTP/HUS, either pure cases or overlapping conditions, from other cases initially diagnosed with SPET/HELLP syndrome, in order to avoid the devastating end-results of TTP/HUS syndrome; especially that in adults TTP/HUS typically follows a progressive course that will results in irreversible renal failure, progressive neurologic damage, ischemic heart disease, and eventually death [17]. The mortality rate before the era of plasma exchange was nearly 90 percent [18]. Plasma exchange is simply replacement the patient’s plasma with a healthy donor’s plasma by pheresis [13]. Compared with the previous high mortality rate prior to the implementation of plasma exchange, the mortality rate for patients treated with plasma exchange is 25 percent or less [19].
Materials and Method
The authors hypothesis was as follow; patients with either pure or overlapped TTP/HUS features of microangiopathic hemolysis will show neither clinical nor biochemical improvement after delivery and their peripheral blood films will show significant sever degree of hemolysis which is represented with higher schistocytes percentages.
This clinical trial designed to assess, the diagnostic and prognostic value of schistocytes percentage in peripheral blood film in women who were provisionally diagnosed with sever HELLP, aiming at diagnosing women with hidden TTP/HUS features and predicting cases with persisting hemolysis after delivery.
The required sample size has been calculated using the G*Power software version 3.1.7 (Dusseldorf University, Germany). The primary outcome measure was the schistocytes count in peripheral blood smears obtained from women with the HELLP syndrome who worsen after delivery (cases) and those improved after delivery (controls). Since there is currently no available information regarding the outcome measure, the sample size required for this pilot study calculated based on targeting an effect size that would be clinically relevant. Based on local institutional data, approximately 25 parturient with the HELLP syndrome worsen after delivery present to the Maternity Hospital of Ain Shams University per year. So, it is estimated that recruiting 25 cases (expectedly over a period of one year) and 75 matched controls (i.e., case to control ratio = 1: 3) would have a power of 81% (type II error, 0.19) to detect a statistically significant difference between the two study groups as regards the schistocytes count for a medium-to-large effect size of Cohen’s d = 0.65, which is equivalent to a difference of 0.65 SD in the schistocytes count between cases and controls. This difference has been chosen as it could be regarded as a clinically relevant difference to seek in this pilot study. This calculation used a two-sided unpaired t test and assumed a two-tailed type I error of 0.05, i.e., a confidence level of 95%.
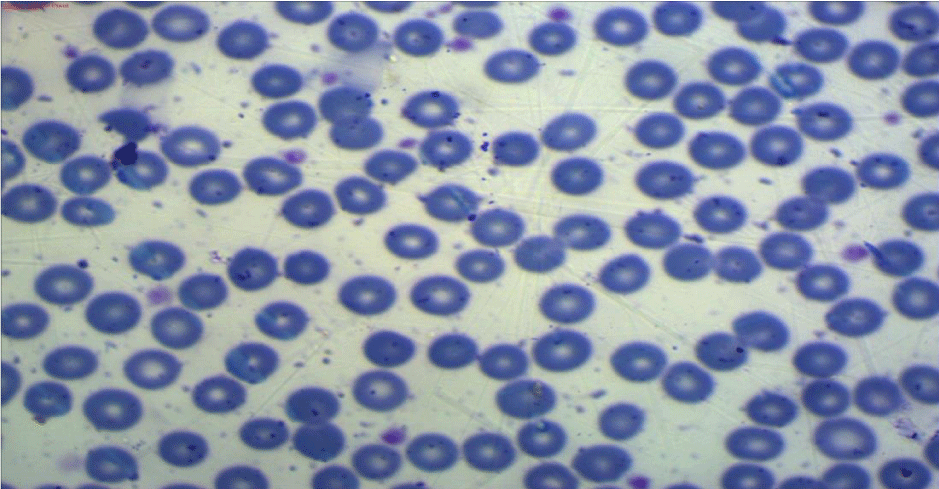
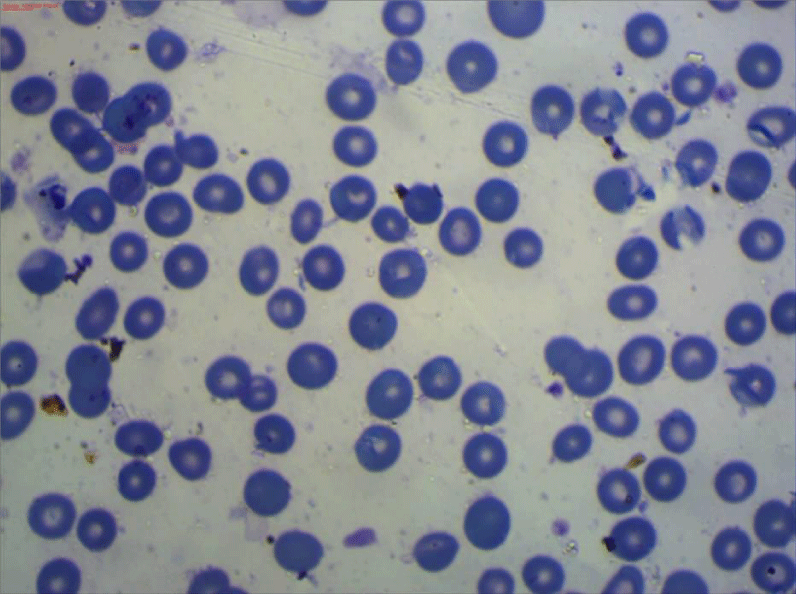
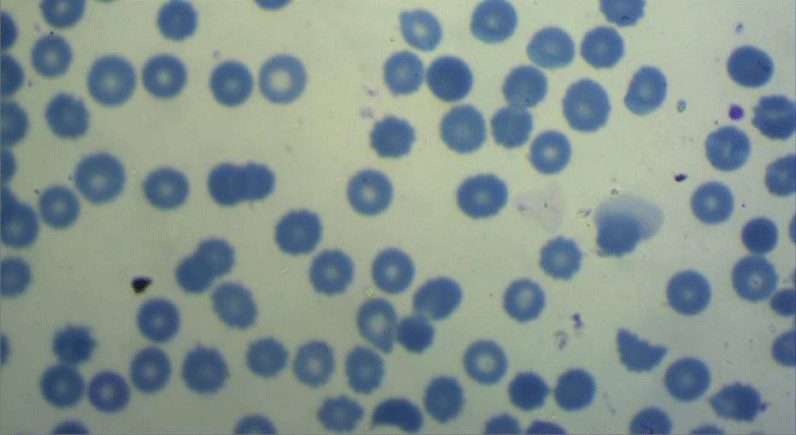
All the participants fulfilled the criteria for the diagnosis of severe preeclampsia [20], and fulfilled the criteria for the diagnosis of HELLP syndrome [6]. All included women have been subjected to peripheral blood smears taken within 12 hours before delivery and subsequently been prepared within two hours of blood collection using the EDTA-anticoagulated blood sample. The blood film then been spread, air-dried, fixed and stained according to standard procedures with panoptical Romanwsky stain [21]. The results of microscopic examination had been expressed as a percentage, after counting at least 1000 RBCs in optimal areas of the film, (Figures 1-3). Interpretation of the examination results done following the recommendations of the International Council of Standardization in Hematology (ICSH) for identification, diagnostic value, and quantitation of schistocytes. (Table 1) [22].
All other routine SPET/HELLP blood samples such as; full blood count, liver function tests, kidney function tests and coagulation profile, have been taken within 12 hours before the delivery of all admitted women. Then followed up 48 hours after delivery and subdivided into:
• Group 1 [no = 75] (HELLP syndrome group): this group included women who showed clinical and biochemical improvement in hemolytic features within 48 hours of delivery.
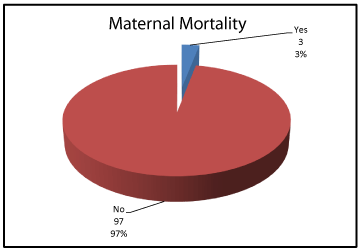
• Group 2 [no = 75] (TTP/HUS). This group included women who sustained clinical and biochemical features of hemolysis or deteriorated after 48 hours of delivery. Three patients from this group died from sever hemolysis.
Results
Statistical analysis and graphing was performed using SPSS for Windows version 20.0 and Microsoft Excel version 2010.
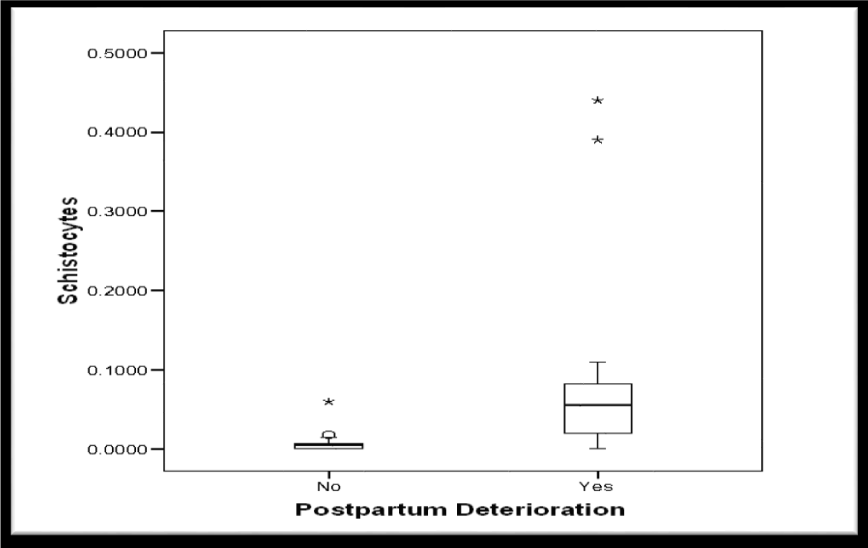
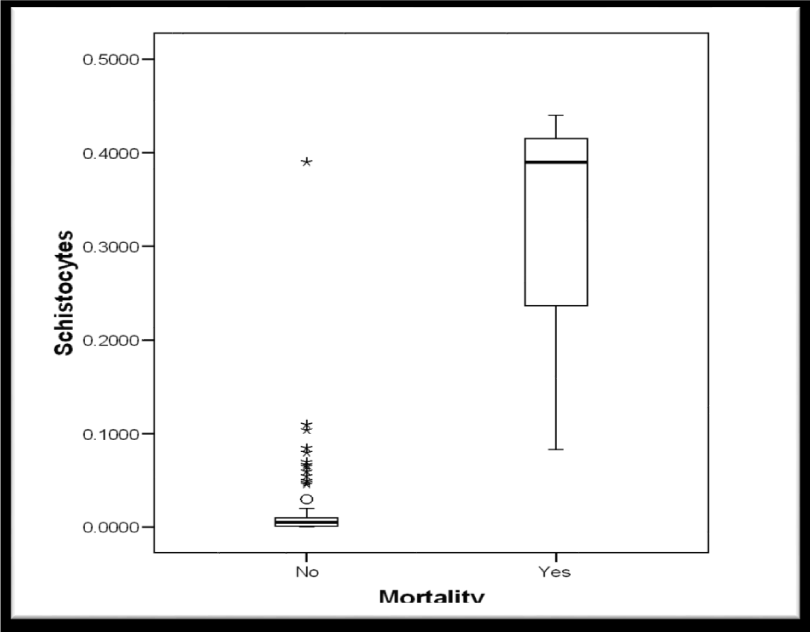
Demographic data showed no significant differences between women who improved postpartum and women who did not regarding age, parity or gestational age, P values: 0.621, 0.972 and 0.407 respectively (Table 2). 75% of the included women improved clinically and biochemically while 25% sustained clinical and biochemical features among them 3 patients died. (Figures 4,5).
No significant differences found between both groups regarding the initial values of systolic blood pressure, diastolic blood pressure, albuminuria, hemoglobin concentration, platelet count or serum creatinine. The initial levels of serum ALT, AST and total bilirubin were, however, significantly, higher in group 2 (Table 3).
The median schistocytes percentage was significantly higher in group 2 (Table 4, Figure 6). Moreover, the median schistocytes percentage was significantly higher in women who died when compared to women who survived. (Table 5, Figure 7).
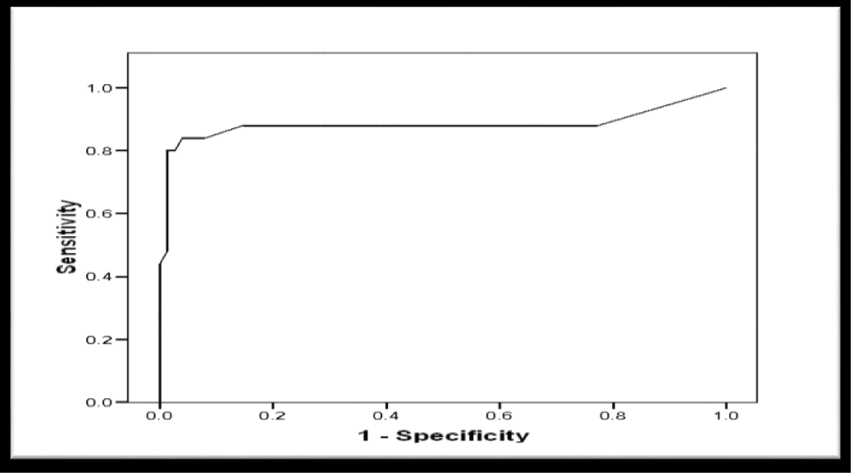
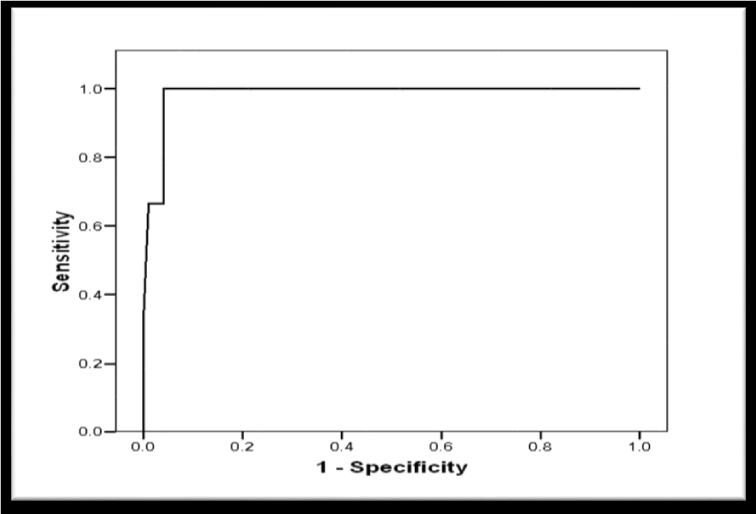
Receiver Operator Characteristic (ROC) curve was constructed for schistocytes percentage as a predictor of postpartum deterioration in included women showed a significant predictability as denoted by the significantly large Area Under the Curve (AUC) = 0.883, 95% CI (0.770 to 0.996), p < 0.001 (Figure 8). Another ROC curve was constructed for schistocytes percentage as a predictor of maternal mortality in included women. The curve showed a significant predictability as denoted by the significantly large Area Under the Curve (AUC) = 0.985, 95% CI (0.956 to 1.103), p = 0.004 (Figure 9).
Validity of Schistocytes as Predictor of Postpartum deterioration in Included Women, at different percentages, the most specific percentage was ≥ 1.9% with sensitivity of 80%, specificity of 98.7%, (PPV) Positive Predictive Value of 95.2%, (NPV) Negative Predictive Value of 93.7%, (FP) False Positive of 1.3%, (FN) False Negative of 20%, (LR+) Positive Likelihood Ratio of 60 (Table 6). However, the validity as a predictor of maternal mortality at a percentage ≥ 8.2% of sensitivity 100%, specificity 95.9%, PPV Positive Predictive Value 42.95%, NPV Negative Predictive Value 100%, FP False Positive 4.1%, FN False Negative 0%, LR+ Positive Likelihood Ratio 24.3 (Table 7).
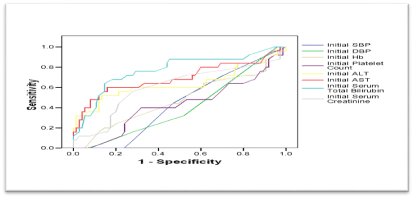
ROC curves were constructed for initial clinical and laboratory data as predictors of postpartum deterioration. Among all measured variables, only initial ALT, initial AST and initial total bilirubin were significant predictors of postpartum deterioration. Their AUCs, though being significantly large, were smaller than the AUC for schistocytes; indicating that the latter is a more significant and better predictor for sustained symptoms (Figure 10). And the validity of initial values of ALT, AST, and total bilirubin, as predictors of postpartum deterioration, were remarkably lower than the corresponding parameters of Schistocytes (Table 8).
Discussion
Differentiating between SPET/HELLP syndrome and other causes of TMAs especially TTP/HUS always poses a challenge for obstetricians and hematologist. That is mainly due to the difficulty in the diagnosis of TTP/HUS in pregnancy and the presence of many overlapping features. Owing to these difficulties and the devastating end results of missing the diagnosis of such life-threatening conditions, clinicians set certain roles for diagnosing TTP/HUS and refuting HELLP syndrome. For example; the American College of Obstetrics and Gynecology (ACOG) stated that most patients with SPET/HELLP syndrome will show evidence of resolution of the disease process within 48 hours from delivery [7]. That is why it has been recommended to raise a red flag if delivery does not lead to normalization of the biochemical values within 2-3 days or improvement in the neurologic, hematological and the nephrological symptoms in women with a provisional diagnosis of SPET/HELLP syndrome. This agreed with the current study in which most patients from the second group, who did not show evidence of resolution of the disease within 48 hours, had initial significant higher values of schistocytes [7].
In 2004 Burns et al. had studied and compared the schistocytes percentages between patients diagnosed with TTP and other causes of TMAs such as; chronic renal failure, metallic heart valve replacement and SPET/HELLP. And they concluded that schistocytes were found in all patients diagnosed with TTP and ranged from 1.0% to 18.4% of red cells. However, in all other TMAs the schistocytes percentages were less than 0.5% and been found in 80% to 100 % of cases [15]. Nearly similar results were observed by Stella et al. in 2009 who concluded that the schistocytes percentages in patients with TTP/HUS were 2-5% and were significantly higher than in women with SPET/HELLP syndrome as the percentages were < 1% [14].
Moreover, in 2009 Stella et al. concluded that Thrombotic Thrombocytopenic Purpura-Hemolytic Uremic Syndrome (TTP/HUS) should be considered in all pregnant women with severe thrombocytopenia, severe anemia, and elevated lactate dehydrogenase enzyme [14]. In 2012 Scully et al. discussed the same idea with more details and concluded that if a TMAs couldn’t be fully explained by a non-TTP pregnancy-related Thrombotic Micro Angiopathy (TMA), TTP then must be considered and plasma-exchange should be started as soon as possible. [13].
Interestingly, Kremer et al. in 2010 recommended that not only postpartum TTP/HUS but also persistent HELLP are absolute indications for plasma-exchange therapy because it is the safest curative treatment [19]. Even more, eculizumab, a complement protein C5 targeted inhibitor has been tried as a treatment for severe HELLP syndrome which prove the overlapping TMAs features in severe cases of HELLP syndrome [14].
In the current study the median of Schistocytes percentages were significantly higher in group 2 and the validity of Schistocytes percentage at different cutoff values as predictor of postpartum deterioration in included women were:
(1) Schistocytes percentage of ≥ 0.95 % with sensitivity of 88%, specificity of 85.5%.
(2) Schistocytes percentage ≥ 1.4% with sensitivity of 84%, specificity of 96%.
(3) Schistocytes percentage ≥ 1.9% with sensitivity of 80%, specificity of 98.7 %, (PPV) Positive Predictive Value of 95.2%, (NPV) Negative Predictive Value of 93.7%, (FP) False Positive of 1.3%, (FN) False Negative of 20%, (LR+) Positive Likelihood Ratio of 60.
Which means that the likelihood ratio of worsening hemolysis in women provisionally diagnosed with HELLP syndrome increases significantly with every 0.5% increase in the schistocytes percentage, and so we will be able to predict cases with either pure of overlapped TTP. Additionally, mortality from severe hemolysis was strongly correlated with the initial percentage of schistocytes; these schistocytes percentages were significantly higher in women who died (Table 7).
This study aimed to red flag undiagnosed cases of TTP who are misinterpreted as SPET/HELLP syndrome, in order to start the appropriate investigation and care early. The current recommendations are in favor of starting early and prompt investigation and treatment, with plasma exchange, in patients with suspected TTP, in order to reduce long term morbidities and mortality; these recommendations stated clearly by the International Council for Standardization in Hematology (ICSH) which has recommended that the finding of schistocytes without other features of RBC abnormalities, should lead to a prompt investigation for the presence of TMAs. TTP particularly carries the worst prognosis without starting the specific treatment immediately. The efficacy, feasibility and the life-saving potential of early plasma exchange in cases with TTP have increased the diagnostic importance of the schistocytes percentage as a diagnostic tool even before the establishment of overt symptoms and complications [22].
Conclusion
Schistocytes percentage in peripheral blood smear in cases provisional diagnosed as HELLP Syndrome might predict cases of sustained symptoms in order to red flag cases of possible thrombotic microangiopathy as TTP/HUS who will benefit from early diagnosis and multidisciplinary team management.
Acknowledgement
Ethical Disclosure
The whole study was carried out at Ain Shams University Maternity Hospital in 2015. The study protocol and the final thesis have been discussed and approved by the research review board of the faculty of medicine Ain Shams University. All participants have consented for the sampling procedure and approved using the results for teaching and scientific purposes.
Registration
ClinicalTrial.gov ID: NCT03246542.
- Neiger R, Contag SA, Coustan DR. The resolution of preeclampsia related thrombocytopenia. Obstet Gynecol. 1991; 77: 692-695. https://goo.gl/baSqmv
- Sibai B. Diagnosis, controversies and management of the syndrome of hemolysis, elevated liver enzymes and low platelets count. Obstet Gynecol. 2004; 103: 981-991. https://goo.gl/iBjkTj
- Benedetto C, Marozio L, Tancredi A, Picardo E, Nardolillo P, Tavella AM, et al. Biochemistry of HELLP syndrome. Adv Clin Chem. 2011; 53: 85-104. https://goo.gl/u1JAPv
- Jebbink J, Wolters A, Fernando F, Afink G, van der Post J, Ris Stalpers C. Molecular genetics in preeclampsia and HELLP syndrome-a review. Biochim Biophys Acta. 2012; 1822: 1960-1969. https://goo.gl/11mLDu
- Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with Hemolysis, Elevated Liver Enzymes and Low Platelets (HELLP syndrome). Am J Obstet Gynecol. 1993; 169: 1000-1006. https://goo.gl/89bxML
- Martin JN Jr, Bailey AP, Rehberg JF, Owens MT, Keiser SD, May WL. Thrombotic thrombocytopenic purpura in 166 pregnancies 1955-2006. Am J Obstet Gynecol. 2008; 199: 98-104. https://goo.gl/jhVrrj
- Diagnosis, controversies and management of the syndrome of hemolysis, elevated liver enzymes and low platelet count. American College of Obstetrics and Gynecology. 2004; 103: 5. https://goo.gl/o2oo1K
- Page LM, Girling JC. A novel cause for abnormal liver function tests in pregnancy and the purperium: non-alcoholic fatty liver disease. BJOG. 2011; 118: 1532-1535. https://goo.gl/v9SbFe
- Moake J. Thrombotic micrangiopathies. N Engl J Med. 2002; 347: 589-600. https://goo.gl/QGRtDL
- Tsai H. The molecular biology of thrombotic microangiopathy. Kidney Int. 2006; 70: 16-23. https://goo.gl/sT11xs
- Lammle B, Kremer Hovinga JA, Alberio L. Thrombotic thrombocytopenia purpura. J Thromb Haemost. 2005; 3: 1663-1675. https://goo.gl/rddgbm
- Fakhouri F, Vercel C, Fremeaux-Bacchi V. AKI and thrombotic microangiopathies in pregnancies. Clin J Am Soc Nephrol. 2012; 7: 2100-2106. https://goo.gl/mvcjtA
- Scully M, Hunt BJ, Benjamin S, Liesner R, Rose P, Peyvandi F, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic pupura and other thrombotic microangiopathies. Br J Haematol. 2012; 158: 323-335. https://goo.gl/yE9DmG
- Stella CL, Dacus J, Guzman E, Dhillon P, Coppage K, How H, et al. The diagnostic dilemma of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome in the obstetric triage and emergency department: lessons from four tertiary hospitals. Am J Obstet Gynecol. 2009; 200: 381. https://goo.gl/zZqQij
- Burns ER, Lou Y, Pathak A. Morphologic diagnosis of thrombotic thrombocytopenic purpura. Am J Hematol. 2004; 75: 18-21. https://goo.gl/xTozhr
- Brain M, Dacie J, Hourihane D. Microangiopathic hemolytic anaemia: the possible role of vascular lesions in pathogenesis. Br J Haematol. 1962; 8: 358-374. https://goo.gl/XxC2tJ
- Remuzzi G. HUS and TTP. Variable expression of a single entity. Kidney Int. 1987; 32: 292-308. https://goo.gl/GThgdm
- Von Baeyer H. Plasmapheresis in thrombotic microangiopathy-associated syndrome: review of outcome data derived from clinical trials and open studies. Ther Apher. 2002; 6: 320-328. https://goo.gl/kLWivs
- Kremer Hovinga JA, Vesely SK, Terrell DR, Lammle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010; 115: 1500-1511. https://goo.gl/huNxyt
- Task force on hypertension in pregnancy. Obstet Gynecol. 2013; 122: 1122. https://goo.gl/pfVhR6
- ICSH reference method for staining of blood and bone marrow films by azure B and eosin Y (Romanowsky stain). International Committee for Standardization in Haematology. Br J Haematol. 1984; 57: 707-710. https://goo.gl/5DZFYZ
- Zini G, D'Onofrio G, Briggs C, Erber W, Jou JM, Lee SH, et al. ICSH recommendations for identification, diagnostic value and quantification of schistocytes. Int J Lab Hematol. 2012; 34: 107-116. https://goo.gl/bfXE8q


Sign up for Article Alerts