Reproductive Challenges and Outcome after Treating Intrauterine Adhesions
Ayman Abdelkader1*, Magdy Moustafa2 and Mohamed Amer3
3Senior clinical fellow, Frimley Park Hospital and Lecturer at Ain Shams University
3Consultant Frimley Park Hospital
3Professor of Ob/Gyn and Head of the Hysteroscopy department at Ain Shams University
*Address for Correspondence: Ayman Abdelkader, Frimley Park Hospital, UK and Department of Obstetrics and Gynaecology, Ain Shams University, Egypt, Tel: +44-077-149-848-34; E-mail: aymanabdelkader18@yahoo.com
Submitted: 31 October 2019; Approved: 17 December 2019; Published: 19 December 2019
Citation this article: Abdelkader A, Moustafa M, Amer M. Reproductive Challenges and Outcome after Treating Intrauterine Adhesions. Int J Reprod Med Gynecol. 2019;5(2): 046-051.
Copyright: © 2019 Abdelkader A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Introduction
Intra Uterine Adhesion (IUA) is the devastating end results that occurs after common gynaecological and obstetric procedures or infections and usually poses a challenge for gynaecologist in both diagnosis and treatment. IUA is not necessarily symptomatic, if symptomatic, in this case the condition will be known as Asherman’s syndrome [1]. However, it is estimated that several degrees of adhesions may be found in almost 1.5% of the asymptomatic population [2].
Risk Factors
The endometrium is highly susceptible to trauma and scarring during pregnancy and especially postpartum, the reason for that is the post-partum decrease in oestrogen level; which renders the basalis layer of the endometrium thin and hence more exposed to trauma during the puerperium [3-5]. Therefore, pregnancy-associated endometrial trauma is considered to be the main risk factor for IUAs.
It has been very difficult to estimate the exact prevelance of IUA due to the inter-observer variation. However, a meta-analysis published in 2014 estimated that the prevelance of IUAs after miscarriage was nearly 20 percent [6]. Nevertheless, the number, timing and the gestational age of the miscarriage are also contributing factors. For example, the risk is doubled if the patient has undergone more than one curettage, and an increased prevalence of IUAs has been observed among patients who underwent curettage after a pregnancy loss at a more advanced gestational age compared with patients with earlier pregnancy loss [7]. Moreover, endometrial curettage later in the puerperium carries the highest risk of adhesion formation mainly due to worsening infection and inflammation [8].
It seems that not only direct endometrial trauma poses a great risk, but also indirect trauma can also lead to almost a similar risk; e.g., compression sutures such as B-Lynch suture, a common procedure that is used for controlling post-partum haemorrhage, were unfortunately linked to adhesion formation in 19 to 27% of patients [9,10]. The authors did not find enough evidence from the literature about the risk of adhesions formation after using an intrauterine balloon in cases of post-partum haemorrhage.
Non-pregnancy associated endometrial trauma is also associated, but to a lesser extent, with IUAs. This includes surgical hysteroscopy for myomectomy especially when using a diathermy resectoscope and in cases with multiple myomas, or for intrauterine septum resection; intrauterine septum resection has an estimated risk of 7% [11-13].
Pelvic Inflammatory Disease (PID) and endometritis carry minimal risk if managed appropriately. In contrast, the risk of adhesions after genital tuberculosis is remarkable and is often presents as severe stage [14,15]. The global incidence of Tuberculosis (TB) is growing at approximately 0.4% per year, and is increasing much faster in sub-Saharan Africa [16]. Pelvic TB usually affects the fallopian tube and endometrium, mainly as a secondary haematological infection from lung TB; it is rarely transmitted sexually from affected partner [16] (Table 1).
Clinical Picture
The clinical picture is always subtle; the most common presentation is abnormal uterine bleeding that accompanied IUAs in 70-95% of cases [2,17]. Secondary amenorrhoea is the most common form of abnormal bleeding that occurs in 37% of cases [2]. Chronic pelvic pain occurs in 3.5% and only in cases of severe adhesions; chronic pelvic pain is secondary to obstruction of the menstrual flow [5]. Pelvic TB usually presented with both abnormal bleeding and pelvic pain in nearly 30% of cases and occurs several years after an acute infection [16]. Infertility is the ruinous end result of IUAs with a reported incidence 7-40% [2,17], and even after pregnancy, recurrent pregnancy loss complicates approximately 13% of treated cases [5].
Diagnosis
Diagnosis depends mainly on high degree of suspicion based on the presence of major risk factors such as pelvic TB or pregnancy associated endometrial trauma. In this case, a trans vaginal scan will be a reasonable test for screening, thin endometrium that is unsynchronised with the endometrial cycle and/or endometrial irregularity are highly suggestive of IUAs; current evidence does not suggest using sono-hysterogram for screening or as a diagnostic tool [18,19].
IUAs can appear incidentally during investigations for bleeding disorders, pelvic pain, infertility and/or recurrent miscarriage [2]. For example, hysterosalpingography as an investigation for infertility might show filling defects or a honey comb appearance in the uterine cavity, or it might show a highly suggestive picture of pelvic TB such as tubal beading or sacculation and/or pipestem shape [20]. Symptoms of TB including night fever and weight loss, might give a clue to start an investigation, especially if the woman has travelled to or is coming from an endemic area [21]. Confirmatory test in this case is a direct biopsy, then either ziehl-Neelsen staining or TB culture media of either the menstrual blood or endometrial sample is performed [20].
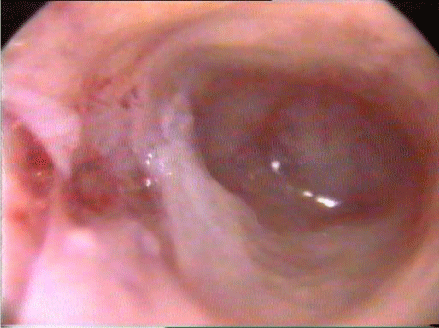
The gold standard tool for diagnosis and classification of IUAs is hysteroscopy [18,22]. A gynaecologist with a special expertise can assess the density and the extent of the adhesions; those are the main categories of the most commonly used classification of IUAs which is the American Society for Reproductive Medicine (ASRM) classification. The scoring system of ASRM classification depends on three variables; the density of the adhesions, the extent of cavity obliteration and the menstrual irregularity figure 1 and table 2 [2,19].
Prevention
Based on the high chances of adhesions after surgical management of miscarriage or evacuation of retained products of conception, it seems reasonable that the primary prevention will be adopting non-surgical management of these cases as first line of treatment in our local guidelines. Especially, that the success rate of medical management of miscarriage is 91% which is comparable to the success rate of 96% with the surgical option [23]. Prolonged retained products of conception carry the highest risk; this is why current evidence is in favour of hysteroscopic guided evacuation, rather than blind curettage.
In 2017 both the American Association of Gynaecological Laparoscopy (AAGL) and the European Society of Gynaecological Endoscopy, (ESGE) have jointly published an illustrated report. In the report different preventive measures that can aid in minimizing IUAs after operative hysteroscopy for common gynaecological procedures have been appraised and the recommended evidence has been summarised below:
• Endometrial injury only is less adheso-genic compared to both endometrial and myometrial trauma; exposing the endometrium in opposing surfaces carries the highest risk [22].
• Cold loop surgery e.g., myosure is safer than electrocautery e.g., resectoscope [22,24].
• Hysteroscopic polyp resection is better than blind polyp avulsion or even curettage guided by the ultrasound [25].
• Gel adhesions barriers e.g., oxidised regenerated cellulose, Carboxymethylcellulose and auto cross-linked hyaluronic acid after potentially traumatic hysteroscopic surgeries are effective in reducing the short-term adhesion formation but have no effect on the long-term adhesion; there is no enough evidence on its effect on pregnancy [22].
• Estrogen treatment compared with placebo treatment was studied in a recent meta-analysis after hysteroscopic septoplasty; no significant benefit was found [26].
Management
Treatment of IUA is mainly for fertility purpose, and the plan for such treatment should focus on lysis of the current adhesions to restore the shape and the size of the uterus [5] and should assess the patient for any preventive measures that should be taken to prevent further adhesions, e.g., using adhesion barriers or building up the endometrium with hormonal treatment.
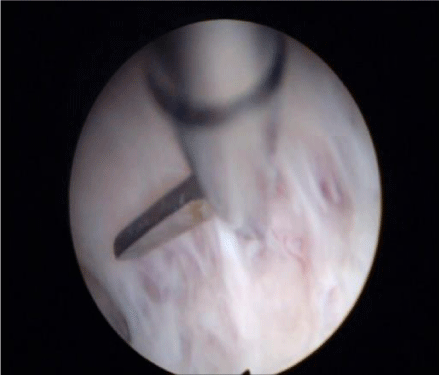
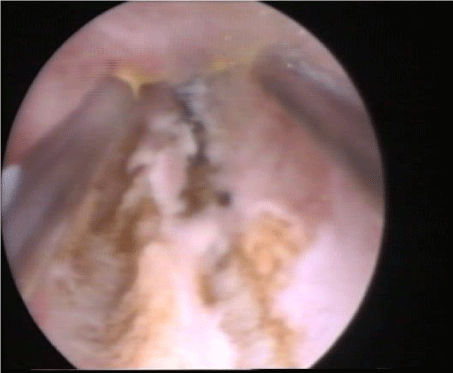
The current evidence stated that the ideal method for adhesiolysis is by using cold, non-electrocautery sharp hysteroscopic scissor [22,28], figure 2. In cases of dense fibrous bands, bipolar electro surgery may be used [29], figure 3. Moreover, in women with severe adhesions and complete agglutination of the cavity, the risk of false passage and subsequently perforation is high; thus, the AALG recommends laparoscopic guidance or even hysterotomy whenever needed [22].
Post Lysis Management
IUAs recurrence rate after adhesiolysis treatment is estimated to be from 30 to 66% [22]. Extensive studies have focused on finding the optimum post lysis preventive therapy [26].
Theoretically, administration of oestrogen will aid in endometrial regeneration after lysis. However, no agreed-upon evidence about its benefit has been found in different meta-analysis; the reason for this was the great variations in the studies regarding the formulas, doses and even the duration of therapy [30,31].
Mechanical barrier methods such as balloon catheter and intra uterine device, have been used for years and were extensively studied; there is good evidence about their efficacy in reducing the recurrence of IUAs but, there is no agreement on the duration of those therapies [32,33]. However, it is agreed that women should be covered with broad spectrum antibiotics during this period [22].
An Amniotic Membrane Graft (AM), whether fresh amnion or freeze-dried amnion, was studied as a barrier method for adhesions recurrence. This method has been studied extensively for years by the authors and the theory of its efficacy in treating endometrial adhesions based on its evident role of adhesion prevention and epithelialization promotion in different surgical and ophthalmological fields [34]. Moreover, it has been suggested that an amnion graft might be a possible source of Endometrial Stromal Cells (ESCs) that promote endometrial regeneration [35]. Recently in 2017, a systematic review of randomized controlled trials concluded that adding freeze-dried amnion on a balloon catheter was most likely able to reduce the recurrence of IUAs [36].
Fertility after Treatment
Systematic reviews reported nearly 80% improvement in the menstrual flow after treatment. However, the recurrence rate varies between 30 to 60% [30]. Because the aim of the treatment is mainly for fertility reasons women should be counselled that the reported pregnancy rate after treatment varies from 40% to 80% while the live birth rate varied between 30% and 70% [30], this inconsistency depends mainly on the severity of the adhesions. Pregnancy after treatment of severe adhesions should be considered as high-risk pregnancy because of reduced placental blood flow and subsequently the higher rate of recurrent miscarriage, foetal growth restriction and preterm delivery [41]. The most serious negative outcome is the risk of developing a morbidly adherent placenta as the result of the large endometrial areas with scarring and fibrosis; in a published report, it was estimated that placenta accreta complicate 10% of pregnancies after previous treatment of moderate to severe adhesions [33].
Current Researches in this Field
Numerous clinical trials are ongoing, mainly on the role of stem cells in promoting regeneration of healthy endometrium. Some promising therapies have been published but still cannot be used, unless they are part of a research protocol. For example; endometrial angiogenic stem cells extracted from autologous adult stem cells might regenerate the damaged endometrium in severe cases [37]. Injecting stem cells directly into the spiral arterioles by catheterization, is a novel technique done after mobilizing Bone Marrow-Derived CD 133+ Stem Cells (BMDSCs) into the circulation using granulocyte-CSF injection, followed by extracting them from the peripheral blood [38]. Theoretically, platelets-rich plasma might have the capacity to suppress pro-inflammatory gene expression in the endometrium and subsequently reduce the recurrence and promote endometrial regeneration. However, more trials to prove its efficacy are still required [39,40].
Alternatives
Uterus transplantation, surrogacy and adoption
Surrogacy and adoption are reasonable solutions in severe resistant cases. Most women will accept this as an alternative, but they will definitely require psychological support, to positively address the lack of both physical and emotional feeling of pregnancy and delivery, in order to create a healthy maternal bond with the adopted or surrogated baby [42]. Nevertheless, women from certain cultures and religious background, will not find these options acceptable based on the belief in the absolute necessity of a genetic parenthood.
After the delivery of the first baby from a transplanted uterus in Sweden in 2014 [43]. Uterine transplantation became an option for all women who emotionally or religiously cannot accept surrogacy or adoption. Additionally, uterine transplantation might represent a possible treatment for one in every 500 women worldwide; this number includes cases of congenitally absent uterus, post treatment for genital tract malignancies and post hysterectomy for benign conditions [44].
Major ethical issues specific to uterus transplantation include the following:
• Some would argue that there is no good reason to justify carrying on a major and extensive 10-13 hours surgery with a high rate of complications, especially for the donor, to transplant a uterus in a procedure that is not considered a lifesaving surgery [42]. Transplantation of deceased-donor organs instead is more challenging in terms of consenting and procurement [45].
• The recipient will go through a cascade of major surgeries starting from the transplantation, in vitro fertilization, Caesarean delivery and finally the hysterectomy after completing the family. All of these surgeries pose great risks and complications. Additionally, all of these women will be receiving immune-suppressant therapies to prevent graft rejection [42].
• The safest treatment for severe graft rejection in pregnancy would be a hysterectomy. That is why we should think of all the emotional, legal and ethical consequences when carrying out a hysterectomy, with a living foetus inside, as a treatment for graft rejection [46].
On the other hand, supporters of uterus transplantation would argue that our current health system funds other life enhancing organ transplantations, such as kidney transplantation and corneal transplantation in order to improve the quality of life despite the presence of alternatives, such as haemodialysis [45].
- Asherman JG. Traumatic intra-utarine adhesions. J Obstet Gynaecol Br Emp. 1950; 17: 892. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14804168
- Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010; 17: 555-569. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20656564
- Chen Y, Chang Y, Yao S. Role of angiogenesis in endometrial repair of patients with severe intrauterine adhesions. Int J Clin Exp Pathol. 2013; 6: 1343-1350. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23826415
- March C. Management of Asherman's syndrome. Reprod Biomed Online. 2011; 23: 63-76. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21549641
- Yu D, Li TC, Xia E, Huang X, Liu Y, Peng X. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman's syndrome. Fertil Steril. 2008; 89: 715. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17681324
- Hooker AB, Lemmers M, Thurkow AL, Heymans MW, Opmeer BC, Brolmann HA, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long term reproductive outcome. Hum Reprod Update. 2014; 20: 117. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24082042
- Adoni A, Palti Z, Milwidsky A, Dolberg M. The incidence of intrauterine adhesion following abortion. Int J Fertil. 1982; 27: 117. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6126446
- Eriksen J, Kaestel C. The incidence of uterine atresia after post-partum curettage. A follow up examination of 141 patients. Dan Med Bull. 1960; 7: 50-51. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/13820611
- Poujade O, Grossetti A, Mougel L, Ceccaldi PF, Ducarme G, Luton D. Risk of synechiae following uterine compression sutures in the management of major postpartum haemorrhage. BJOG. 2011; 118: 433-439. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21199289
- Ibrahim MI, Raafat TA, Ellaithy MI, Aly RT. Risk of postpartum uterine synechiae following uterine compression suturing during postpartum haemorrhage. Aust N Z J Obstet Gynaecol. 2013; 53: 37-45. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23163583
- Taskin O, Sadik S, Onoglu A, Gokdeniz R, Erturan E, Burak F, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Lapaosc. 2000; 7: 351. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10924629
- Yang JH, Chen MJ, Wu MY, Chao KH, Ho HN, Yang YS. Office hysteroscopic lysis of intrauterine adhesion after transcervical resection of multiple apposing submucous myomas. Fertil Steril. 2008; 89: 1254-1259. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17686478
- Roy KK, Singla S, Baruah J, Kumar S, Sharma JB, Karmakar D. Reproductive outcome following hysteroscopic septal resection in patients with infertility and recurrent abortions. Arch Gynecol Obstet. 2011; 283: 273-279. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20041257
- Sharma JB1, Roy KK, Pushparaj M, Gupta N, Jain SK, Malhotra N, et al. Genital Tuberculosis: an important cause of Asherman's syndrome in India. Arch Gynecol Obstet. 2008; 277: 37-41. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17653564
- Orhan Bukulmez, Hakan Yarali, Timur Gurgan. Total corporal synechiae due to tuberculosis. Hum Repod. 1999; 14: 1960. http://bit.ly/2RSphyR
- Agarwal J, Gupta JK. Femal genital Tuberculosis-a retrospective clinico-pathologic study of 501 cases. Indian J Pathol Microbiol. 1993; 36: 389. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8157306
- Hanstede MM, van der Meij E, Goedemans L, Emanuel MH. Results of centralized Asherman surgery 2003-2013. Fertil Steril. 2015; 104: 1561. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26428306
- Soares SR, Barbosa dos Reis MM, Camargos AF. Diagnostic accuracy of sonohysterography, transvaginal sonography and hysterosalpingography in patients with uterine cavity disease. Fertil Steril. 2000; 73: 406. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10685551
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, mullerian anomalies and intrauterine adhesions. Fertil Steril. 1988; 49: 944. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3371491
- Oosthuizen AP, Wessels PH, Hefer JN. Tuberculosis of the female genital tract in patients attending an infertility clinic. S Afr Med J. 1990; 77: 562-564. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2345881
- Saracoglu OF, Mungan T, Tanzer F. Pelvic Tuberculosis. Int J Gynecol Obstet. 1992; 37: 115. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1348699
- American Association of Gynecological Laparoscopy. Elevating Gynecologic surgery. J Minim Invasive Gynecol. 2017; 24: 695.
- Patua B, Dasgupta M, Bhattacharyya SK, Bhattacharya S, Hasan SH, Saha S. An approach to evaluate the efficacy of vaginal misoprostol administered for a rapid management of first trimester spontaneous onset incomplete abortion, in comparison to surgical curettage. Arch Gynecol Obstet. 2013; 288: 1243-1248. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23708389
- Mazzon I, Favilli A, Cocco P, Grasso M, Horvath S, Bini V, et al. Does cold loop hysteroscopic myomectomy reduce intrauterine adhesions? Fertil Steril. 2014; 101: 294. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24182410
- Ben-Ami I, Melcer Y, Smorgick N, Schneider D, Pansky M, Halperin R. A comparison of reproductive outcomes following hysteroscopic management versus dilatation and curettage of retained products of conception. Int J Gynecol Obstet. 2014; 127: 86. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24997472
- Healy MW, Schexnayder B, Connell MT, Terry N, DeCherney AH, Csokmay JM, et al. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016; 215: 267. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27173082
- Bosteels J, Weyers S, D'Hooghe TM, Torrance H, Broekmans FJ, Chua SJ M. Anti- adhesion therapy following operative hysteroscopy for treatment of female subfertility. Cochrane Database Syst Rev. 2017; 11: 11110. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29178172
- Robinson JK, Colimon LM, Isaacson KB. Postoperative adhesiolysis therapy for intrauterine adhesions (Asherman's syndrome). Fertil steril. 2008; 90: 409. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18571166
- H Fernandez, A Gervaise, R de Tayrac. Operative hysteroscopy for infertility using normal saline solution and a coaxial bipolar electrode: A pilot study. Hum Reprod. 2000; 15: 1773. http://bit.ly/2RVSVDe
- Johary J, Xue M, Zhu X, Xu D, Velu PP. Efficacy of estrogen therapy in patients with intrauterine adhesions. J Minim Invasive Gynecol. 2014; 21: 44. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23933351
- Di Spiezio Sardo A, Mazzon I, Bramante S, Bettocchi S, Bifulco G, Guida M, et al. Hysteroscopic myomectomy: a comprehensive review of surgical technique. Hum Reprod Update. 2008; 14: 101. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18063608
- Orhue AA, Aziken ME, Igbefoh JO. A comparison of two adjunctive treatments for intrauterine adhesions following lysis. Int J Gynaecol Obstet. 2003; 82: 49-56. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12834941
- Lin X, Wei M, Li TC, Huang Q, Huang D, Zhou F, et al. A comparison of intrauterine balloon, intrauterine contraceptive device and hyaluronic acid gel in the prevention of adhesion reformation following hysteroscopic surgery for Asherman's syndrome: a cohort study. Eur J Obstet Gynecol Reprod Biol. 2013; 170: 512. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23932377
- Amer MI, Abd-El-Maeboud KH. Amnion graft following hysteroscopic lysis of intrauterine adhesions. J Obstet Gynecol. 2006; 32: 559-566. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17100817
- Mohamed I. Amera, KarimAbd El Maebouda, Amal Alloubb. Amnion graft as a possible source of stem cells for endometrial regeneration after lysis of severe intrauterine adhesions. Middle East Fertil Soc J. 2012; 17: 54-56. http://bit.ly/2RUZXYY
- Yan Y, Xu D2. The effect of adjuvant treatment to prevent and treat intrauterine adhesions: a network Meta-Analysis of Randomized Controlled Trials. J Minim Invasive Gynecol. 2018; 25: 589-599. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28893657
- Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient with severe Asherman's syndrome. J Hum Reprod Sci. 2011; 4: 43-48. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21772740
- Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016; 1087-1096. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27005892
- Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod. 2017; 21: 54. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28333034/
- Marini MG, Perrini C, Esposti P, Corradetti B, Bizzaro D, Riccaboni P, et al. Effects of platelet-rich plasma in a model of bovine endometrial inflammation in vitro. Reprod Biol Endocrinol. 2016; 14: 58. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27619959
- Feng Z, Yang B, Liu S. Diagnostic and therapeutic hysteroscopy for traumatic intrauterine adhesions after induced abortion: clinical analysis of 365 cases. Gynaecological Endoscopy. 1999; 8: 95.
- Testa G, Koon EC, Johannesson L. Living donor uterus transplant and surrogacy: ethical analysis. Am J Transplant. 2017; 17: 912. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27754594
- Brannstrom M, Johannesson L, Bokstrom H. Livebirth after uterus transplantation. The Lancet. 2014; 607-616. http://bit.ly/2S3fO7E
- Johannesson L, Dahm-Kahler P, Eklind S, Brannstrom M. The future of human uterine transplantation. Womens health (Lond Eng). 2014; 10: 455-67. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25259905
- Alghrani A. Yes, uterus transplantation should be publicly funded. J Med Ethics. 2016; 42: 566-567. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26941252
- Johannesson L, Kvarnstrom N, Molne J, Dahm Kahler P, Enskog A, Diaz Garcia C, et al. Uterus transplantation trial: 1 year outcome. Fertil Steril. 2015; 103: 199. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25439846

Sign up for Article Alerts