Assessment of Food Consumption in Women with Chronic Pelvic Pain Secondary to Endometriosis
Joyce Beatriz da Silva, Maria Beatriz Ferreira Gurian, Carla Barbosa Nonino, Omero Benedicto Poli Neto, Antonio Alberto Nogueira, Francisco Jose Candido dos Reis and Julio Cesar Rosa e Silva*
Department of Gynecology and Obstetrics, School of Medicine of Ribeirao Preto, University of Sao Paulo, Ribeirao Preto, Brazil
*Address for Correspondence: Julio Cesar Rosa e Silva, Department of Gynecology and Obstetrics, School of Medicine of Ribeirao Preto, University of Sao Paulo, Ribeirao Preto, Brazil, Tel: +551-636-022-488; E-mail: juliocrs@usp.br
Submitted: 11 November 2019; Approved: 24 January 2020; Published: 27 January 2020
Citation this article: da Silva JB, Ferreira Gurian MB, Nonino CB, Poli Neto OB, Nogueira AA, et al. Assessment of Food Consumption in Women with Chronic Pelvic Pain Secondary to Endometriosis. Int J Reprod Med Gynecol. 2020;6(1): 001-009.
Copyright: © 2020 da Silva JB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Chronic pelvic pain; Food analysis; Lipid profile; Pain intensity; Anxiety; Depression
Download Fulltext PDF
Introduction: To assess food consumption and to analyze the lipid profile of women with a clinical diagnosis of Chronic Pelvic Pain (CPP) secondary to endometriosis and to other causes of the disease.
Methods: This was an observational case-control study conducted on 91 women, 46 of them with CPP secondary to endometriosis and 45 with CPP secondary to other causes. The subjects were submitted to a dietary survey by an indirect method (24-hour food recall) in order to assess their nutritional status and food intake. A 5-ml blood sample was collected for the analysis of lipid profile, clinical pain intensity was determined by a Visual Analogue Scale (VAS), and anxiety and depression symptoms were determined using the Hospital Anxiety and Depression (HAD) scale.
Result: The groups did not differ in terms of mean age, BMI or percent body fat. Assessment of food, calorie and micronutrient consumption revealed that lipid intake differed between groups (p = 0.0347), with a mean value of 58.29 ± 40.78 g for the endometriosis group and a mean value of 48.10 ± 31.53 g for the group with CPP secondary to other causes. Regarding the micronutrients analyzed, there was a difference in zinc, with a mean value of 11.46 ± 9.98 mg for the group with CPP secondary to endometriosis and a mean value of 8.17 ± 7.77 mg for the group with CPP secondary to other causes (p = 0.0417) and in the amino acid tryptophan, with a mean intake of 506.55 ± 407.89 for the group with CPP secondary to endometriosis and a mean intake of 373.57 ± 3982.48 mg for the group with CPP secondary to other causes (p = 0.0494).
Conclusion: Patients with CPP secondary to endometriosis have a higher intake of lipids, zinc and tryptophan that may be associated with this disease.
Introduction
Chronic Pelvic Pain (CPP) is a frequent complaint in gynecological practice, producing suffering, compromising the quality of life of women and involving high costs for health care services [1]. The prevalence of CPP has not been well established, but the estimate is that 3.8% of women aged 15 to 73 years and 14 to 24% of women of reproductive age have CPP [2,3].
One of the gynecological issues related to CPP is endometriosis, whose major clinical problem is pain syndrome, manifesting as dysmenorrhea, acyclic pelvic pain, dyspareunia and painful defecation, in addition to infertility [4], contributing to high costs for the health care system and representing a substantial physical and psychological burden for affected women [5].
The presence of endometriosis exceeds 30% among women with CPP submitted to laparoscopy [3]. The mechanisms involved in its etiopathogenesis and the association with its symptoms are still unclear. Endometriosis has a complex and multifaceted etiology that is difficult to understand, but it probably involves hormonal, anatomical, genetic, immune and inflammatory factors [6]. Evidence suggests that pain may be caused by peritoneal inflammation [7], a higher concentration of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6, which activates macrophages, inducing the formation of lipid peroxidation and others products of the interaction between the apolipoproteins and peroxides, triggering the production of antibodies [8,9].
Few studies have explored the influence of food on the risk of developing endometriosis, however, it is known that some specific nutrients may have a role in the etiopathology of the disease, which makes the diet a potentially modifiable risk factor. Some studies suggest that endometriosis may originate or be associated with oxidative stress, which is the imbalance between the production of free radicals and antioxidants [10].
Zinc (Zn) is a mineral that participates in the regulation of the chronic inflammatory state, reducing inflammatory cytokines and oxidative stress, playing a substantial role in growth and development, acting as a signal, through its three functions: catalytic, structural and regulatory. Its high concentration is associated with a compromised lipid profile and higher risk of metabolic syndrome [11].
In addition, tryptophan, which is an indispensable amino acid provided by dietary proteins and serotonin precursor, performs several physiological functions [12]. It has been associated to the production of metabolites and can be involved in the control of inflammation, being associated with aging and regulation of energy homeostasis and mood improvement [13].
The dietary pattern of the general population has undergone several changes from natural foods rich in nutrients, antioxidants and phytochemicals to refined, processed and ultra-processed foods, making Western diets often caloric without adequate nutrients and causing an imbalance that can contribute to the onset or worsening of diseases such as endometriosis and recurrent spontaneous abortions [14].
A diet rich in fats (45% of the daily calorie requirements), can increase oxidative stress and the inflammatory process, increasing the risk of developing endometriosis. On the other hand, consumption of omega-3 can decrease the risk of endometriosis by up to 50%, for every 1% of energy from Omega-3 [15].
The planning of an effective treatment in all its aspects may help an early identification of the problems of CPP secondary to endometriosis, representing a very important intervention for the reduction and relief of the suffering of affected women. Within this context, the identification of dietary factors as well as other factors associated with the life style of these patients, may influence both the symptoms and evolution of this disease.
Thus, the objective of the present study was to assess the food consumption and lipid profile of women with a clinical diagnosis of CPP secondary to endometriosis and to compare these patients to women with CPP secondary to other causes.
Materials and Methods
The description of the present study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (http://www.strobe-statement.org) [16]. This was an observational study of the case-control type, which was approved by the Ethics Committee of the Faculty of Medicine of Ribeirao Preto, University of de Sao Paulo (FMRP-USP). All subjects included gave written informed consent to participate in the study. Women diagnosed with CPP at the Pelvic Pain Outpatient Clinic of the University Hospital (HCFMRP-USP) were invited to participate in the study before starting any treatment.
Inclusion criteria were: women aged 18 to 49 years, in menarche, with a diagnosis of CPP characterized by a duration of at least six months and of sufficient intensity to interfere with habitual activities, requiring clinical or surgical treatment. Women older than 50 years and in menopause, smokers, pregnant women, women breastfeeding during the last six months and women who refused to participate were excluded from the study. Informed consent was obtained before intervention.
Women who agreed to participate in the study were scheduled for evaluation between days 3 to 7 of the menstrual cycle and instructed not to modify their eating behavior, to come to the clinic after a 12 hour fast [17], and not to ingest caffeine-containing products such as coffee, tea or chocolate, [18] alcoholic drinks [19] or medications [20] during a 24-hour period before blood collection. Percent body fat was determined by electronic analysis (bioelectrical impedance) and a blood sample was obtained for the determination of lipid profile. Questionnaires were filled out and data were collected on the day of assessment.
The intensity of clinical pain was assessed using a Visual Analogue Scale (VAS) [21,22] which consists of an uninterrupted 10 cm long line where the patient is instructed to mark the point that corresponds to her reported pain, with the beginning of the scale (0) corresponding to “absence of pain” and the end (10) corresponding to the “worst pain ever experienced or imagined”. For the classification of pain according to the VAS, we considered 5-44 mm to be mild pain, 45-74 mm to be moderate pain, and 75 to 100 mm to be severe pain [23].
The Hospital Anxiety and Depression scale (HAD) was used to assess anxiety and depression symtpoms. The scale consists of 14 items divided into two subscales of seven well-defined items each for each mood disorder. Seven items correspond to anxiety (HAD-A) and seven to depression (HAD-D). Four alternatives are available for each item, with a score ranging from 0 to 3. The sum of scores obtained for the items of each subscale provides a total score ranging from 0 to 21, with a cut-off point of 8 for anxiety and of 9 for depression [24]. Socio demographic data such as schooling, marital status and parity were also recorded on the same day.
The dietary survey is an indirect method for the assessment of food intake by each subject. In the present study we used the 24-hour food recall (R24h) [25] which consists of verbal information provided by the subject about her food intake in the last 24 hours preceding the interview, with data about habitually consumed foods and drinks being also obtained [26].
Data were analyzed statistically with the aid of the SAS University® software, Institute Inc., SAS Campus Drive, Cary, NC (2016) [27] and the results are reported as mean ± standard deviation or median and range, according to distribution. An exploratory analysis of the data was first carried out by measuring the central position of dispersal and by constructing normality graphs. Due to the asymmetry of distribution of some variables, the nonparametric Mann-Whitney test was used to compare the distributions of the quantitative variables in relation to the study groups.
Food consumption was analyzed quantitatively using the Diet Smart software - (Smardata solucoes inteligentes, Mococa) (2015) [28]. Energy and macronutrient intake was calculated and compared to the Acceptable Macronutrient Distribution Ranges (AMDRs) [29-31], which recommend a calorie intake of 45 to 65% from carbohydrate, 10 to 35% from proteins, and 20 to 35% from lipids. Micronutrient intake data were compared to the recommendations of the Dietary Reference Intakes (DRIs) [32] and to the Estimated Average Requirements (EAR), which recommend the following quantities of nutrients for subjects of the age and sex analyzed: calcium (800-100 mg/day), iron (5-6 mg/day), magnesium (255-265 mg), zinc (6.8 mg), selenium (45 µg), vitamin A (500 µg), vitamin C (60 mg), vitamin D (10 µg), vitamin E (12 mg), and tryptophan (4 mg/kg).
Results
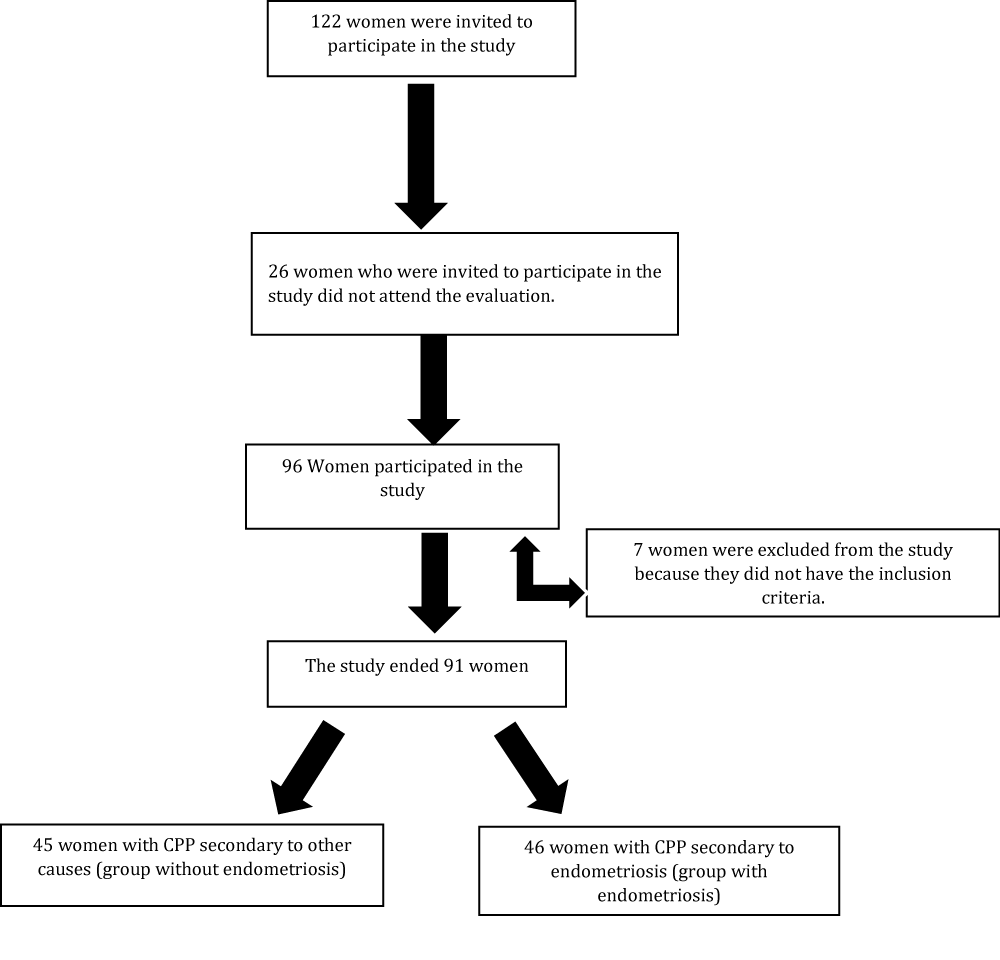
A total of 122 women with a clinical diagnosis of CPP were invited to participate in the study. Ninety-six were evaluated and 7 of them were excluded because they did not meet the inclusion criteria of the study or they were in menopause, a period that interferes with body fat percentage. Thus, 91 women were left, 45 of them diagnosed with CPP secondary to other causes, forming the group of CPP without endometriosis, and 46 diagnosed with CPP secondary to endometriosis, forming the group of CPP with endometriosis. As shown in the flowchart (Figure 1), the women were recruited and evaluated during the period from April 2014 to October 2015.
Table 1 lists the baseline demographic data for the two groups. Comparison of baseline data between groups revealed a significant difference (p < 0.05) in parity and in VAS data, with the endometriosis group showing a higher mean pain value on the scale than the group without endometriosis.
Table 2 lists analysis of the food recall of women with CPP with and without endometriosis regarding macronutrients revealed no difference in calorie or carbohydrate intake between groups, whereas lipid consumption was greater among the women with endometriosis.
The intake of most micronutrients did not differ between groups, except for zinc and tryptophan, whose intake was higher among women with endometriosis.
The lipid profile revealed that both groups had mean serum concentrations of total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides within the normal ranges as recommended by the SBC Directives (2017) [33], with no difference between groups.
Table 3 lists percent consumption according to Recommended Dietary Allowances (RDA) and DRIs [32]. It can be seen that the highest percentage of both groups (CPP secondary to endometriosis and CPP secondary to other causes) consumed appropriate amount of both carbohydrates and lipids. Regarding proteins, a higher percentage of both groups consumed quantities above recommendations, with no difference between groups (p > 0.05).
Regarding micronutrients, both groups consumed lower levels than recommended of vitamins A, C, D and of minerals such as calcium, iron, magnesium, and selenium. The group with endometriosis had a greater percentage of zinc consumption lower than recommended, whereas in the group without endometriosis the highest percentage of consumption was above recommended levels.
Discussion
The present study assessed the food consumption and lipid profile of women with a clinical diagnosis of CPP secondary to endometriosis and secondary to other causes. Analysis of food intake revealed a greater intake of lipids, zinc and tryptophan by women with endometriosis.
Lipids are involved in a variety of biological functions [34], are absorbed in the organism as fatty acids and have been shown to modulate the acute and chronic nociceptive response in animal studies [35]. Zinc is essential for the activity of some antioxidant enzymes, playing a role in the modulation of homeostasis [36]. Tryptophan is an essential amino acid which is not produced by our organism but which is obtained through the diet by the consumption of protein-rich foods [37]. Tryptophan also is a precursor of metabolites such as serotonin, an important neurotransmitter that modulates various physiological behaviors and functions such as sleep, mood, appetite, learning, and memory [37].
Some studies have suggested that the diet can make a positive contribution to the reduction of symptoms and to the control of systemic oxidative stress by providing a high consumption of antioxidant nutrients or that these nutrients can be provided in the form of supplements [12].
Western diets have high calorie levels and a low nutritional quality due to the exacerbated consumption of refined foods, fast foods and industrialized foods. The composition of the foods and the eating differences due to the seasonality of each region impair the assessment of the dietary habits of the population. Thus, methods such as food frequency questionnaires, periodic food recalls, and food weighing, among others, can facilitate this dietary evaluation [13].
In addition, pain can influence eating habits, causing a reduction of appetite and consequent changes in food choices [13]. Studies have demonstrated that a large proportion of patients with chronic pain do not consume daily amounts of vitamins and mineral provided by the intake of fruits, legumes and vegetables [35].
Epidemiological data regarding endometriosis and the diet are scarce [36] Parazzini et al, using the application of a food frequency questionnaire, observed that the intake of fruits, vegetables and legumes is associated with a low risk of developing endometriosis due to the high fiber content of these foods, whereas the consumption of sausage-like meats was identified as a risk factor for the development of the disease [37]. In a case-control study, Trabert et al, observed that an increased number of fruit portions consumed per day was connected to a higher risk of endometriosis, although these authors did not determine a possible link between endometriosis and vegetable consumption [38]. In the present study we did not assess the frequency of each group of foods consumed by women with CPP with and without endometriosis, but we obtained the 24-hour food recall, which does not permit to predict the consumption of vegetables, legumes and fruits by these women as done in the cited studies.
The diet is a potentially modifiable risk factor for endometriosis, which is considered to be a chronic and progressive condition. Few studies have focused on the understanding and the mechanistic relationship of a specific dietary intake with the risk of endometriosis. However, a series of epidemiological studies have considered specific diets for endometriosis in the general population. For example, the consumption of fish rich in fatty acids such as omega 3, and of green vegetables, fruits and dairy products is not associated with an increased risk of endometriosis. In contrast, the intake of red meat, trans fats, saturated fats and dioxins may increase the risk of developing the disease [38-40], the same applying to the intake of alcohol, coffee and a diet rich in saturated fats and in foods exposed to environmental toxins [41].
Fatty acids increase the systemic levels of IL-6 and other inflammatory markers, which are present at higher concentrations in women with endometriosis [40]. Thus, a diet rich in antioxidants may protect women against the development of the disease. However, the link between excess weight, diet and risk of endometriosis continues to be uncertain [42,43]. Dysmenorrhea and pelvic pain can be relieved by the quality of food intake [44], with a possible reduction of inflammation being caused by the consumption of polyunsaturated fatty acids, by a diet poor in saturated fats and by the control of vitamin B1, magnesium, and vitamin E [45]. According to the present data, the mean lipid intake of women with CPP and endometriosis was higher, although we did not determine whether the fatty acids involved were polyunsaturated or saturated.
The mean lipid intake of women with CPP and endometriosis was higher than that of women with CPP without endometriosis, although this difference was not associated with the mean fat percentage of the sample, since the group without endometriosis had a greater percentage and a lower intake of calories from lipids, a fact also observed with respect to total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides in the group without endometriosis. The similarity between the two groups in relation to cholesterol levels and their fractions, is probably because the two groups presents an inflammatory process related to CPD, secondary to endometriosis or secondary to other causes, despite having significantly different lipid intake. Fats may influence the concentrations of prostaglandins, possibly affecting ovarian function [46]. The diet has a strong impact on the elements of the pathogenesis of endometriosis such as oxidative stress, estrogen levels and prostaglandin metabolism [11] since the risk of endometriosis may be increased by exposure to estrogen and a fat-rich diet may increase the circulation of estrogens [47].
Lipid peroxidation, a phenomenon that contributes to the development and progression of chronic diseases with inflammatory characteristics such as endometriosis, can be prevented by the intake of antioxidant nutrients such as vitamins A, C and E, which are also involved in the removal of free radicals and ROS that involve the growth and adhesion of endometrial cells to the peritoneal cavity of women with endometriosis [11]. Other nutrients considered to be antioxidants are flavonoids and carotenoids, zinc, magnesium, manganese, chrome and selenium [48]. The antioxidants are produced by our organism and by enzymes or are absorbed from the diet and from nutrients, which are divided into two groups considered to form a defense line. One group acts by detoxifying the agent before it can cause damage and consists of reduced Glutathione (GSH), Superoxide Dismutase (SOD), catalase, Glutathione Peroxidase (GSH -Px), and vitamin E. The function of the other group is to repair the lesion by means of vitamin C and glutathione reductase (GSH-Rd) [49] According to Bosetti, et al. a diet rich in green vegetables and fruits includes various micronutrients, especially high levels of vitamin C, carotenoids, folic acid, and lycopene, which protect against cell proliferation [50]. The administration of a diet rich in the antioxidant vitamins A, C and E led to a reduction of markers of oxidative stress in patients with endometriosis [51]. Although our study did not assess the food frequency of women with and without endometriosis, our analysis of food intake showed that both groups ingested amounts of vitamin C exceeding daily recommendations, with no difference between groups.
The use of oxidant supplements in order to reduce oxidative stress has been investigated in various studies, but greater elucidation is needed to determine this benefit [51] According to Evans, Halliwel, the function of antioxidants is to equilibrate the system of protection of tissues and body fluids impaired by oxidative stress [52]. Excess oxidative stress may cause a depletion of antioxidants and their supplementation can be beneficial.
Studies have demonstrated an association of the severity of endometriosis with oxidative stress, age and serum vitamin E and lipids. High free radical concentrations occur through the inflammatory process in the pelvic region resulting from oxidative stress, reducing the potential of ROS [11]. In the present study we assessed the dietary intake of vitamin E rather than its serum concentration and observed that both CCP groups (with and without endometriosis) ingested lower vitamin E amounts than recommended by the RDA.
In addition to being studied for its known action on bone metabolism, vitamin D has also been investigated for its anti-inflammatory, immune-modulatory and anti-proliferative effects [13]. In their study, Lasco, Catalano, Benvenga administered a dose of 300,000 IU vitamin D before the menstrual period and observed a reduction of pain and of the use of Non-steroidal Anti-Inflammatory Drugs (NSAIDs) during the period compared to the placebo group, with a better response by patients who had reported a greater intensity of pain before the beginning of the study [53]. In the present study there was no difference in vitamin D intake between the two study groups, although the CPP group without endometriosis ingested expressive quantities of zinc compared to the CPP group with endometriosis.
The present study showed that, although intake was within recommended levels, women with endometriosis ingested a higher quantity of zinc than women without endometriosis. Studies have suggested that intracellular zinc depletion increases the expression of the proinflammatory cytokines Interleukin (IL) -6 and IL-8 via a nuclear pathway dependent on the kappa β factor (NF-β) and other markers of inflammation in vitro and in vivo [33].
Studies have shown a negative association between whole blood zinc concentration and the presence of endometriosis, in agreement with a report showing that mean daily zinc intake is lower in women with endometriosis than in women without the disease [51]. This differs from the present study, in which the group of women with CPP and endometriosis had a higher sink intake than women with CPP without the disease.
In the present study, mean pain level was increased in the CPP group with endometriosis compared to the CPP group without endometriosis. Women with endometriosis have a high concentration of lipid peroxidation markers in blood and peritoneal fluid, which promotes cell adhesion and macrophage activation. Macrophages, in turn, release oxygen and nitrogen, leading to stress formation [13]. The predominance of M2 macrophages indicates an anti-inflammatory action and promotes angiogenesis and tissue restoration, whereas the predominance of M1 macrophages promotes inflammation, inhibiting angiogenesis and tissue remodeling. The greater the quantity of body fat present, the higher the predominance of M1 macrophages, with the possible presence of greater pain [54]. CPP patients without endometriosis have a higher fat percent and, according to the literature, the higher the fat percent, the greater the pain, explaining the fact that, although these patients represent a control group, they are also the group with CPP secondary to other causes, and even the CPP group with endometriosis has a fat percent higher than appropriate levels.
5-Hydroxytryptophan (5-HTP) is a neurotransmitter that plays an essential role in nociception and mood regulation [55] having been associated with central and peripheral regulation of the nociceptive signal [54-56]. Changes in 5-HTP have been reported in patients with chronic pain and the present study showed a difference in mean tryptophan intake according to the food recall analyzed, with the group with CPP and endometriosis showing a greater intake and also a higher pain score compared to the CPP group without endometriosis.
Changes in the serum concentrations of cholesterol and its fractions (LDL - cholesterol and HDL - cholesterol) and of triglycerides may also contribute to the etiopathogeny of endometriosis due to increased oxidative stress, since women with endometriosis have elevated levels of antibodies that are markers of oxidative stress [57-60]. The present study did not show a difference between the groups evaluated, both of which were within SBC recommendations (2017) [30].
Conclusion
Patients with CPP secondary to endometriosis have a greater intake of lipids, zinc and tryptophan than patients with CPP secondary to other causes. However, an in-depth analysis of food consumption is needed for women with endometriosis, since few studies have explored it, mainly in terms of the interaction of minerals and amino acids (tryptophan) with endometriosis, in order to clearly determine whether the changes reported here are relevant and can be of help for treatment or for the reduction of symptoms.
- Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstetrics & Gynecology. 1996; 87: 321-327. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8598948
- Gelbaya TA, El Halwagy HE. Focus on primary care: chronic pelvic pain in women. Obstetrical & Gynecological Survey. 2001; 56: 757-764. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11753178
- Howard FM. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstetrical & Gynecological Survey. 1993; 48: 357-387. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8327235
- Farquhar C. Endometriosis. BMJ. 2007; 334: 249-253. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17272567/
- Simoens S, Hummelshoj L, D Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007; 13: 395-404. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17584822
- Trussel J, Hatcher RA, Cates W Jr, Stewart FH, Kost K. Contraceptive failure in the United States: An update. Stud Fam Plann. 1990; 21: 51-54. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2180135
- Vercellini P. Endometriosis: what a pain it is. Semin Reprod Endocrinol. 1997; 15: 251-261. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8566252
- Amaral VF, Rosa e Silva JC, Nakao LS, Rosa e Silva AC, Ferriani RA. Pelvic endometriosis and systemic oxidative stress measured by serum thiols. Eur J Obstet Gynecol Reprod Biol. 2005; 123: 38.
- Campos Petean C, Ferriani RA, dos Reis RM, de Moura MD, Jordao AA Jr, Navarro PA. Lipid peroxidation and vitamin E in serum and follicular fluid of infertile women with peritoneal endometriosis submitted to controlled ovarian hyper stimulation: a pilot study. Fertil Steril. 2008; 90: 2080-2085. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18249400
- Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008; 90: 247-257. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18672121
- Olechnowicz J, Tinkov A, Skalny A, Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci. 2018; 68: 19-31. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28965330
- Le Floc'h N, Otten W, Merlot E. Aminoacidos. 2011; 41: 1195.
- Sorgdrager FJH, Naude PJW, Kema IP, Nollen EA, Deyn PP. Tryptophan metabolism in inflammation: from biomarker to therapeutic target. Front Immunol. 2019; 10: 2565. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31736978/
- Halpern G, Schor E, Kopelman A. Nutritional aspects related to endometriosis. Rev Assoc Med Bras (1992). 2015; 61: 519-523. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26841161
- Jurkiewicz Przondziono Joanna, Lemm Magdalena, Kwiatkowska Pamula Anna, Ziolko Ewa, Wojtowicz Mariusz K. Influence of diet on the risk of developing endometriosis. Ginekol Pol. 2017; 88: 96-102. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28326519
- Probing STROBE. Epidemiology. 2007; 18: 789-790. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18049189
- Machado RB, Tachotti F, Cavenague G, Maia E. Effects of two different oral contraceptives on total body water: a randomized study. Contraception. 2006; 73: 344-347. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16531163
- Straneva PA, Maixner W, Light KC, Pedersen CA, Costello NL, Girdler SS. Menstrual cycle, beta-endorphins, and pain sensitivity in premenstrual dysphoric disorder. Health Psychology. 2002; 21: 358-367. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12090678
- Fernandez G, Weis S, Stoffel Wagner B, Tendolkar I, Reuber M, Beyenburg S, et al. Menstrual cycle-dependent neural plasticity in the adult human brain is hormone, task, and region specific. J Neurosci. 2003; 23: 3790-3795. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12736349
- Bajaj P, Arendt Nielsen L, Bajaj P, Madsen H. Sensory changes during the ovulatory phase of the menstrual cycle in healthy women. Eur J Pain. 2001; 5: 135-144. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11465979
- Campbell WI, Lewis S. Visual analogue measurement of pain. Ulster Med J. 1990; 59: 149-154. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2278111/
- Kane RL, Bershadsky B, Rockwood T, Saleh K, Islam NC. Visual Analog Scale pain reporting was standardized. J Clin Epidemiol. 2005; 58: 618-623. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15878476
- Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003; 4: 407-414. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14622683
- Botega, Neury Jose Ponde, Milena Pereira Medeiros, Pledson Lima, Manuela Garcia Guerreiro, Carlos Alberto Mantovani. Validation of the hospital anxiety and depression scale (HAD) in outpatients with epilepsy. Brazilian Journal of Psychiatry. 1998; 7: 285-289. http://bit.ly/2RrLrY1
- Sales RL, Silva MMS, Costa NMB, Euclydes MP, Eckhardt VF, Rodrigues CMA, et al. Development of a questionnaire to assess food intake of population groups. Rev Nutr. 2006; 19: 539-552. http://bit.ly/30Sx2ah
- Ribeiro AC, Savio KEO, Rodrigues MLCF, Costa THM, Schmitz BAS. Validation of a food frequency questionnaire for the adult population. Rev Nutr. 2006; 19: 553-562. http://bit.ly/2RMSsSj
- SAS-Statistical Analysis System. Statistical analysis system user´s guide. 2016 Version 9.1. Cary: Statistical Analysis System Institute. http://bit.ly/2GmByEG
- SMARTDATA. Diet smart. Smart Data Solutions.
- Food and Nutrition Board. Institute of Medicine Dietary reference intakes. 2002 Washington (DC): National Academy Press. http://bit.ly/2RrsxQQ
- Padovani, RM, Amaya Farfan, J, Colugnati Fernando Antonio Basile, Domene Semiramis Martins Alvares. Dietary reference intakes: applicability of tables in nutritional studies. Rev Nutr. 2006; 19: 741-760.
- INSTITUTE OF MEDICINE. Dietary reference intakes; the essential guide to nutrient requirements. 2006. Washington (DC): National Academy Press. http://bit.ly/2Gmsccc
- Faludi AA, Izar MCO, Saraiva JFK, Chacra APM, Bianco HT, Afiune Neto A, et al. Update of the Brazilian dyslipidemia and atherosclerosis prevention directive - 2017. Arq Bras Cardiol. 2017; 109: 1-76.
- Loh HH, Law PY. The role of membrane lipids in receptor mechanisms. Annu Rev Pharmacol Toxicol. 1980; 20: 201-234. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6247958
- Tokuyama S, Nakamoto K. Unsaturated fatty acids and pain. Biol Pharm Bull. 2011; 34: 1174-1178. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21804202
- Lai GL, Yeh CC, Yeh CY, Chen RY, Fu CL, Chen CH, et al. Decreased zinc and incresead lead blood levels are associated with endometriosis in Asian Women. Reprod Toxicol. 2017; 74: 77-84. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28889936
- Schatzberg AF. Noradrenergic versus serotonergic antidepressants: predictors of treatment response. J Clin Psychiatry. 1998; 59: 15-18. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9818626
- Wonodi, R. Schwarcz. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in schizophrenia. Schizophr Bull. 2010; 36: 211-218. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20147364
- Meleger AL, Froude CK, Walker J 3rd. Nutrition and eating behavior in patients with chronic pain receiving long-term opioid therapy PM R. 2014; 6: 7-12. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23973502
- Britten P, Cleveland LE, Koegel KL, Kuczynski KJ, Nickols Richardson SM. Updated US Department of Agriculture Food Patterns meet goals of the 2010 Dietary Guidelines. J Acad Nutr Diet. 2012; 112: 1648-1655.
- Parazzini F, Chiaffarino F, Surace M, Chatenoud L, Cipriani S, Chiantera V, et al. Selected food intake and risk of endometriosis. Hum Reprod. 2004; 19: 1755-1759. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15254009
- Trabert B, Peters U, De Roos AJ, Scholes D, Holt VL. Diet and risk of endometriosis in a population-based case-control study. Br J Nutr. 2011; 105: 459-467. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20875189
- Heard ME, Melnyk SB, Simmen FA, Yang Y, Pabona JM, Simmen RC. High-fat diet promotion of endometriosis in an Immunocompetent mouse model is associated with altered peripheral and ectopic lesion redox and inflammatory status. Endocrinology. 2016; 157: 2870-2882. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27175969
- Missmer SA, Hankinson SE, Spiegelman D, Barbieru RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyles. Am j Epidemiol. 2004; 160: 784-954. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15466501
- Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002; 17: 426-431. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11821289
- Carvalho LF, Samadder AN, Agarwal A, Fernandes LF, Abrao MS. Oxidative stress biomarkers in patients with endometriosis: systematic review. Arch Gynecol Obstet. 2012; 286: 1033-1040. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22791380
- Proctor ML, Murphy PA. Herbal and dietary therapies for primary and secondary dysmenorrhea. Cochrane Database Syst. 2001; 3: 21-24. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11687013
- Harel Z, Biro FM, Kottenhahn RK, Rosenthal SL. Supplementation with omega-3 polynsaturated fatty acids in the management os dysmenorrhea in adolescentes. Am J Obstet Gynecol. 1996; 174: 1335-1338. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8623866
- Smith JR, Nakanishi M, Robetorye RS, Venable ST, Pereira Smith O M. Studies demonstrating the complexity of regulation and action of the growth inhibitory gene SD11. Exp Gerontol. 1996; 31: 327-335.
- Gorbach SL, Goldin BR. Diet and the excretion and enterohepatic cycling of estrogens. Prev Med. 1987; 16: 525 -531. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3628202
- Bianchi MLP, Antunes LMG. Free radicals and the main dietary antioxidants. Rev Nutr. 1999; 12: 123-130. http://bit.ly/2TWkahW
- Ferreira ALA, Matsubara LS. Free radicals: concepts, related diseases, defense system and oxidative stress. Rev Assoc Med Bras. 1997; 43: 61-68. http://bit.ly/2Gou8kq
- Bosetti C, Altieri A, La Vecchia C. Diet and environmental carcinogenesis in breast/gynecological cancers. Cur Opin Obstet Gynecol. 2002; 14: 13-18. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11801871
- Mier Cabrea J, Aburto Soto T, Burrola Mendez S, Jimenez Zamudio L, Tolentino MC, Casanueva E, et al. Women with endometriosis improved their peripheral antioxidant markers after application of a high antioxidant diet. Reprod Biol Endocrinol. 2009; 7: 54. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19476631
- Evans P, Halliwel B. Micronutrients: oxidant/antioxidant status. Brit J Nutr. 2001; 67: 74. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11509092
- Lasco A, Catalano A, Benvenga S. Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: results of a randomized, double-blind, placebo-controlled study. Arch Intern Med. 2012; 172: 366-367. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22371927
- Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM, et al. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014; 17: 109. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24013945
- Patetsos E, Horjales Araujo E. Treating chronic pain with SSRIs: What do we know? Pain Res Manag. 2016; 2016: 2020915. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27445601
- Millan MJ. Serotonin (5-HT) and pain: a reappraisal of its role in the light of receptor multiplicity,” Seminars in Neuroscience. 1995; 7: 409-419. http://bit.ly/30Qvei0
- Esfandiari N, Ai J, KHazaei M, Javed M, Gotlieb L, Casper RF. Effect of a statin on an in vitro model of endometriosis. Fertil Steril. 2005; 84: 123. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17097652
- Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci. 2002; 955: 89-100. https://www.ncbi.nlm.nih.gov/pubmed/11949968

Sign up for Article Alerts