Significance of Uterine Cavity Fibroids and Polyps in Reproductive Medicine?
Vincent Y.T. Cheung*
Department of Obstetrics and Gynecology, Queen Mary Hospital, University of Hong Kong, Hong Kong
*Address for Correspondence: Vincent Y.T. Cheung, Department of Obstetrics and Gynecology, Queen Mary Hospital, University of Hong Kong, 102 Pokfulam Road, Hong Kong, Tel: +852-22553914; Fax: +852-25173278; E-mail: vytc@hku.hk
Submitted: 30 December 2016; Approved: 25 January 2017; Published: 07 February 2017
Citation this article: Cheung VYT. Significance of Uterine Cavity Fibroids and Polyps in Reproductive Medicine. SRL Reprod Med Gynecol. 2017;3(1): 004-009.
Copyright: © 2017 Cheung VYT. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Uterus; Fibroids; Endometrial polyps; Assisted reproduction; Ultrasound; Sonohysterography; Uterine artery embolization; High-intensity focused ultrasound
Download Fulltext PDF
Uterine fibroids and endometrial polyps are common lesions in the female genital tract, and often they are asymptomatic. However, if the lesions are occupying and distorting the uterine cavity, they may cause symptoms such as abnormal uterine bleeding, subfertility and pregnancy losses. This review will discuss the impact of uterine cavity fibroids and polyps on subfertility and pregnancy losses and whether the removal of these lesions will improve reproductive outcome.
Lesions occupying the uterine cavity such as endometrial polyps, submucous fibroids, uterine septa, and intrauterine adhesions are frequently seen on an ultrasonographic examination performed for various reasons in asymptomatic patients. The exact nature of these lesions can be determined by various imaging techniques including high-resolution transvaginal ultrasonography and sonohysterography. However, some of those may need to be confirmed by hysteroscopic directed excision. These lesions are found in 10-15% of subfertile women, which cause distortion of the uterine cavity and thus suggested to be one of the causes of subfertility [1]. This review will discuss the impact of uterine cavity fibroids and endometrial polyps on subfertility and pregnancy losses and whether the removal of these lesions will improve the reproductive outcome.
Uterine Fibroids
Uterine fibroids are the most common tumors of the female genital tract, which are found in 20-40% of women [2]. It can be present in any part of the uterine body or cervix and can be confined to the muscle wall (intramural), can form under the endometrial lining (submucosal), or under the serosal covering of the uterus (subserosal). It is estimated that fibroids may be associated with subfertility in 5-10% of women and are possibly the sole cause of subfertility in 2-3% of women [3]. Submucous, and to a lesser extent intramural fibroids are thought to have a negative impact on fertility and implantation [4-6]. Depending on the degree of uterine cavity distortion, fibroids are classified as type 0 if they pedunculate 100% into the cavity; as type I if more than 50% of the mass is within the cavity; and as type II if they have less than 50% of the mass within the cavity [2].
Impact of fibroids on fertility
Since the vast majority of women with fibroids are fertile and asymptomatic, it is clear that the mere presence of one or more fibroid(s) in a woman does not mean that her reproductive capability is compromised. However, fibroids do know to occur more frequently in women with a history of subfertility. Therefore, there is still a great deal of uncertainty as to whether and how the presence of fibroid(s) may affect a women’s fertility. Several mechanisms have been postulated to explain how fibroids can impair fertility:
Anatomical distortion of the uterine cavity: Fibroids that distort the uterine cavity, as in the case of submucosal (type 0) or intramural fibroid partly extended into the cavity (types I and II) can have an adverse effect on implantation [2]. A systemic review had shown that submucosal fibroids may decrease the implantation rates from 11.5% to 3% [5]. Therefore, it was shown that women who have undergone hysteroscopic resection of submucosal fibroids have a significant improvement in pregnancy [6,7]. Also, depending on the location, a submucosal fibroid may obstruct sperm migration from the cervix to the fallopian tube, or cause narrowing or kinking of the fallopian tube.
Implantation: In addition to the distortion of the uterine cavity, endometrium overlying a uterine fibroid is likely to have reduced vascularity, which may lead to implantation failure. However, the precise effects of fibroids on implantation are still uncertain, and studies on these aspects give conflicting results [2,3].
Dysfunctional uterine contractility: Fibroids occupying the uterine cavity can lead to dysfunctional uterine contractility and thereby affect sperm migration, tubal contractility and embryo nidation [3].
Other mechanisms such as changes in endometrial cavity milieu, abnormal uterine vascularization, and chronic endometrial inflammation have been suggested [3,4].
The issue of whether fibroids can be the sole cause of subfertility has been poorly understood. This is because of the lack of prospective, randomized, and controlled studies separating out other subfertility factors. A randomized and prospective study evaluating spontaneous conception in subfertile women with and without fibroids found a significant discrepancy in pregnancy rate for subfertile women (11% with fibroids versus 25% without fibroids) [8]. In a recent consensus statement developed by a group of Australasian subspecialists in reproductive endocrinology and infertility (the ACCEPT group), it was commented that subserosal fibroids did not appear to impact on fertility outcomes; intramural fibroids may be associated with reduced fertility, and submucosal fibroids were associated with reduced fertility. However, the relative effect of the fibroid number and size upon fertility outcomes remains uncertain [9].
Fibroids and assisted reproductive technologies
The potential mechanisms by which fibroids could compromise reproductive function have already been described. It is postulated that uterine fibroids are associated with poorer reproductive outcomes in Assisted Reproductive Technologies (ART), but the evidence to support this is scanty and often controversial [3]. Overall, the evidence supports the concept that submucosal and intramural fibroids which distort the uterine cavity have a negative effect on outcomes of ART [4]. The location of the fibroids is of critical importance in ART outcomes [10]. Submucosal fibroids, in particularly, significantly reduce implantation and pregnancy rates of ART. It has been shown that submucosal fibroids that distort the uterine cavity have been found to carry a relative risk of 0.3 for pregnancy and 0.28 for implantation after ART, compared with subfertile women without fibroids [6,10]. Other authors have also demonstrated reduced success following ART with an odds ratio of 0.3 for conception and 0.3 for delivery in the presence of submucosal fibroids [11]. The effect is not as pronounced for intramural fibroids with an odds ratio of 0.62 for implantation and 0.69 for delivery per transfer cycle [12]. Subserosal fibroids have a negligible impact on fertility with ART [8,11].
Fertility following myomectomy
Myomectomy involves removal of the fibroid(s) with preservation of the uterus and thus fertility. However, the actual effects of myomectomy on fertility remain uncertain [4]. On the other hand, myomectomy is associated with a risk of postoperative adhesion formation, which may result in further compromise of reproductive capacity, chronic pelvic pain, and an increased risk of ectopic pregnancy if conception is achieved. In addition, fibroids have the potential to recur such that 20-25% of women who undergo myomectomy require a secondary procedure [13]. Despite the lack of evidence from randomized studies, it does appear that surgical intervention for uterine fibroids does increase pregnancy rates, with an estimated 50% of women conceiving following myomectomy for fibroid-associated infertility [11]. Studies have shown that removal of fibroids increased the spontaneous pregnancy rate from 25% to 42% [8], which supports the fact that fibroids influence fertility. Myomectomy can be performed abdominally, laparoscopically, and hysteroscopically. The relative effects of laparoscopic versus abdominal myomectomy on fertility outcomes are unknown although a recent multicenter randomized controlled study compared myomectomy performed by laparoscopy or mini-laparotomy found that both techniques were safe, and the size and location of the fibroid(s) were the strongest predictors of surgical outcome [14]. However, several studies had reported that hysteroscopic resection of submucous fibroids leads to improved pregnancy rates [3]. A systemic review also reported that hysteroscopic myomectomy for submucosal fibroid appeared likely to improve fertility outcomes [6]. Since the risks of hysteroscopic myomectomy are relatively few when compared to abdominal or laparoscopic myomectomy, the resection of submucosal fibroids to enhance fertility in a woman with no other obvious cause of subfertility is generally advocated by most reproductive medicine specialists [3]. Also, there is insufficient evidence to determine whether myomectomy for intramural fibroids improves fertility outcomes [9]. A recent Cochrane Database of Systematic Reviews (2012), showed no evidence for a significant effect of myomectomy on clinical pregnancy rate for intramural, submucosal, combined intramural and subserosal and combined intramural and submucosal fibroids, and no difference between laparoscopic and open approach on fertility performance [15]. However, this review needs to be viewed with caution due to the small number of studies with insufficient evidence from randomized controlled trials. On the other hand, hysteroscopic myomectomy seems to increase the odds of clinical pregnancy in women with unexplained subfertility and submucosal fibroids, but the evidence is at present still not conclusive [16].
Fibroids and pregnancy losses
Studies have shown that spontaneous miscarriage rates in the first and second trimesters of pregnancy are higher in women with fibroids [4]. More recent studies suggest that the rates of miscarriage are higher in women with submucosal fibroids [4,9]. The underlying mechanisms that could lead to subfertility, as outlined previously, could also be applicable to how fibroids could cause miscarriage. It seems reasonable to suppose that if implantation occurs on a submucosal fibroid, or on an intramural fibroid located close to and distorted the uterine cavity, could predispose to miscarriage. A miscarriage rate of 60% has been reported with fibroids which were reduced to 24% after myomectomy [17].
Endometrial Polyps
Endometrial polyps and fertility
Endometrial polyps are a common structural uterine pathology, with a wide variation in the reported incidence among subfertile women, ranging from 1.4% to 41%, depending on the methods used for diagnosis and the characteristics of the populations studied [18,19]. However, the relationship between endometrial polyps and subfertility has been a matter of debate. Moreover, it is not clear whether removal of endometrial polyps improves implantation and thus the live birth rate [18]. The mechanism by which endometrial polyps may interfere with implantation is not clear. It is possible that polyps can lead to defective embryo implantation, suggested to be due to altered endometrial receptivity [19] or increased plasma glycodelin concentration [18]. The presence of an endometrial polyp may also induce local inflammatory changes or distort the uterine cavity, thus interfering with normal implantation and embryonic development [18]. Mittal and coauthors [20] also reported that the glands and stroma in endometrial polyps are unresponsive to progesterone stimulation.
The usual practice of removal and histological examination of a polyp in subfertile women is without evidence-based support. It is widely accepted to remove a polyp before commencing any form of fertility treatment, because there is nothing to lose. Particularly, polypectomy has been recommended to women in whom endometrial polyp is the only obvious cause for their subfertility. Some benefit from hysteroscopic polypectomy for women who were trying to conceive naturally were suggested in a small perspective study, with a wide variation in the reported pregnancy and live birth rates [21,22].
It is not clear; however, what is the best treatment option for a polyp identified at the time of controlled ovarian stimulation and prior to fresh embryo transfer. The two options are either to proceed with fresh embryo transfer and consider polypectomy if treatment was unsuccessful or to freeze all embryos, remove the polyp and proceed with fresh embryo transfer three months later.In a retrospective case-control study, Lass, et al. [23] compared implantation and clinical pregnancy rates in women undergoing fresh embryo transfer without removal of the polyp with those who had the polyp removed followed by frozen embryo transfer. Only women with polyps of less than 20 mm were included in the study. Although there was a trend towards higher implantation, clinical pregnancy and live birth rates after polypectomy, the difference was not statistically significant. In a retrospective study involving 83 subfertile women with a history of the menstrual disorder, hysteroscopic polypectomy appeared to improve fertility and pregnancy rates irrespective of the size or number of the polyps [24]. In contrast, data from another retrospective case-control study suggested that polyps less than 1.5 cm diagnosed by ultrasound scan do not require treatment prior to IVF with ICSI as they do not appear to have any negative effects on pregnancy and implantation rates [25]. In a more recent systemic review, favorable effects of polypectomy on pregnancy outcome can be demonstrated in that the hysteroscopic removal of endometrial polyps prior to IUI increases the odds of clinical pregnancy rate [16]. The location of the endometrial polyp may also influence pregnancy rate and fertility outcome. In a retrospective study involving 230 subfertile women undergoing hysteroscopy and polypectomy, the pregnancy rate after surgery was much better for polyps located at the utero-tubal junction (57.4%) than for uterine polyps in the posterior (28.5%), lateral (18.8%) and anterior (14.8%) uterine walls [26].
While there is some evidence to suggest a detrimental effect of polyps on fertility, the evidence is scarce and conflicting. In general, if a polyp is seen prior to commencing ovarian stimulation for assisted reproduction, the woman should be advised for hysteroscopy and polypectomy. If a polyp is suspected during the course of ovarian stimulation prior to fresh embryo transfer, further management should be individualized given the number of embryos created, the previous reproductive history of the patient and the individual clinics’ success rates for their frozen embryo program. Further studies are needed to determine the best treatment option.
Endometrial polyps and pregnancy losses
Anatomic abnormalities account for 10% to 15% of cases of recurrent pregnancy losses and are generally thought to cause pregnancy losses by interrupting the vasculature of the endometrium, and prompting abnormal and inadequate placentation [27]. These anatomic abnormalities include congenital uterine anomalies, intrauterine adhesions, and uterine fibroids or polyps. In a recent study, endometrial polyps were identified form hysteroscopy in 7.9 % of women following miscarriages [28]. Hysteroscopic resection is generally suggested if these anatomic abnormalities are identified in a woman with recurrent pregnancy losses [27].
Ultrasonography
Uterine fibroids
The best imaging technique for visualization of fibroids is using the transvaginal scanning (TVS), with a curvilinear, multifrequency, endocavity transducer with a central frequency of 6.5 MHz. However, if the uterus is significantly enlarged, TVS may fail to visualize the whole uterus and a transabdominal scan is required. The transabdominal scan is performed using a multifrequency curvilinear abdominal transducer with a central frequency of 3.5 MHz.
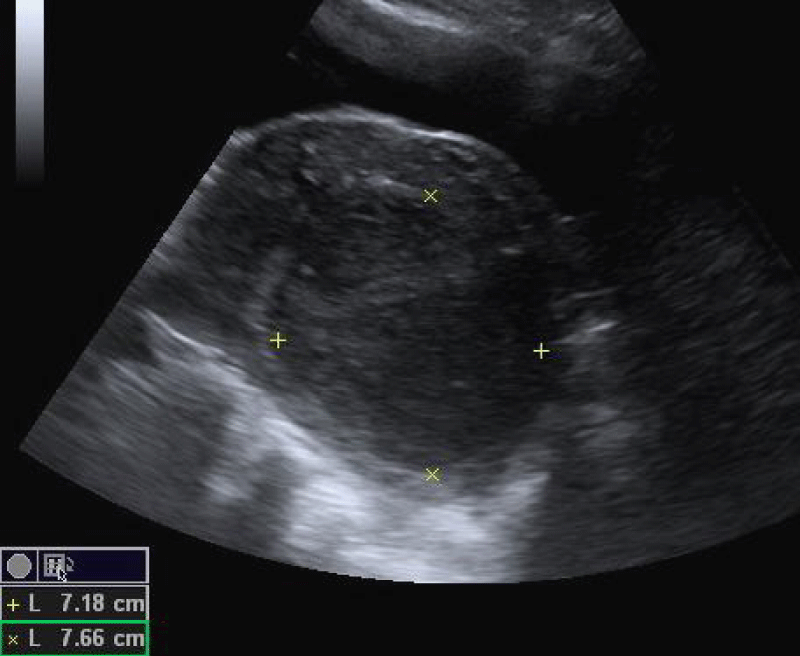
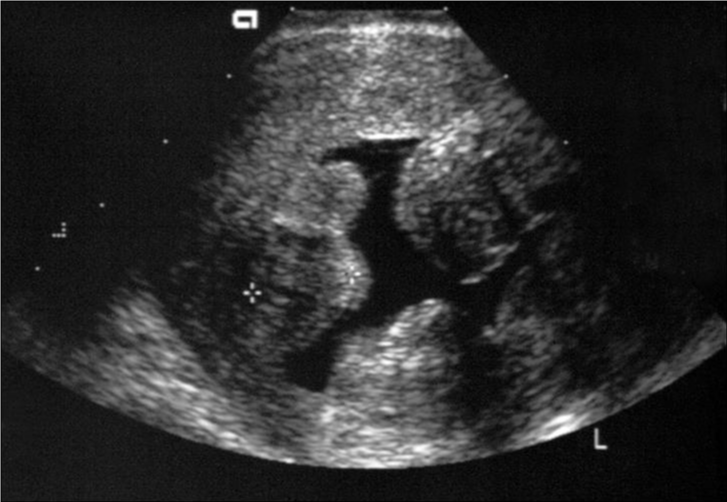
The outline of a fibroid is usually well visualized, because of the presence of the pseudo capsule. Ultrasonographically, fibroids appear as heterogeneous highly attenuating echo pattern. Fibroids are commonly hypoechoic compared with the adjacent myometrium (Figure 1). However, if they undergo fatty or fibrous changes, they can be isoechoic or even hyperechoic. Cystic degeneration can be seen on ultrasound as a central anechoic area, which may sometimes contain internal echoes and fluid levels. Calcification can be identified by the presence of echogenic foci or a bright outer rim, which often produce posterior acoustic shadowing.
Endometrial polyps
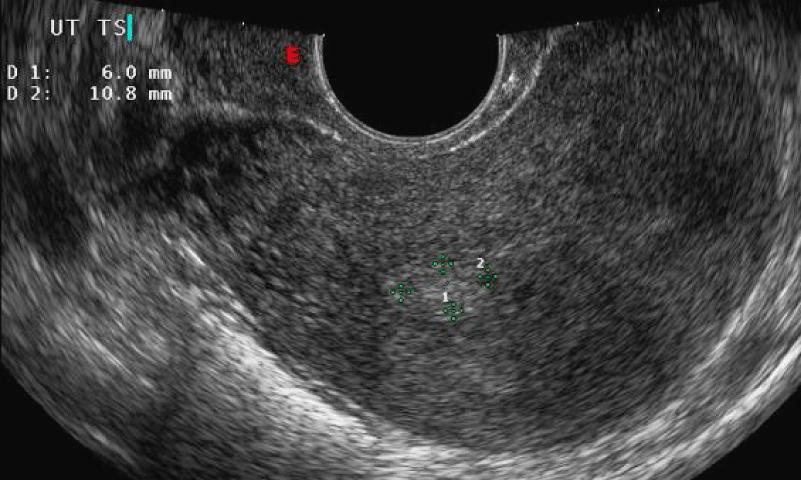
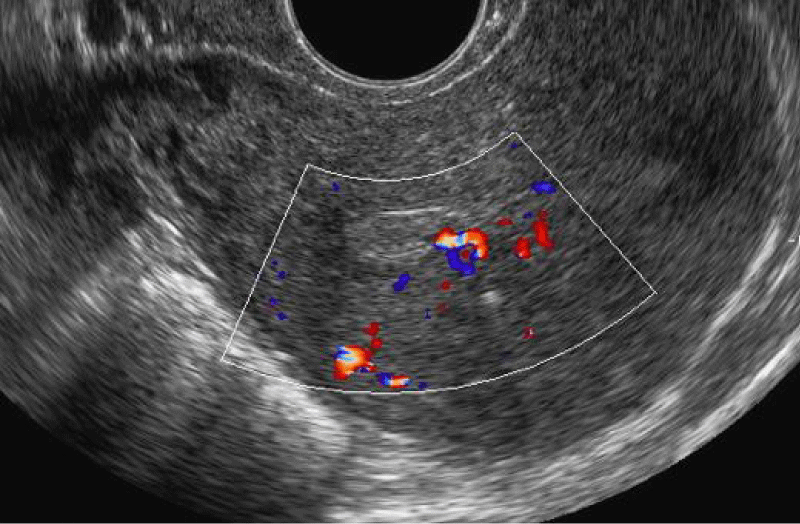
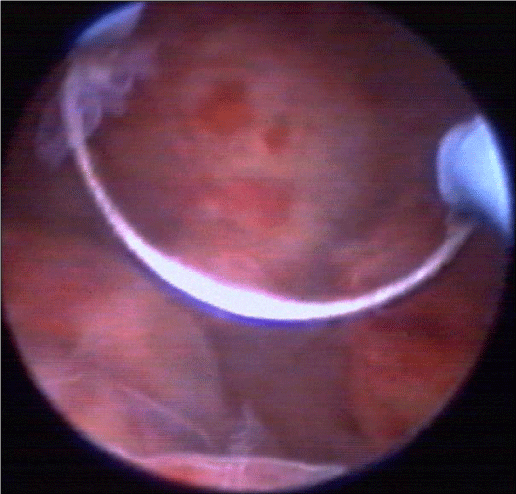
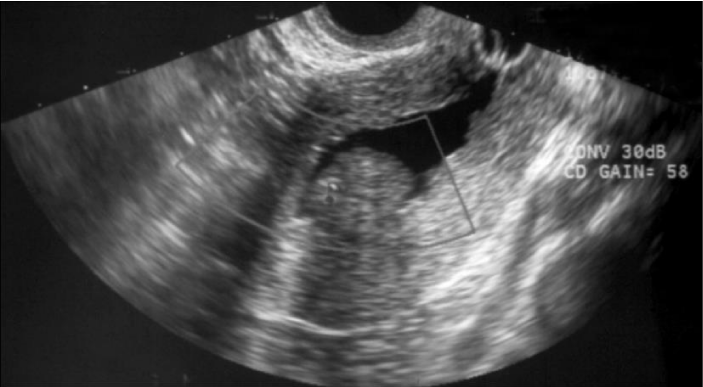
Ultrasonographically, an endometrial polyp should be suspected when ultrasound demonstrates a focal area of endometrial thickening. Endometrial polyps are typically echogenic in appearance (Figure 2), but may appear as complex lesions with echogenic tissue and cystic appearance. Color Doppler imaging of a polyp typically reveals a central feeding vessel (Figure 3), which helps to distinguish that from a submucous fibroid.
3D scanning
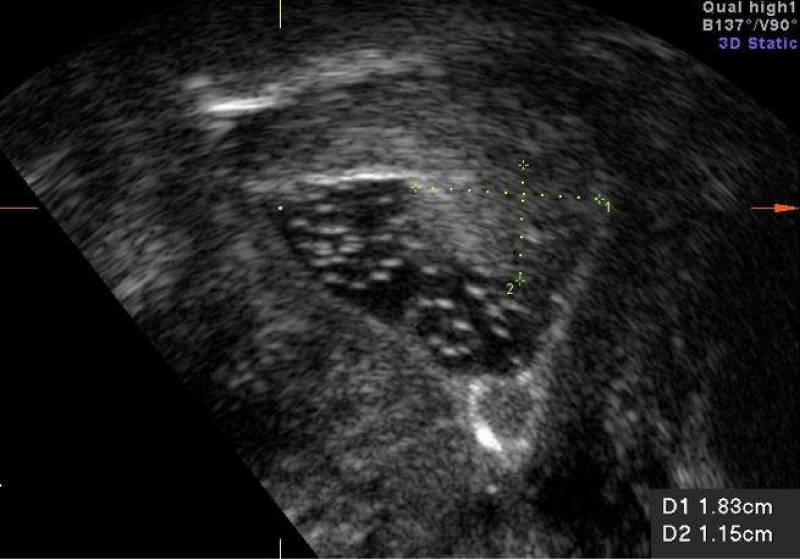
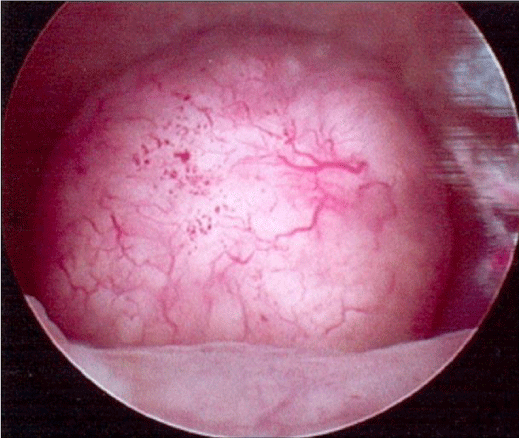
3D scanning demonstrates the uterine cavity and thus the fibroids and the endometrial polyps in the coronal plane. It is particularly useful when assessing the exact location, and size of the fibroid and the polyp, especially when done in conjunction with sonohysterograpy (Figure 4).
Saline / gel infusion sonohysterography
Saline infusion sonohysterography involves the instillation of normal saline into the endometrial cavity followed by transvaginal ultrasound examination of the uterus. It has been widely used in the evaluation of patients with abnormal uterine bleeding. The distension of endometrial cavity by normal saline improves visualization of the endometrial lining and therefore enhances the ability to detect intrauterine pathology compared to conventional transvaginal sonography. Lesions occupying the endometrial cavity such as submucous fibroids (Figure 5A & 5B) and endometrial polyps (Figure 6A & 6B) can be easily and clearly visualized [29]. However, saline infusion sonohysterography has been found to suppress the color signal within the endometrium at color Doppler examination [30]. Recently, the gel has been proposed as an alternative to saline for instillation sonography [31]. Because gel has a high viscosity, gel-instillation sonography is associated with less back flow through the cervix and less transtubal flow than is saline contrast sonohysterography. This results in a more stable filling of the uterine cavity. Also, gel infusion does not seem to affect the power Doppler signal in patients with endometrial polyps.
Color and power Doppler
Color and power Doppler can be a useful tool in the assessment of the blood supply of uterine cavity lesions. It can be used to help distinguish between an endometrial polyp and a submucosal fibroid. The vascular pattern of endometrial polyps is described as a feeding vessel beginning from the base and going through the central part of the endometrium, referred to as the ‘pedicle artery sign’ [32] or as the ‘single-vessel pattern’ [33] (Figure 3). The power Doppler characteristic of submucosal fibroids is described as a ‘rim-like Doppler pattern’, which shows a circular or semicircular vessel surrounding the focal endometrial lesion [34].
Intervention Radiological Treatment
Uterine artery embolization
Uterine artery embolization involves the introduction of a catheter into the uterine artery via the femoral artery. An embolic agent (small synthetic particles) such as microspheres (tris-acryl gelatin) or PVA material (an embolus) is injected to embolize the arterial branches supplying the fibroid. Studies have indicated a potential improvement in fertility after this treatment modality [35]. However, for submucous fibroids which can easily be resected hysteroscopically, this treatment option is generally not recommended.
High-intensity focused ultrasound
High-intensity focused ultrasound therapy has received increasing interest in the management of solid malignancies and benign tumors. In the management of uterine fibroids, high-intensity focused ultrasound induces focal thermocoagulation of the fibroids. Magnetic resonance imaging has been used to define the target for controlling and monitoring the ablation. Recently, sonographically guided high-intensity focused ultrasound has been introduced to monitor the ablation process [36]. Results obtained by various research groups have shown that using sonographically guided high-intensity focused ultrasound for treatment of fibroids is safe, effective, and highly acceptable to patients [37]. A Recent study demonstrated significant shrinkage of submucous fibroid with the alleviation of symptoms using ultrasound-guided high-intensity focused ultrasound [38]. Several reports have suggested an improvement in fertility after high-intensity focused ultrasound treatment due to the resolution of uterine cavity distortion [39,40].
- Bosteels J, Kasius J, Weyers S, Broekmans FJ, Mol BW, D'Hooghe TM. Treating suspected uterine cavity abnormalities by hysteroscopy to improve reproductive outcome in women with unexplained infertility or prior to IUI, IVF, or ICSI. Gynecol Surg. 2013; 10: 165-167.
- Clough A, Khalaf Y. Ultrasonography of uterine fibroids. In: Rizk BRMB, ed. Ultrasonography in Reproductive Medicine and Infertility. New York: Cambridge University Press; 2010. p. 87-96.
- Mukhopadhaya N, Asante GP, Manyonda IT. Uterine fibroids: impart on fertility and pregnancy loss. Obstetrics, Gynaecology and Reproductive Medicine. 2007; 17: 311-317.
- Khaund A, Lumsden MA. Impact of fibroids on reproductive function. Best Pract Res Clin Obstet Gynaecol. 2008; 22: 749-760.
- Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008; 198: 357-366.
- Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009; 91: 1215-1223.
- Shokeir TA. Hysteroscopic management in submucous fibroids to improve fertility. Arch Gynecol Obstet. 2005; 273: 50-54.
- Bulletti C, De Ziegler D, Polli V, Flamigni C. The role of leiomyomas in infertility. J Am AssocGynecolLaparosc. 1999; 6: 441-445.
- Kroon B, Johnson N, Chapman M, Yazdani A, Hart R. Australasian CREI Consensus Expert Panel on Trial evidence (ACCEPT) group. Fibroids in infertility--consensus statement from ACCEPT (Australasian CREI Consensus Expert Panel on Trial evidence). Aust N Z J Obstet Gynaecol. 2011; 51: 289-295.
- Guo XC, Segars JH. The impact and management of fibroids for fertility: an evidence-based approach. Obstet Gynecol Clin North Am. 2012; 39: 521-533.
- Somigliana E, Vercellini P, Daguati R, Pasin R, De Giorgi O, Crosignani PG. Fibroids and female reproduction: a critical analysis of the evidence. Hum Reprod Update. 2007; 13: 465-476.
- Benecke C, Kruger TF, Siebert TI, Van der Merwe JP, Steyn DW. Effect of fibroids on fertility in patients undergoing assisted reproduction. A structured literature review. Gynecol Obstet Invest. 2005; 59: 225-230.
- Nezhat FR, Roemisch M, Nezhat CH, Seidman DS, Nezhat CR. Recurrence rate after laparoscopic myomectomy. J Am AssocGynecolLaparosc. 1998; 5: 237-240.
- Palomba S, Zupi E, Russo T, Falbo A, Marconi D, Tolino A, et al. A multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: short-term outcomes. Fertil Steril. 2007; 88: 942-951.
- Metwally M, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev. 2012; 11: CD003857.
- Bosteels J, Kasius J, Weyers S, Broekmans FJ, Mol BW, D'Hooghe TM. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2015; 2: CD009461.
- Li TC, Mortimer R, Cooke ID. Myomectomy: a retrospective study to examine reproductive performance before and after surgery. Hum Reprod. 1999; 14: 1735-1740.
- Afifi K, Anand S, Nallapeta S, Gelbaya TA. Management of endometrial polyps in subfertile women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2010; 151: 117-121.
- Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity. Fertil Steril. 2011; 95: 2690-2692.
- Mittal K, Schwartz L, Goswami S, Demopoulos R. Estrogen and progesterone receptor expression in endometrial polyps. Int J Gynecol Pathol. 1996; 15: 345-348.
- Spiewankiewicz B, Stelmachów J, Sawicki W, Cendrowski K, Wypych P, Swiderska K. The effectiveness of hysteroscopicpolypectomy in cases of female infertility. Clin Exp Obstet Gynecol. 2003; 30: 23-25.
- Varasteh NN, Neuwirth RS, Levin B, Keltz MD. Pregnancy rates after hysteroscopicpolypectomy and myomectomy in infertile women. Obstet Gynecol. 1999; 94: 168-171.
- Less A, Williams G, Abusheika N, Brinsden P. The effect of endometrial polyps on outcomes of in vitro fertilization (IVF) cycles. J Assist Reprod Genet. 1999; 16: 410-415.
- Stamatellos I, Apostolides A, Stamatopoulos P, Bontis J. Pregnancy rates after hysteroscopicpolypectomy depending on the size or number of the polyps. Arch Gynecol Obstet. 2008; 277: 395-399.
- Isikoglu M, Berkkanoglu M, Senturk Z, Coetzee K, Ozgur K. Endometrial polyps smaller than 1.5 cm do not affect ICSI outcome. Reprod Biomed Online. 2006; 12: 199-204.
- Yanaihara A, Yorimitsu T, Motoyama H, Iwasaki S, Kawamura T. Location of endometrial polyp and pregnancy rate in infertility patients. Fertil Steril. 2008; 90: 180-182.
- Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009; 2: 76-83.
- Cogendez E, Dolgun ZN, Sanverdi I, Turgut A, Eren S. Post-abortion hysteroscopy: a method for early diagnosis of congenital and acquired intrauterine causes of abortions. Eur J Obstet GynecolReprod Biol. 2011; 156: 101-104.
- Cheung VYT. Sonohysterography in the diagnosis of uterine abnormalities. Hong Kong Journal of Gynaecology, Obstetrics and Midwifery. 2002; 3: 98-103.
- Epstein E, Valentin L. Gray-scale ultrasound morphology in the presence or absence of intrauterine fluid and vascularity as assessed by color Doppler for discrimination between benign and malignant endometrium in women with postmenopausal bleeding. Ultrasound Obstet Gynecol. 2006; 28: 89-95.
- Van Den Bosch T, Van Schoubroeck D, Luts J, Bignardi T, Condous G, Epstein E, et al. Effect of gel-instillation sonography on Doppler ultrasound findings in endometrial polyps. Ultrasound Obstet Gynecol. 2011; 38: 355-359.
- Timmerman D, Verguts J, Konstantinovic ML, Moerman P, Van Schoubroeck D, Deprest J, et al. The pedicle artery sign based on sonography with color Doppler imaging can replace second-stage test in women with abnormal bleeding. Ultrasound Obstet Gynecol. 2003; 22: 166–171.
- Alcázar JL, Castillo G, Mínguez JA, Galán MJ. Endometrial blood flow mapping using transvaginal power Doppler sonography in women with postmenopausal bleeding and thickened endometrium. Ultrasound Obstet Gynecol. 2003; 21: 583-588.
- Cil AP, Tulunay G, Kose MF, Haberal A. Power Doppler properties of endometrial polyps and submucosal fibroids: a preliminary observational study in women with known intracavitary lesions. Ultrasound Obstet Gynecol. 2010; 35: 233-237.
- Walker WJ, Bratby MJ. Magnetic resonance imaging (MRI) analysis of fibroid location in women achieving pregnancy after uterine artery embolization. Cardiovasc Intervent Radiol. 2007; 30: 876-881.
- Cheung VY. Sonographically guided high-intensity focused ultrasound for the management of uterine fibroids. J Ultrasound Med. 2013; 32: 1353-1358.
- Ren XL, Zhou XD, Yan RL, Liu D, Zhang J, He GB, et al. Sonographically guided extracorporeal ablation of uterine fibroids with high-intensity focused ultrasound: midterm results. J Ultrasound Med. 2009; 28: 100-103.
- Wang W, Wang Y, Wang T, Wang J, Wang L, Tang J. Safety and efficacy of US-guided high-intensity focused ultrasound for treatment of submucosal fibroids. EurRadiol. 2012; 22: 2553-2558.
- Bouwsma EV, Gorny KR, Hesley GK, Jensen JR, Peterson LG, Stewart EA. Magnetic resonance-guided focused ultrasound surgery for leiomyoma-associated infertility. Fertil Steril. 2011; 96: e9-e12.
- Hanstede MM, Tempany CM, Stewart EA. Focused ultrasound surgery of intramural leiomyomas may facilitate fertility: a case report. Fertil Steril. 2007; 88: 497. e5-7.

Sign up for Article Alerts