When does Hysterectomy Replace Myomectomy in Benign Uterine Pathology??
L. Mettler* and I. Alkatout
Department of Obstetrics & Gynecology, University Hospitals Schleswig-Holstein, Campus Kiel, 24105 Kiel, Germany
*Address for Correspondence: Liselotte Mettler, Department of Obstetrics & Gynecology, University Hospitals Schleswig-Holstein, Campus Kiel, 24105 Kiel, Germany, Tel: +49 431 500 21450; E-mail: lmettler@email.uni-kiel.de
Submitted: 06 December 2016; Approved: 20 April 2017; Published: 28 April 2017
Citation this article: Mettler L, Alkatout I. When does Hysterectomy Replace Myomectomy in Benign Uterine Pathology? SRL Reprod Med Gynecol. 2017;3(1): 010-019.
Copyright: © 2017 Mettler L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Etiology of fibroids; Laparoscopic myomectomy; Laparoscopic hysterectomy
Download Fulltext PDF
Uterine fibroids are the most frequent benign tumors of the female genital tract. Fibroids are associated with a variety of clinical problems, e.g. pain, bleeding disorders, bulk-related symptoms or infertility. For women wishing to preserve their uterus, fibroids can be surgically removed by hysteroscopy, laparoscopy or laparotomy. While hysterectomy remains the only definitive solution, many alternative treatment possibilities of uterine preservation are available today. The indication for treatment has to be taken carefully after weighing up alternative treatment methods, such as expectant management, medical treatment or interventional radiologic methods, and after obtaining informed consent. The optimal method of treatment takes into account the patient’s interests and wishes and the practical feasibility in the clinical setup. Surgical skills and experience play an important role as surgical procedures on the uterus are not without risk and can lead to severe complications. The decision to operate anticipates an improvement of the initial situation and, therefore, the ideal surgical approach is of utmost importance.
Introduction
One of the multiple treatment possibilities for myomasis hysterectomy which can be performed as Total Laparoscopic Hysterectomy (TLH) or Subtotal Laparoscopic Hysterectomy (SLH) (Figure 1). As it is a less invasive procedure, patients with myomas can consider a subtotal approach (SLH); however, only Total Laparoscopic Hysterectomy can protect 100% from new fibroid formations, avoid later sarcoma formation, uncontrolled bleedings or any other problems arising from the uterus.
The etiology of fibroid formation remains unclear in spite of numerous theories. Nevertheless, a genetic disposition must be given, as Africans have a much higher frequency of multiple myomas than Caucasians. Although certain up- and down-regulations in the genes of patients with and without myomas have been described, no clear strategy for the prevention of fibroids is available. It is known that patients who have close relatives with fibroids are more likely to develop fibroids themselves. In some patients, fibroids are clustered with other disorders. Hereditary leiomyomatosis and renal cell carcinoma syndrome is a rare syndrome involving fibroids. Individuals with the gene that leads to both fibroids and skin leiomyomas are at increased risk for a rare case of kidney cell cancer (papillary renal cell carcinoma).
Understanding which genes are involved in fibroids does not automatically tell us why fibroids develop or how to control them. From our understanding of fibroid behavior, we would guess that genes involved in estrogen or progesterone production, metabolism, or action would be involved. Unfortunately, science is seldom that straightforward. Most guesses regarding these “candidate genes” turn out to be wrong and many studies are usually required to find out how these genes lead to disease. There are also small variations called polymorphisms in genes that may play a role in influencing the risk of fibroids. Both polymorphisms and mutations are changes in the sequence of genes, but the difference is in the degree of change. A mutation makes a major change in the gene that leads to a change in the protein the gene is coding for, e.g. it can change the amino acid from alanine to glycine or cause the protein to be prematurely cut off.
Evidence for the role of genes in fibroid development and growth
Studies of women with fibroids suggest several reasons to suspect that genes play a role in fibroid formation [1,2]. The first is that both women in a pair of identical twins are twice as likely to have had a hysterectomy as both women in a pair of fraternal (non identical) twins. Identical twins share 100 percent of their genes, while fraternal twins share only 50 percent of their genes. This suggests that the genes that identical twins share make them more likely to form fibroids, since both identical and non identical twins have equal exposure to environmental factors. This difference between identical and fraternal twins has been observed in a general population of women undergoing hysterectomy and a population of women with fibroids leading to hysterectomy [3,4].
There is also evidence that women who have close relatives, such as a mother or sister, with fibroids are much more likely to have fibroids themselves [5,6]. This propensity is called familial aggregation. Just as with breast cancer, if you have many relatives affected by fibroids, your risk of disease is likely to be increased.
Molecular genetics and genome wide scan for fibroid genes
In the age of molecular genetics, we can look for genes involved in a disease which is effectively looking for a needle in a haystack. This process is called a genome-wide scan. This is a common approach to finding genes in complex diseases, such as diabetes, asthma, and heart disease. With a genome-wide scan, women who are sisters and both have fibroids (an affected sibling pair) are recruited to participate in the study. Their DNA is studied for common genes. If hundreds of women are studied, each region of every chromosome can be examined, and it can be determined which genes are shared by the sisters who share the fibroid phenotype but are different in many other respects. This approach often produces novel genes that were not previously thought to be involved in the disease process [7-10].
Microscopic facts and fibroid viability
Fibroids are composed primarily of smooth muscle cells. The uterus, stomach and bladder are all organs made of smooth muscle. Smooth muscle cells are arranged so that the organ can stretch, instead of being arranged in rigid units like the cells in skeletal muscle in arms and legs that are designed to “pull” in a particular direction. In women with fibroids, tissue from the endometrium typically looks normal under the microscope. Sometimes, however, over sub mucosal fibroids there is an unusual type of uterine lining that does not have the normal glandular structures. The presence of this abnormality, called aglandularfuntionalis (functional endometrium with no glands), in women having bleeding disorders is sometimes a clinical clue for their doctors to look more closely for a sub mucosal fibroid [11]. A second pattern of endometrium, termed chronic endometritis, can also suggest that there may be a sub mucosal fibroid, although this pattern can also be associated with other problems such as retained products of conception and various infections of the uterus. Once we move beyond hysterectomy as a one-size-fits-all solution to fibroids, distinctions in size, position, and appearance will likely be important for treating fibroids. Once we understand these issues, we may be able to tell why some women have severe bleeding and other women with a similarly sized fibroid have no problem.
Costs of Fibroids
In fact, accurately capturing all the costs attributable to uterine fibroids will help us move toward more, and more effective, innovative therapies. When deciding whether or not to launch a new concept, companies typically look at the amount currently spent for other treatments. The economics of fibroids has chiefly been discussed in terms of the health care costs of hysterectomy. This in itself is a huge amount of money. According to a recent estimate, in the United States, more than $2 billion every year is spent on hospitalization costs due to uterine fibroids alone [12]. Additionally, one study estimates that the health care costs due to uterine fibroids are more than $4,600 per woman per year [13].
When you incorporate all the costs of fibroids, however, the total is even more significant.
• The costs of myomectomy, Uterine Artery Embolization (UAE), and other minimally invasive therapies
• The costs of birth control pills and other hormonal treatments to control bleeding
• The costs of tampons, pads, and the adult diapers many women require to contain the bleeding
• The costs of alternative and complementary therapies
• The cost of doing nothing (for many women this means missing work or working less productively during their period).
Why should a patient have a hysterectomy today when so many alterative treatment options are available? Firstly, up to a certain size of the enlarged uterus, laparoscopic subtotal hysterectomy completely solves the problem and if women want to eliminate every risk of recurrent fibroids, hysterectomy is their only choice. Secondly, hysterectomy also cures coexisting problems, such as adenomyosis, endometriosis, endometrial polyps or cervical dysplasia. There is also no danger of ever leaving a sarcoma behind.
Review of all uterine-preserving treatment possibilities for fibroids (medical and surgical options)
The surgical treatment of fibroids can be differentiated between less invasive and more invasive surgical techniques. Time and type of treatment have to be chosen individually and are dependent on the patient and the treating gynecologist (Tables 1,2).
Expectant management
Wait-and-see is a possibility if patients are asymptomatic, decline medical or surgical treatment or have contraindications to any kind of treatment. However, existing data describe the possibility that fibroids can shrink substantially either by optimizing endocrinological disorders, such as hypothyroidism, or during the postpartum period [14,15].
To pursue the idea of expectant management, the pelvic mass must definitely be classified as a fibroid and differentiated from an ovarian mass. The complete blood count should be regular, especially in patients with severe symptoms, such as menorrhagia or hypermenorrhea. The women must also be informed that the risk of miscarriage, premature labor and delivery, abnormal fetal position and placental abruption is increased [16].
Medical therapy
The benefit of medical treatment in the management of women with symptomatic fibroids is still difficult to prove. Medical therapy can provide adequate symptom relief, especially in cases where hypermenorrhea is the leading problem. The benefit of symptom improvement decreases in long-term treatment periods so that more than 50% undergo surgery within two years. Ulipristalacetate in an application of 5 mg daily proved to be a satisfying treatment in many clinical studies [17].
Nevertheless, there has been a shift in traditional thinking that medical treatment of fibroids is solely based on the manipulation of steroid hormones. A deeper analysis and understanding of specific genes or pathways associated with leiomyomatosis may open new possibilities for prevention and medical treatment [18].
Alternative treatment methods
If the patient does not want to undergo surgery or there are contraindications to surgery, there are alternative procedures:
Uterine artery embolization: This minimally invasive therapeutical option allows an occlusion of the specific arteries supplying blood to the fibroids. A catheter is introduced via the femoral artery under local anesthesia and particles are injected to block the blood flow to the fibroid. This can be an effective treatment option if the uterus should not be removed, surgery is contraindicated and family planning is completed. It results in myoma shrinkage of up to 46%. Nevertheless, there is still a significant rate of post interventional complications [19,20].
Magnetic resonance-guided focused ultrasound: This is a more recent treatment method for uterine fibroids in premenopausal women. Again, the patients must have completed their family planning. In a noninvasive thermo ablative technique multiple waves of ultrasound energy are converged on a small volume of tissue, resulting in maximal thermal destruction. The limiting factors are size, vascularity and access [21,22].
Uterine preserving surgical treatment of fibroids
Indications: Surgical treatment of fibroids is still the main pillar in the treatment of leiomyomas. Hysterectomy is the only definitive solution and can be performed as supracervical or total hysterectomy. Myomectomy performed by hysteroscopy, laparoscopy, abdominal access or with robotic assistance is an alternative surgical method.
Indications for surgical therapy of uterine fibroids are table 1:
1. Abnormal uterine bleeding disorders (hypermenorrhea, dysmenorrhea, menorrhagia- and metrorrhagia)
2. Bulk-related symptoms
3. Primary or secondary infertility and recurrent pregnancy loss.
Counseling and informed consent: Patients undergoing an operative procedure have to be informed of the risks and potential complications as well as alternative operating methods. Counseling before surgery should include discussion of the entry technique and the associated risks: injury of the bowel, urinary tract, blood vessels, omentum and other surrounding organs and, at a later date, wound infection, adhesion-associated pain and hernia formation.
Counseling needs to integrate the individual risk dependent on the BMI of the patient. Depending on the medical history, it is important to consider anatomical malformations, number of vaginal births, midline abdominal incisions, a history of peritonitis or inflammatory bowel disease [23].
Myomectomy: Myomectomy is a surgical treatment option for women who have not completed their family planning or who wish to retain their uterus for any other reasons. The enucleation of fibroids by any method is an effective therapy for bleeding disorders or displacement pressure in the pelvis. Nevertheless, the risk of recurrence remains after myomectomy. Furthermore, if any other pathologies might be causative or only co-causative for the symptoms (such as adenomyosis uteri), these problems will persist [24]. Enucleated myomas and pregnancy - related complications have been investigated extensively. All operating possibilities, especially laparoscopic versus laparotomic, but recently also laparoscopic versus robotic - assisted myomectomy have been evaluated. Uterine rupture or uterine dehiscence is rare and occurs in only 2% - 4% of laparoscopic cases and even less seldom in robotic - assisted and laparotomic cases. Careful patient selection and secure preparation and suture techniques appear to be the most important variables for myomectomy in women of reproductive age [25,26]. Uteri with multiple fibroids have an increased number of uterine arterioles and venules. Therefore, myomectomy can lead to a significant blood loss and corresponding arrangements should be made [27].
Hysteroscopic myomectomy: Sub mucosal fibroids have their origin in myometrial cells underneath the endometrium and represent about 15 to 20% of all fibroids. Before the establishment of hysteroscopy as a minimally invasive and effective treatment method, these myomas were removed by hysterotomy or even hysterectomy. Increased surgical training, improvement of technology and the widespread use of hysteroscopic myomectomy have made it a safe, fast, effective and cheap method of fibroid resection while preserving the uterus [28].
Patient selection concentrates on intra cavitary sub mucous and some intramural fibroids. More than 50% of the fibroid circumference needs to be protruding into the uterine cavity. Deep myometrial leiomyomas require advanced operative skills and have an increased risk for perioperative complications and incomplete resection. The depth of myometrial penetration correlates with the volume of distension fluid absorbed [29,30]. Few data are available on the size of myoma that prevents the use of the hysteroscopic approach. The European Society of Hysteroscopy suggests to limit the myoma size to 4 cm but the few existing data report a significant increase of complications in fibroids that are > 3cm. Surgical skills determine the size and number of myomas that can be resected [31].
Prior to hysteroscopy, knowledge of the patient’s medical history is important e.g. history of caesarean section or any other reason to expect an anatomical disorder. A vaginal ultrasound scan must be performed to precisely determine the uterus location, size and all cervical and uterine pathologies [32]. If available and feasible, fluid hysteron - sonography should be performed to better differentiate the relationship of leiomyoma to the endometrial cavity and the myometrium. No prophylactic antibiotic is required to prevent surgical site infection.
The first step is the dilation of the cervical channel with Hegar dilators up to Hegar 9. The most commonly used instrument for fibroid resection is the monopolar or bipolar wire loop. Using a monopolar device the fluid medium must be non-electrolytic, using a bipolar device the fluid medium is isotonic [33]. A continuous flow allows the clearance of blood out of the uterine cavity to improve visualization. Furthermore, the resected pieces can be retracted. Nevertheless, the surface of the myoma and the time needed for resection increase the risk of excessive fluid absorption [34].
The resectoscope is inserted through the cervix into the uterine cavity and after distension with fluid the uterine cavity is carefully inspected. The monopolar resectoscope requires a cutting current of 60 to 120 watts. Bipolar resectoscopes offer the possibility of simultaneous cut and coagulation. The wire loop passes easily through the tissue. The incision starts at the highest point of the myoma. Only in pedunculated fibroids might the incision cut the peduncle first. The loop is then moved towards the surgeon using the spring mechanism and simultaneously the entire resectoscope is gently pulled backwards. The wire loop must be in view of the surgeon during the whole procedure. This motion is repeated until the whole myoma has been resected and the surrounding myometrium (depth) and endometrium (side) can be differentiated. All resected specimen is sent to the pathologist. In cases of heavy bleeding and reduced vision the endometrium and the cutting surface have to be reinspected. These areas can be desiccated with the coagulating current.
The resected area will be recovered by the surrounding endometrium during the following weeks. The complication rate is low (0.8% – 2.6%) [34,35]. Complications that can occur, especially after extensive resection, are uterine perforation or excessive fluid absorption. Absorption of distension fluid might result in hyponatremia or volume overload [36]. The recurrence rate is about 20% in a follow - up period of more than 3 years [31].
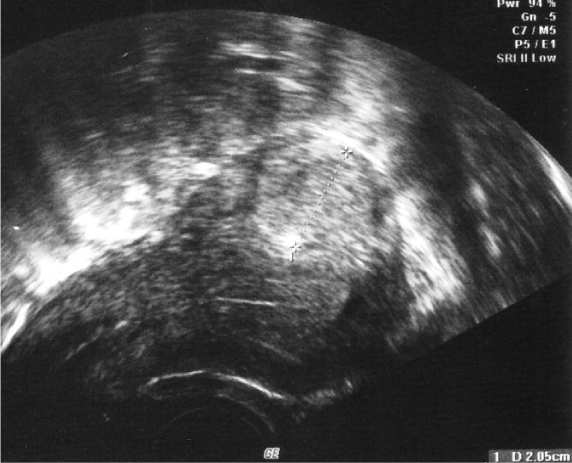
Laparoscopic myomectomy: With the improvement of laparoscopic techniques and skills, myomectomy can be performed laparoscopically in most women. The laparoscopic approach is usually used for intramural or subserosal fibroids. The main advantage compared to abdominal myomectomy is decreased morbidity and a shorter recovery period. Nevertheless, laparoscopic myomectomy is limited by surgical expertise and especially laparoscopic suturing skills [37,38]. Selection criteria for laparoscopic myomectomy are location, size and number of fibroids. Nevertheless, these characteristics are variable in relation to the surgical expertise. Preoperative imaging is performed by vaginal ultrasound to assess the precise features of the leiomyomas [32,39-41].
Laparoscopic myomectomy starts with the usual placement of ports and trocars. After placement of the initial port in the umbilicus, two ancillary trocars are placed in the lower abdomen about 2 cm medial of each iliac crest [41-43]. Myomectomy can lead to severe bleeding that will complicate the procedure due to reduced vision. Vessel bleeding is controlled by bipolar electrosurgical paddles. Intraoperative bleeding can be reduced using vasopressin or other vasoconstrictors. Vasopressin is diluted (e.g. 20 units in 100 ml of saline) and injected into the planned uterine incision site. Vasopressin constricts the smooth muscle in the walls of capillaries, small arterioles and venules. Nevertheless, due to side effects the surgeon should pull back the plunger of the syringe before insertion to check that the needle is not inserted intravascularly [44-46]. Alternatively, misoprostol can be administered vaginally about one hour before surgery to reduce blood loss [47].
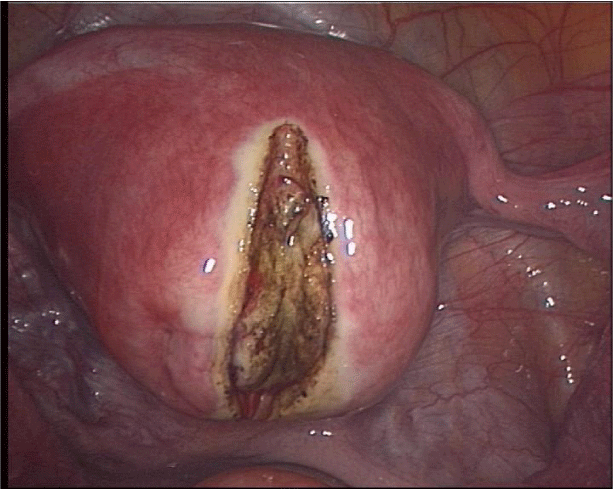
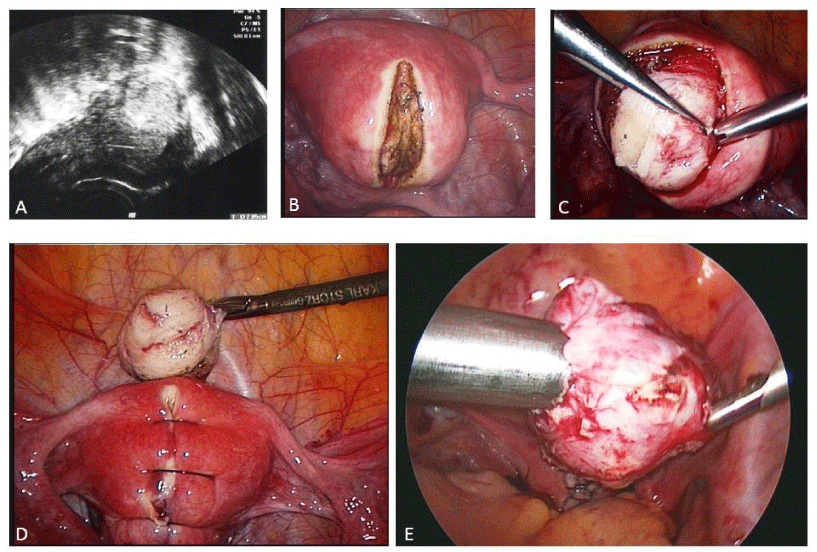
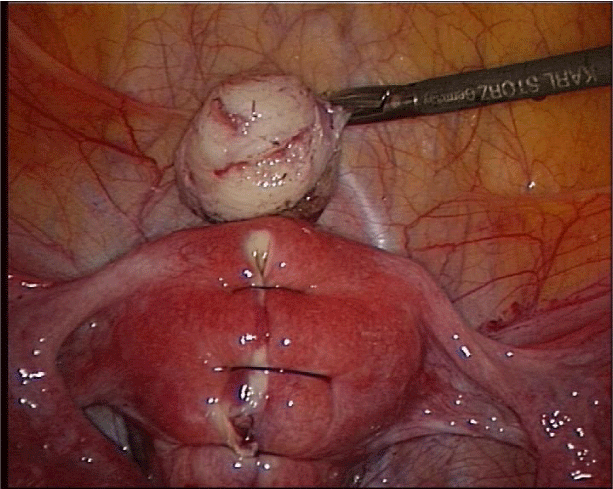
The uterine incision is preferably made vertically as this allows a more ergonomic suturing of the defect. The incision is performed with a monopolar hook directly over the fibroid and carried through deeply until the entire myoma tissue has been reached. After exposure of the myoma, it is grasped with a tenaculum or sharp forceps and traction and countertraction are applied. The removal of the myoma can easily be performed with blunt and sharp dissecting devices. Capsular vessels should be coagulated before complete removal of the myoma as coagulation becomes more difficult if traction is unsuccessful and bipolar coagulation occurs in the remaining myometrium wall. Subsequent to removal, the myoma is morcellated with an electromechanical device under direct vision and at a safe distance to all structures, such as the small bowel, to avoid inadvertent injury. The myoma tissue is removed and sent for pathologic evaluation. The uterine defect is closed with delayed absorbable sutures in one or two layers, depending upon the depth of the myometrial defect. It is important that the suture starts at the deepest point to avoid any cavity that might lead to a weak uterine wall. Furthermore, we tie the knot extra corporeally so that the knot can be pushed into the deep layers with full strength (Figures 1-4). Alternatively, barbed sutures, such as V-lock, can be used to tighten the tissue or a third ancillary trocar can be inserted to hold the suture tight. The security of the uterine closure has bearing on the risk of uterine rupture in subsequent pregnancy. Different kinds of adhesion prevention barriers can be applied [48-50]. Women should wait at least 4 to 6 months before attempting to conceive [51].
Abdominal myomectomy: Abdominal or open myomectomy has its origin in the early 1900s as a uterus- preserving procedure. Today, it is mostly performed for women with intramural or subserosalmyomas and less frequently for sub mucosal localization. Since the introduction of endoscopic procedures, the indication for abdominal myomectomy has become rare. It becomes an option if hysteroscopic or laparoscopic myomectomy is not feasible or if a laparotomy is required for any other reason. The indication to exclude uterine sarcomas has to be taken very strictly; however, uterine sarcoma is a very rare malignancy and the rate of sarcoma after clinical diagnosis of myoma is very low. The risk of severe complications in association with open surgery is higher than with hysteroscopic or laparoscopic moymectomy. Prophylactic antibiotics should be given for any abdominal fibroid operation [52,53]. After the Pfannenstil incision either a vertical or transverse uterine incision is performed [54]. The myoma enucleation is performed by traction on the myometrial edges, e.g. with Allis clamps. After exposure of the fibroid it can be extirpated. The pseudo capsule is typically dissected bluntly. The uterine defects are closed with sutures in several layers to reapproximate the tissue and achieve hemostasis without excessive bipolar coagulation.
Robotic myomectomy: Robot-assisted myomectomy is a relatively new approach. The advantages of robotic surgery are three-dimensional imaging, mechanical improvement, including 7 degrees of freedom for each instrument, stabilization of the instruments within the surgical field and improved ergonomics for the surgeon. Technical difficulties are decreased as suturing is easier than during conventional laparoscopy; however, there are few data comparing robot-assisted with conventional laparoscopic myomectomy [55-57]. The advantages compared to abdominal myomectomy are decreased blood loss and shorter recovery time. Nevertheless, operation duration and operating costs are much higher than for conventional procedures. Furthermore, robotic devices are large and bulky. Robotic surgery is limited by the lack of tactile feedback and additional team training is necessary to minimize the risk of mechanical failure [58]. To date no advantage compared to conventional laparoscopy could be demonstrated regarding blood loss or operative duration. A more secure myometrial closure has not yet been proven [56]. In obese patients robot-assisted surgery might be beneficial [59].
Hysterectomy as Treatment for Myomas
As fibroids are the most common indication for hysterectomy (30% of hysterectomies in white and 50% of hysterectomies in black women), specific focus is given to hysterectomies within this article. The decision for a hysterectomy in a multifibroid uterus depends on the wish of the patient, her health status and whether childbearing has been completed. Only if the patient suffers from metrorrhagia does the disorder need to be examined pre-operatively in more detail as this may be a sign of endometrial cancer or sarcoma. The combined evaluation of MRI and tumor makers pre-operatively leads to a more specific diagnosis of rapidly growing uterine masses or adnexas in the case of a leiomyomatous uterus or adnexal tumors. Only in those cases where malignancy is not suspected is a simple TLH or SLH recommended, otherwise an oncological approach has to be selected. Hysterectomy, as TLH or SLH, is recommended for the following indications:
- Acute hemorrhage with non-response to other therapies
- Completion of family planning and current or increased future risk of other diseases, such as cervical intraepithelial neoplasia, endometrial hyperplasia or an increased risk of uterine or ovarian cancer. Precondition for the indication for hysterectomy is that these risks can be eliminated or decreased by hysterectomy.
- Failure of previous treatment.
- Completion of family planning and significant symptoms (e.g. multiple fibroids or adenomyosis) and the desire for a definitive solution.
The main advantage of hysterectomy over all other therapeutical possibilities is the definitive solution in eliminating all existing symptoms and the risk of recurrence. Nevertheless, the advantage of a definitive solution that allows freedom from future problems can be an obstacle if family planning has not been completed or the patient has a personal inhibition against the removal of the central genital female organ [60]. These issues must be discussed in advance with the patient before the decision for a hysterectomy is taken. Furthermore, for a solitary sub mucous, subserous, pedunculated or intramural myoma, the complication rate of a hysterectomy has to be compared with the complication rate of a myomectomy. The operational risks have to be compared to the operational risks of hysteroscopy, laparoscopic enucleation or conservative management. With the advances in cervical cancer screening the prevention of future cervical or uterine pathologies is no longer a relevant indication for hysterectomy. The decision must be tailored to meet the needs of each individual patient.
Laparoscopic hysterectomy was first introduced in 1989 with the aim of reducing the morbidity and mortality of abdominal hysterectomy to the level reached with vaginal hysterectomy. Laparoscopic assistance for vaginal hysterectomy can be of advantage if there is a need for adhesiolysis, a need to treat endometriosis simultaneously, a need to treat large leiomyomas and to ensure an easier and safer adnexectomy. If feasible, vaginal hysterectomy allows a more rapid and less painful recovery than open or laparoscopic surgery and is much cheaper [61].
Removal of ovaries and/ or fallopian tubes
Generally, the ovaries are not removed when a hysterectomy is performed for uterine fibroids. Removing the uterus alone will cure the bleeding and the size-related symptoms caused by the fibroids. When treating fibroids it is not necessary to remove the ovaries as is sometimes the case when treating other diseases, such as endometriosis or gynecologic cancers.
According to new research presented at the Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in 2013, bilateral salpingectomy at hysterectomy, with preservation of the ovaries, is considered a safe way of potentially reducing the development of ovarian serous carcinoma. Removing the fallopian tubes does not cause the onset of menopause, as does the removal of the ovaries. Furthermore, prophylactic removal of the fallopian tubes during hysterectomy or sterilization would rule out any subsequent tubal pathology, such as hydrosalpinx, which is observed in up to 30% of women after hysterectomy. Women undergoing hysterectomy with retained fallopian tubes or sterilization have at least double the risk of subsequent salpingectomy. Removal of the fallopian tubes at hysterectomy should therefore generally be recommended [62].
Should the ovaries be removed or left in place?
Many physicians were taught that at a set age (which varies between 35 and 50) women should be told that removal of the ovaries is recommended as part of the surgery, with the speculation of “while we are there, we may as well.” The general teaching had been that ovaries have no function after menopause and the risk of ovarian cancer increases with increasing age, so removing the ovaries near the time of menopause was a no-lose proposition. This was especially true if hormone replacement therapy could be used to help younger women transition to the time when they would naturally go through menopause.
However, more recent research suggests that although after menopause the ovaries produce little estradiol (the major estrogen in premenopausal women), they make a tremendous amount of androgens (usually thought of as male hormones) [63]. It is thought that these androgens may be important in maintaining mood and sex drive [64-66]. In addition, the risks of hormone replacement have become clearer, and many women choose to use hormones following menopause [67,68]. (Most women are aware that there has been research form the Women`s Health Initiative demonstrating significant complications with postmenopausal hormone replacement therapy. However, it is not widely known that the risks are lower for women without a uterus, who are able to take estrogen alone [68]. Recently the association of premature loss of ovarian function and the increasing risk of heart disease has also been explored [69].
Considering all these factors, there are good reasons to retain the ovaries if possible. The major reason to remove them at the time of fibroid surgery is if the woman has a high risk of ovarian cancer.
Should the fallopian tubes be resected in cases of hysterectomy?
Once the reproductive function is completed, the tubes of a female should be removed within the reproductive age while ovaries need to stay to support the female wellbeing. Beyond the reproductive age, the fallopian tubes should always be removed with the uterus while ovaries are routinely removed only above the age of 65 years.
According to new research presented at the 2013 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists, bilateral salpingectomy, the removal of both fallopian tubes during hysterectomy while preserving the ovaries, is considered a safe way of potentially reducing the development of ovarian serous carcinoma, the most common type of ovarian cancer. Increasing evidence points toward the fallopian tubes as the origin of this type of cancer. Removing the fallopian tubes does not cause the onset of menopause, as does removal of the ovaries.
Prophylactic removal of the fallopian tubes during hysterectomy or sterilization would rule out any subsequent tubal pathology, such as hydrosalpinx, which is observed in up to 30% of women after hysterectomy. Moreover, this intervention is likely to offer considerable protection against later tumor development, even if the ovaries are retained. Thus, we recommend that any hysterectomy should be combined with salpingectomy. Women undergoing hysterectomy with retained fallopian tubes or sterilisation have at least a doubled risk of subsequent salpingectomy. Removal of the fallopian tubes at hysterectomy should therefore be recommended [62,70].
As the indication for abdominal hysterectomy in benign diseases has become very rare, it is not discussed in this article. Even as robot-assisted laparoscopy has not yet shown any advantage for the experienced surgeon in the treatment of hysterectomy and therefore is not recommended by the American Association of Gynecologic Laparoscopists (AAGL), we are sure it will one day replace conventional laparoscopic or vaginal procedures [71].
Vaginal hysterectomy
Before starting a vaginal hysterectomy a bimanual pelvic examination is performed to assess uterine mobility and descent, and to exclude unsuspected adnexal pathology. Only then can a final decision be made whether to proceed with a vaginal or abdominal approach. The operation starts with entry into the cul-de-sac. The uterosacral ligaments are identified and clamped, including the lower portion of the cardinal ligaments. In the next step the vesicovaginal space is opened and after identification of the peritoneal fold it is cut and the cardinal ligaments are lighted, including the uterine vessels. Most adnexa can be removed by grasping the ovary and clamping the infundibulopelvic ligament. The uterus can then stepwise be enucleated from the remaining peritoneal fold at a safe distance from the bladder. The peritoneum can either be closed or left open and the vaginal epithelium is reapproximated in either a vertical or a horizontal manner. A myomatous uterus has to be morcellated in a piecemeal manner. Sometimes it is necessary to enucleate solitary large myomas or to perform intramyometrial coring, especially in cases of diffusely enlarged uteri [72,73].
Subtotal Laparoscopic Hysterectomy (SLH)
The supracervical hysterectomy was first described in 1990 by Lyons and in another technique by Semm. The operative technique is similar to the total laparoscopic hysterectomy. Only after occluding the ascending branch of the uterine artery is the uterine corpusresected as reverse conus down to the endocervical canal [74]. For SLH and TLH the trocar placement is the same as for laparoscopic myomectomy (see above). There is no need to perform ureterolysis at the beginning of the operation as the ureter is at a safe distance if the suturing line is kept strictly at the uterine wall. The infundibulo pelvic ligament and the round ligament are divided from the pelvic side wall and, if the adnexa are to remain in situ, division of the adnexa from the uterus. The broad ligament is then opened, dissected and each leaf separately coagulated. The bladder is separated from the uterus by opening the vesico uterine ligament and pushing the bladder downwards for about 1-2 cm. This is followed by presentation of the ramus ascendens of the uterine artery and division of the uterine pedicles with the same stepwise dissection of the left adnexa. A thorough inspection of the cervix then takes place. The cervix is separated from the uterus with the help of the electric cutting loop or any other cutting instrument. This is followed by coagulation of the cervical canal and closure of the peritoneum over the remaining cervical stump for infection and adhesion prevention. Afterwards morcellation of the uterine body is performed and, if the adnexa are also resected, they should be put into an endo bag for extraction.
Total Laparoscopic Hysterectomy (TLH)
SLH should be avoided if adenomyosis uteri are suspected because parts of the endometrial glands remain in the cervical and paracervical channel. These can cause an early recurrence or persistence of the symptoms although the few existing data offer no direct confirmation of this view [75,76].
The surgical steps of the TLH are identical to the SLH, the only difference being that a uterine manipulator is placed in the vagina before the operation. After separation of the bladder from the uterus, the bladder is pushed and dissected down 2-3 cm to clearly visualize the rim of the cervical cap. In cases of post-cesarean section, a gentle, blunt and intermediate sharp dissection has to be carried out. The uterus is lateralized by pushing up the manipulator. The uterine artery and vein with collateral arteries are completely coagulated near the cervix and dissected. The vagina is resected from the cervix with the monopolar hook by firmly stretching the manipulator cranially and carefully performing an intrafascial dissection leaving the sacro-uterine ligaments almost completely in place. This is in accordance with the Classic Intrafascial Supracervical Hysterectomy (CISH) technique introduced by Kurt Semm [77]. The uterus is then retracted through the vagina while still fixed to the manipulator. If the uterus is too large, it has to be morcellated either intra-abdominally or transvaginally. The vagina is closed with 2 corner sutures and 1 or 2 sutures in between the corner sutures. The sacro-uterine ligaments and the middle portion of the vagina are stitched and elevated to prevent vaginal prolapse or enterocele formation at a later time. Peritonealization and drainage are not required.
With the improvement of endoscopic surgery and above all the improvement in endoscopic suturing laparoscopic-assisted vaginal hysterectomy has become obsolete, especially as this technique does not include a suspension of the cardinal and sacro-uterine ligaments.
Conclusions
Treatment options for uterine leiomyomas vary. The choice of treatment should be made on an individual basis taking into account the following factors: the patient’s level of suffering due to bleeding disorders or displacement-caused pain, the status of family planning and the patient’s preferences regarding the different treatment options.
In asymptomatic women expectant management is suggested except for hydronephrosis caused by displacement or hysteroscopically resectable submucous fibroids in women who pursue pregnancy.
In postmenopausal women without hormonal therapy fibroids usually shrink and become asymptomatic. Therefore, expectant management is the method of choice. However, sarcoma should be excluded if a new or an enlarging pelvic mass occurs in a postmenopausal woman. Surgical treatment is the option of choice if the leiomyomas are symptomatic. If there are contraindications to operative procedures or hysterectomy is declined by the patient for personal reasons, any of the alternative treatment options can be considered (medical, embolization or guided ultrasound).
In premenopausal women appropriate submucosal leiomyomas should be resected hysteroscopically if the women wish to preserve their childbearing potential and / or they are symptomatic (e.g. bleeding, miscarriage). Intramural and subserosal leiomyomas in women who wish to preserve their fertility can be removed laparoscopically. Nevertheless, an appropriate surgical technique and advanced laparoscopic skills are necessary. If this cannot be guaranteed, abdominal myomectomy has to be recommended or referral to a laparoscopic center to maximize the possibility and safety of pregnancy after uterine reconstruction. The risk of uterine rupture in pregnancy following myomectomy needs to be discussed with the patient.
Robotic assistance might make laparoscopic suturing easier; however, to date there are too few data to support this contention.
For women who have completed their family planning, hysterectomy is the definitive procedure for relief of symptoms and prevention of recurrence of fibroid-related problems. With increasing experience in laparoscopic hysterectomies, the risk of side effects has become manageable. In relation to the compliance and individuality of the patient, the suitable solution can be laparoscopic supracervical or total laparoscopic hysterectomy.
- Watson JD, Crick FH. Molecular structure of nucleic acids: a structure for deoxyribose nucleic acid. Nature. 1953; 171: 737-738. https://goo.gl/osiYd8
- Watson JD, Crick FH. Genetical implications of the structure of deoxyribonucleic acid. Nature. 1953; 171: 964-967. https://goo.gl/ib72iM
- Treloar SA, Martin NG, Dennerstein L, Raphael B, Heath AC. Pathways to hysterectomy: insights from longitudinal twin research. Am J Obstet Gynecol. 1992; 167: 82-88. https://goo.gl/wxAjGt
- Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman's reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998; 83: 1875-1880. https://goo.gl/60Iy37
- Vikhlyaeva EM, Khodzhaeva ZS, Fantschenko ND. Familial predisposition to uterine leiomyomas. Int J Gynaecol Obstet. 1995; 51: 127-1231. https://goo.gl/vOh8or
- Van Voorhis BJ, Romitti PA, Jones MP. Family history as a risk factor for development of uterine leiomyomas. Results of a pilot study. J Reprod Med. 2002: 47: 663-669. https://goo.gl/NZVmMG
- Al-Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. J Soc Gynecol Investig. 2006; 13: 136-144. https://goo.gl/1cwEmk
- Tsibris JC, Segars J, Coppola D, Mane S, Wilbanks GD, O'Brien WF, et al. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril. 2002; 78: 114-121. https://goo.gl/qz2F7e
- Wang H, Mahadevappa M, Yamamoto K, Wen Y, Chen B, Warrington JA, et al. Distinctive proliferative phase differences in gene expression in human myometrium and leiomyomata. Fertil Steril. 2003; 80: 266-276. https://goo.gl/3PH9MB
- Gross K, Morton C, Stewart E. Finding genes for uterine fibroids. Obstetrics and Gynecology. 2000; 95: 60. https://goo.gl/oXYrHu
- Patterson-Keels LM, Selv aggi SM, Haefner HK, Randolph JF Jr. Morphologic assessment of endometrium overlying submucosal leiomyomas. J Reprod Med. 1994; 39: 579-584. https://goo.gl/vs0zPj
- Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gynecol. 2006; 195: 955-964. https://goo.gl/itln1d
- Hartmann KE, Birnbaum H, Ben-Hamadi R, Wu EQ, Farrell MH, Spalding J, et al. Annual costs associated with diagnosis of uterine leiomyomata. Obstet Gynecol. 2006; 108: 930-937. https://goo.gl/tEFc4k
- Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A. 2008; 105: 19887-19892. https://goo.gl/SszvQ6
- Laughlin SK, Hartmann KE, Baird DD. Postpartum factors and natural fibroid regression. Am J Obstet Gynecol. 2011; 204: 496. https://goo.gl/dCKih0
- Zaima A, Ash A. Fibroid in pregnancy: characteristics, complications, and management. Postgrad Med J. 2011; 87: 819-828. https://goo.gl/SY3d2W
- Donnez J, Vazquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril. 2014; 101: 1565-1573. https://goo.gl/I9jXuD
- Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. 2004; 191: 1621-1631. https://goo.gl/HPwEKh
- Edwards RD, Moss JG, Lumsden MA, Wu O, Murray LS, Twaddle S, et al. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007; 356: 360-370. https://goo.gl/3IoDqH
- Van der Kooij SM, Bipat S, Hehenkamp WJ, Ankum WM, Reekers JA. Uterine artery embolization versus surgery in the treatment of symptomatic fibroids: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011; 205: 317. https://goo.gl/7cQCpp
- Kim HS, Baik JH, Pham LD, Jacobs MA. MR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: long-term outcomes. Acad Radiol. 2011; 18: 970-976. https://goo.gl/lJBpmQ
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance - guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009; 34: 584-589. https://goo.gl/s4PKqQ
- Preventing entry-related gynecological laparoscopic injuries. RCOG Green-top Guideline. 2008; 49: 1-10. https://goo.gl/9T7oDA
- Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004; 104: 393-406. https://goo.gl/HJhfyl
- Myo Sun Kim, You Kyoung Uhm, Ju Yeong Kim, Byung Chul Jee, Yong Beom Kim. Obstetric outcomes after uterine myomectomy: Laparoscopic versus laparotomic approach. Obstet Gynecol Sci. 2013; 56: 375-381. https://goo.gl/hzHsvu
- Lonnerfors C, Persson J. Pregnancy following robot-assisted laparoscopic myomectomy in women with deep intramural myomas. Acta Obstet Gynecol Scand. 2011; 90: 972-977. https://goo.gl/ljUEt6
- Mettler L, Schollmeyer T, Tinelli A, Malvasi A, Alkatout I. Complications of Uterine Fibroids and Their Management, Surgical Management of Fibroids, Laparoscopy and Hysteroscopy versus Hysterectomy, Haemorrhage, Adhesions, and Complications. Obstet Gynecol Int. 2012; 2012: 791248. https://goo.gl/bkanVb
- Di Spiezio Sardo A, Mazzon I, Bramante S, Bettocchi S, Bifulco G, Guida M, et al. Hysteroscopic myomectomy: a comprehensive review of surgical techniques. Hum Reprod Update. 2008; 14: 101-119. https://goo.gl/Pvi8bm
- Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997; 68: 881-886. https://goo.gl/Pi3yYO
- Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993; 82: 736-740. https://goo.gl/OKOUD6
- Hart R, Molnár BG, Magos A. Long term follow up of hysteroscopic myomectomy assessed by survival analysis. Br J Obstet Gynaecol. 1999; 106: 700-705. https://goo.gl/0HXWPX
- Mettler L, Sammur W, Alkatout I, Schollmeyer T. Imaging in gynecologic surgery. Womens Health (Lond Engl). 2011; 7: 239-248. https://goo.gl/1AxtHN
- Varma R, Soneja H, Clark TJ, Gupta JK. Hysteroscopic myomectomy for menorrhagia using Versascope bipolar system: efficacy and prognostic factors at a minimum of one year follow up. Eur J Obstet Gynecol Reprod Biol. 2009; 142: 154-159. https://goo.gl/0T1apq
- Loffer FD, Bradley LD, Brill AI, Brooks PG, Cooper JM. Hysteroscopic fluid monitoring guidelines. The ad hoc committee on hysteroscopic training guidelines of the American Association of Gynecologic Laparoscopists. J Am Assoc Gynecol Laparosc. 2000; 7: 167-168. https://goo.gl/Rz91GI
- Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000; 96: 266-270. https://goo.gl/TL7C2E
- Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000; 96: 517-520. https://goo.gl/0nko0m
- Parker WH, Rodi IA. Patient selection for laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1994; 2: 23-26. https://goo.gl/jTmP2Z
- Lefebvre G, Vilos G, Allaire C, Jeffrey J, Arneja J, Birch C, et al. The management of uterine leiomyomas. J Obstet Gynaecol Can. 2003; 25: 396-418. https://goo.gl/rjm8vO
- Alkatout I, Bojahr B, Dittmann L, Warneke V, Mettler L, Jonat W, et al. Precarious preoperative diagnostics and hints for the laparoscopic excision of uterine adenomatoid tumors: two exemplary cases and literature review. Fertil Steril. 2011; 95: 1119. https://goo.gl/mq3jKE
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol. 2002; 186: 409-415. https://goo.gl/wLC7XG
- Alkatout I, Honemeyer U, Strauss A, Tinelli A, Malvasi A, Jonat W, et al. Clinical diagnosis and treatment of ectopic pregnancy. Obstet Gynecol Surv. 2013; 68: 571-581. https://goo.gl/nY2XJ6
- Alkatout I, Schollmeyer T, Hawaldar NA, Sharma N, Mettler L. Principles and safety measures of electrosurgery in laparoscopy. JSLS. 2012; 16:130-139. https://goo.gl/NPA11T
- Alkatout I, Stuhlmann-Laeisz C, Mettler L, Jonat W, Schollmeyer T. Organ-preserving management of ovarian pregnancies by laparoscopic approach. Fertil Steril. 2011; 95: 2467-2470. https://goo.gl/yIj1AK
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2011: p. CD005355. https://goo.gl/p7QzBy
- Zhao F, Jiao Y, Guo Z, Hou R, Wang M. Evaluation of loop ligation of larger myoma pseudocapsule combined with vasopressin on laparoscopic myomectomy. Fertil Steril. 2010; 95:762-766. https://goo.gl/CB1dDA
- Tinelli A, Liselotte M, Malvasi A, Hurst B, Catherino W, Ospan AM, et al. Impact of surgical approach on blood loss during intracapsular myomectomy. Minim Invasive Ther Allied Technol. 2013; 23: 87-95. https://goo.gl/H1qEI3
- Celik H, Sapmaz E. Use of a single preoperative dose of misoprostol is efficacious for patients who undergo abdominal myomectomy. Fertil Steril. 2003; 79: 1207-1210. https://goo.gl/VYI5E5
- Mettler L, Sammur W, Schollmeyer T, Alkatout I, et al. Cross-linked sodium hyaluronate, an anti-adhesion barrier gel in gynaecological endoscopic surgery. Minim Invasive Ther Allied Technol. 2013; 22: 260-265. https://goo.gl/GDH1z2
- Mettler LT. Schollmeyer T, Alkatout I. Adhesions during and after surgical procedures, their prevention and impact on women's health. Womens Health (Lond). 2012; 8: 495-498. https://goo.gl/5hlkOD
- Tulandi TC, Murray C, Guralnick M. Adhesion formation and reproductive outcome after myomectomy and second-look laparoscopy. Obstet Gynecol. 1993; 82: 213-215. https://goo.gl/Ed0omX
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006; 61: 106-110. https://goo.gl/0mwhBW
- D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2009; 116: 131-139. https://goo.gl/DhT14E
- Mukhopadhaya N, De Silva C, Manyonda IT. Conventional myomectomy. Best Pract Res Clin Obstet Gynaecol. 2008; 22: 677-705. https://goo.gl/Z22ugL
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007; 110: 1301-1303. https://goo.gl/4DRQy5
- Pundir J, Pundir V, Walavalkar R, Omanwa K, Lancaster G, Kayani S. Robotic-assisted laparoscopic vs abdominal and laparoscopic myomectomy: systematic review and meta-analysis. J Minim Invasive Gynecol. 2013. 20: 335-345. https://goo.gl/SYqCdU
- Barakat EE, Bedaiwy MA, Zimberg S, Nutter B, Nosseir M, Falcone T. Robotic-assisted, laparoscopic, and abdominal myomectomy: a comparison of surgical outcomes. Obstet Gynecol. 2011; 117: 256-265. https://goo.gl/LZ7zk4
- Mettler L, Clevin L, Ternamian A, Puntambekar S, Schollmeyer T, Alkatout I. The past, present and future of minimally invasive endoscopy in gynecology: a review and speculative outlook.Minim Invasive Ther Allied Technol. 2013; 22: 210-226. https://goo.gl/snh2Nc
- Schollmeyer T, et al. Robotic surgery in gynecology. The Gynecologist. 2011; 44(3): p. 196-201.
- George A, Eisenstein D, Wegienka G. Analysis of the impact of body mass index on the surgical outcomes after robot-assisted laparoscopic myomectomy. J Minim Invasive Gynecol. 2009; 16:730-733. https://goo.gl/as1jjV
- Falcone T, Parker WH. Surgical management of leiomyomas for fertility or uterine preservation. Obstet Gynecol. 2013; 121: 856-868. https://goo.gl/hQm4v0
- Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al. EVALUATE hysterectomy trial: a multicentre randomized trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy. Health Technol Assess. 2004; 8: 1-154. https://goo.gl/NZphQ6
- Dietl J, Wischhusen J, Hausler SF. The post-reproductive Fallopian tube: better removed? Hum Reprod. 2011; 26: 2918-2924. https://goo.gl/YcCCqJ
- Adashi EY. The climacteric ovary as a functional gonadotropin-driven and rogen-producing gland. Fertil Steril. 1994; 62: 20-27. https://goo.gl/qzzz5A
- Shifren JL. The role of androgens in female sexual dysfunction. Mayo Clin Proc. 2004; 79: S19-S24. https://goo.gl/I0rxgV
- Buster JE, Kingsberg SA, Aguirre O, Brown C, Breaux JG, Buch A, et al. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol. 2005; 105: 944-952. https://goo.gl/XeGaTh
- Nyunt A, Stephen G, Gibbin J, Durgan L, Fielding AM, Wheeler M, et al. Androgen status in healthy premenopausal women with loss of libido. J Sex Marital Ther. 2005; 31: 73-80. https://goo.gl/R696Vs
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003; 349: 523-534. https://goo.gl/Vw7u6C
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004; 291: 1701-1712. https://goo.gl/4gUc5O
- Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005; 106: 219-226. https://goo.gl/5SYfI7
- Guldberg R, Wehberg S, Skovlund CW, Mogensen O, Lidegaard O. Salpingectomy as standard at hysterectomy? A Danish cohort study, 1977-2010. BMJ Open. 2013; 3. https://goo.gl/dq1g2u
- AAMIG W. AAGL position statement: Robotic-assisted laparoscopic surgery in benign gynecology. J Minim Invasive Gynecol. 2013; 20: 2-9. https://goo.gl/Wp42V0
- Meeks GR, Harris RL. Surgical approach to hysterectomy: abdominal, laparoscopy-assisted, or vaginal. Clin Obstet Gynecol. 1997; 40: 886-894. https://goo.gl/RERosM
- Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995; 86: 60-64. https://goo.gl/3b4mW8
- Jenkins TR. Laparoscopic supracervical hysterectomy. Am J Obstet Gynecol. 2004; 191: 1875-1884. https://goo.gl/QQIoPy
- Berner E, Qvigstad E, Myrvold AK, Lieng M. Pelvic Pain and Patient Satisfaction After Laparoscopic Supracervical Hysterectomy: Prospective Trial. J Minim Invasive Gynecol. 2014; 21: 406-411. https://goo.gl/arHXat
- Alkatout I, Mettler L, Beteta C, Hedderich J, Jonat W, Schollmeyer T, et al. Combined surgical and hormone therapy for endometriosis is the most effective treatment: prospective, randomized, controlled trial. J Minim Invasive Gynecol. 2013; 20: 473-481. https://goo.gl/WqWK3Z
- Semm K. Hysterectomy via laparotomy or pelviscopy. A new CASH method without colpotomy. Geburtshilfe Frauenheilkd. 1991; 51: 996-1003. https://goo.gl/bHN7Cs

Sign up for Article Alerts