Luminescent Properties of Ti4+-Doped White-Light Glasses Sio2- Al2O3 -Na2O -B2O3-Bao?
Huiwang Lian1 and Yang Li1,2,3*
1School of Physics and Optoelectronic Engineering, Guangdong University of Technology, Guangzhou 510006, China
2Department of Biochemistry and Molecular Pharmacology, University of Massachusetts Medical School, Worcester, Massachusetts 01605, USA
3State Key Laboratory of Luminescent Materials and Devices, School of Materials Science and Technology, South China University of Technology, Guangzhou 510640, China
*Address for Correspondence: Yang Li, School of Physics and Optoelectronic Engineering, Guangdong University of Technology, Guangzhou 510006, China, E-mail: lychris@sina.com
Submitted: 16 April 2018; Approved: 15 May 2018; Published: 18 March 2018
Citation this article: Lian H, Li Y, Luminescent Properties of Ti4+ -Doped White-Light Glasses Sio2- Al2O3 -Na2O -B2O3 –Bao. American J Mater Appl Sci. 2018;1(1): 001-004.
Copyright: © 2018 Li Y, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Luminescent; Glass; Ti4+ Ion; White-Light; Fluorescence Lifetime
Download Fulltext PDF
We investigated the photoluminescence properties and decay curves of SiO2 -AL2O3- Na2O - BaO-B2O3-x TiO2 glasses prepared via the conventional melt quenching method. A broad emission band 400-800 nm peaking at 500 nm was observed in emission spectrum, while a very broad excitation spectral region (from 250 nm to 430 nm) and two main absorption bands at 260 and 400 nm were found. The fluorescence lifetime was measured and all the decay curves were approximated by an exponential decay function. Electron transition occurring between surface defects, oxygen vacancies or self-trapped excitons, support the observed spectroscopic data.
Introduction
Applications of transparent glass materials cover from ordinary apparatuses (such as window glasses and glass containers) to precise optical devices (such as lens and fibers) [1-6]. For example, in photovoltaic (PV) modules for solar energy utility, a thin protective layer of superstrate glass with excellent mechanical rigidity, chemical stability, and optical transparency, is laminated on the semiconductor. The spectral transmittance is well considered to maximize the PV efficiency. In crystalline silicon PV modules, any absorption in the superstrate glass from 400 to 1000 nm will introduce additional loss to the final PV efficiency.
In fact, now, novel glasses possessing special emission band are being extensively developed to resolve this issue. Transition metal and rare earth ions are intentionally doped into glasses as active centers and endow those transparent hosts with luminescent properties at various wavelength [7-10]. Many studies have pointed out the relationships between the structure of the host glass and the properties of the doped ions. The transition metal or rare earth ions doped glasses have attracted a great deal of interest because of their well-defined and sharp energy levels and the modifications of the energy level structure.
White light glasses now have found their applications in visual display and decorative art [11-15]. Generally, white light is synthesized by the excitation of a crystalline phosphorus with an Ultra Violet (UV) In GaN LED chip. However, given that conventional LEDs are encapsulated with an epoxy resin, the increase temperature caused by the LED chip can cause resin deterioration, reducing the quality and the intensity of light emission. To replace the crystalline phosphors, one of the alternatives is the glassy systems doped transition metal or rare earth ions, due to their simple fabrication process, low production costs, high transparency and thermal stability. Here, we develop Ti4+ doped SiO2 -AL2O3 -Na2O –BaO -B2O3 glass which has a broad emission band 400-800 nm. Decay curves and photoluminescence were determined for the prepared glasses.
Materials and Methods
The linear accelerator
The following chemical reagents were used as starting materials: SiO2 (99.0%), AL2O3 (99.9), Na2CO3 (99.8%), BaO (97.0%), H3BO3 (99.5%), TiO2 (99.99%). In the following, sample nomenclature is xSyAzB. E.g., 30S15A20B for x = 3, y=15 and z=20. Batches of starting materials were mixed homogeneously in an agate mortar and melted in a pure alumina crucible at 1050 °C for 1h in the air, and then were quickly poured onto steel plate preheated at 400 °C, and cooled to room temperature to obtain glass. All glass samples were cut and polish in proper shape for further studies. The preparing conditions listed in Table 1 (composition, melting temperature) were determined based on the many experimental trials. The visible luminescence spectra and decay curves were measured with a high-resolution spectrofluorometer (UK, Edinburgh Instruments, and FLS980) equipped with a 500 W Xenon lamp as an excitation source, with a Hamamatsu R928P visible photomultiplier (250 nm-850 nm). The measured spectral ranges for excitation and emission were 250-430 and 400-800 nm, respectively.
Results and Discussion
The photoluminescence analysis is used for measuring of recombination of electron-hole radiative. This spectrum is used here for investigation of defects that created due to adding Titanium ion.
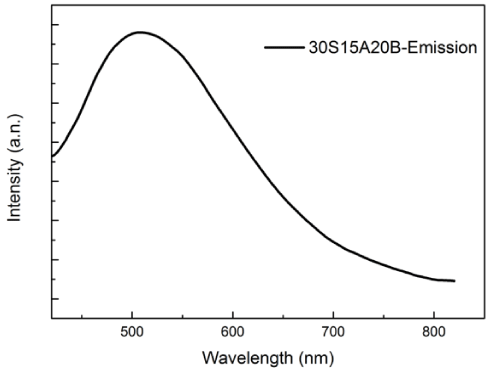
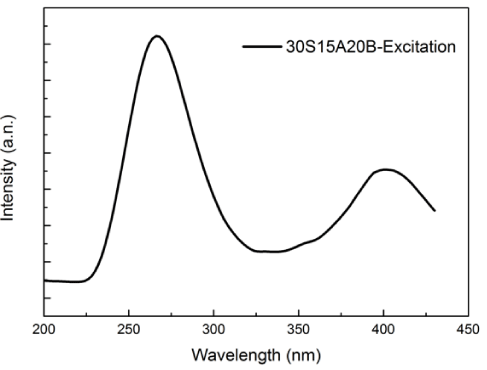
Figure 1 shows the emission spectrum of 30S15A20B glass excited at 254 nm. A single broad emission band was observed. Photoluminescence spectrum can be caused by surface defects, oxygen vacancy or self-trapped excitons [16-19]. In addition, surface chemistry can effect on this spectrum. The emission band at 506 nm is probably related to surface bonds or oxygen vacancy on the surface of TiO2 crystal. Also, it is believed that 30S15A20B glass acts as electron saver for electron absorption that TiO2 produced under light source irradiation and excited by a photon. This phenomenon resulted in decrease electron population at TiO2 and inhibition of electron-hole (e- / h+ pair) recombination. Figure 2 shows the excitation spectrum of 30S15A20B glass. The excitation spectrum covers a very broad spectral region (from 250 nm to 430 nm) and contain two main absorption bands at 264 and 408 nm.
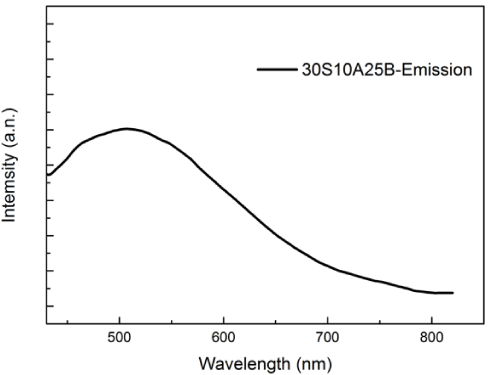
Figure 3 shows the emission spectrum of 30S10A25B glass excited at 254 nm. Under excitation at 254 nm, the glass exhibits the emission peaking at 510 nm in30S10A25B glass. The strong peak that its center is 510 nm is probably due to the dangling bonds or oxygen vacancies in TiO2 crystal surface.
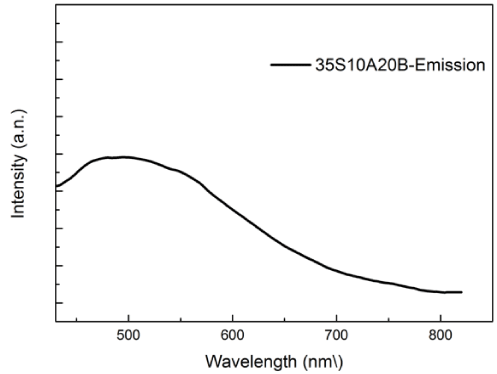
Figure 4 shows the emission spectrum of 35S10A20B glass excited at 254 nm. Under excitation at 254 nm, the glass exhibits the emission peaking at 490 nm in 35S10A20B glass.
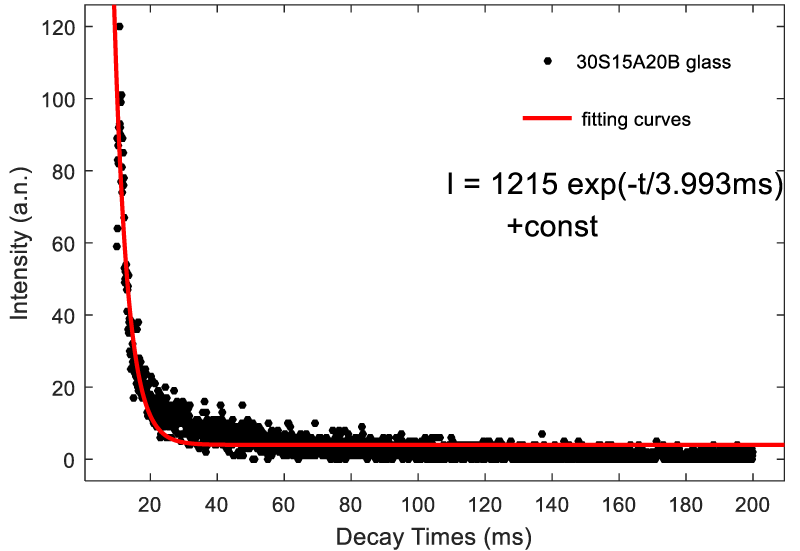
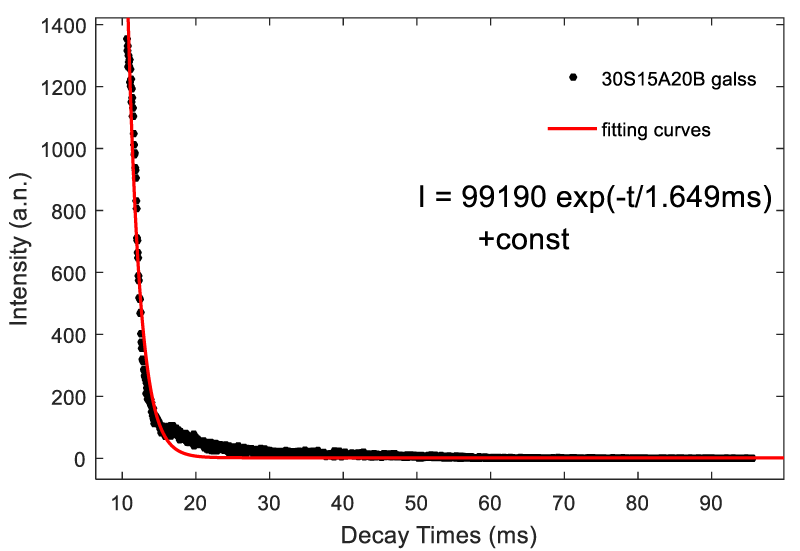
Figure 5 shows the fluorescence decay curves of 30S15A20B glass by monitoring 506 nm emission under the excitation of 264 nm light. The fluorescence lifetime is 3.993ms. Figure 6 shows the fluorescence decay curves of 30S15A20B glass by monitoring 506 nm emission under the excitation of 408 nm light. The fluorescence lifetime is 1.649ms. The decay curves can be well fitted by the exponential function [20]:
I (t) =A1 exp(-t / t1)+I0 (1)
Where I(t) is relative fluorescence intensity at decay time t, A1 and I0 are constants, and τ1 represents the lifetime.
Conclusions
We synthesized Ti4+-doped SiO2 -AL2O3 -Na2O –BaO -B2O3 glasses via the conventional melt quenching method. We investigated the emission spectra and excitation spectra and decay curves of different glass composition. The 30S15A20B, 30S10A25B, 35S10A20B glasses show the broad emission band 400-800 nm peaking at 506 nm, 510 nm and 490 nm, respectively. Moreover, the excitation spectrum covers a very broad spectral region (from 250 nm to 430 nm) and contain two main absorption bands at 260 and 400 nm. The fluorescence decay curves of 30S15A20B glass can be well fitted by the exponential function. The fluorescent lifetime by using 264 nm light excitation (3.993 ms) is larger than that by using 408 nm ligh excitation (1.649 ms).
Acknowledgement
We acknowledges financial support from the National Natural Science Foundation of China (Grant Nos. 51602063, 21671045, 21471038 and 51472091). This work was also supported by the Tip‐Top Scientific and Technological Innovative Youth Talents of Guangdong Special Support Program (2016TQ03R281) and Open Fund of the State Key Laboratory of Luminescent Materials and Devices (South China University of Technology).
- Ataalla M, Afify AS, Hassan M, Abdallah M, Milanova M, Aboul-Enein HY, et al. Tungsten-based glasses for photochromic, electrochromic, gas sensors, and related applications: A review. Journal of Non-Crystalline Solids. 2018; 491: 43-54. https://goo.gl/xohzzt
- Benitez T, Y. Gómez S, de Oliveira APN, Travitzky N, Hotza D. Transparent ceramic and glass-ceramic materials for armor applications. Ceramics International. 2017; 43: 13031-13046. https://goo.gl/SEr2zr
- Dalapati GK, Kushwaha AK, Sharma M, Suresh V, Shannigrahi S, Zhuk S, et al. Transparent heat regulating (THR) materials and coatings for energy saving window applications: Impact of materials design, micro-structural, and interface quality on the THR performance. Progress in Materials Science. 2018; 95: 42-131. https://goo.gl/PB5jVS
- Mariyappan M, Arunkumar S, Marimuthu K. White light emission and spectroscopic properties of Dy3+ ions doped bismuth sodiumfluoroborate glasses for photonic applications. Journal of Alloys and Compounds. 2017; 723: 100-114. https://goo.gl/NC7SgY
- Ouannes K, Lebbou K, Walsh BM, Poulain M, Alombert-Goget G, Guyot Y. Antimony oxide based glasses, novel laser materials. Optical Materials. 2017; 65: 8-14. https://goo.gl/MEMmgn
- Sutha S, Suresh S, Raj B, Ravi KR. Transparent alumina based superhydrophobic self–cleaning coatings for solar cell cover glass applications. Solar Energy Materials and Solar Cells. 2017; 165: 128-137. https://goo.gl/WzNAmi
- Algarni H, Reben M, Almoeed S, Maâlej R, Yousef ES. Luminescence emission of Tm-Dy ions codoped tellurite glasses under visible light excitation. Optik. 2018; 160: 340-347. https://goo.gl/LHm6UP
- Ren J, Wagner T, Oswald J, Orava J, Frumarova B, Frumar M. Spectroscopic properties of Ni2+ and rare-earth codoped Ge–Ga–Sb–S glass. Journal of Physics and Chemistry of Solids. 2010; 71: 30-34. https://goo.gl/1XuRkC
- Sato M, Kim SW, Shimomura Y, Hasegawa T, Toda K, Adachi G. Chapter 278 - Rare Earth-Doped Phosphors for White Light-Emitting Diodes. In: Jean-Claude B, Vitalij K P, editors. Handbook on the Physics and Chemistry of Rare Earths. 49: Elsevier; 2016. 1-128.
- Yang A, Qiu J, Zhang M, Ren H, Zhai C, Qi S, et al. Mid-infrared luminescence of Dy3+ ions in modified Ga-Sb-S chalcogenide glasses and fibers. Journal of Alloys and Compounds. 2017; 695: 1237-1242. https://goo.gl/SQz3iM
- Annapoorani K, Karthikeyan P, Basavapoornima C, Marimuthu K. Investigations on the optical properties of Dy3+ ions doped potassium aluminiumtelluroborate glasses for white light applications. Journal of Non-Crystalline Solids. 2017; 476: 128-136.
- Kaur S, Vishwakarma AK, Deopa N, Prasad A, Jayasimhadri M, Rao AS. Spectroscopic studies of Dy3+ doped borate glasses for cool white light generation. Materials Research Bulletin. 2018; 104: 77-82. https://goo.gl/Jfaz8U
- Lodi TA, Sandrini M, Medina AN, Barboza MJ, Pedrochi F, Steimacher A. Dy:Eu doped CaBAl glasses for white light applications. Optical Materials. 2018; 76: 231-236. https://goo.gl/Ws7mQ5
- Vijayakumar M, Viswanathan K, Marimuthu K. Structural and optical studies on Dy3+:Tb3+ co-doped zinc leadfluoro-borophosphate glasses for white light applications. Journal of Alloys and Compounds. 2018; 745: 306-318. https://goo.gl/3fBxRq
- Vijayalakshmi L, Naveen Kumar K, Rao KS, Hwang P. Bright up-conversion white light emission from Er3+ doped lithium fluoro zinc borate glasses for photonic applications. Journal of Molecular Structure. 2018; 1155: 394-402. https://goo.gl/L1JFfH
- Askari MB, Banizi ZT, Soltani S, Seifi M. Comparison of optical properties and photocatalytic behavior of TiO2/MWCNT, CdS/MWCNT and TiO2/CdS/MWCNT nanocomposites. Optik. 2018; 157: 230-239. https://goo.gl/UzHgKh
- Askari MB, Tavakoli Banizi Z, Seifi M, Bagheri Dehaghi S, Veisi P. Synthesis of TiO2 nanoparticles and decorated multi-wall carbon nanotube (MWCNT) with anatase TiO2 nanoparticles and study of optical properties and structural characterization of TiO2/MWCNT nanocomposite. Optik - International Journal for Light and Electron Optics. 2017; 149: 447-454. https://goo.gl/j6r37d
- Masai H, Okada G, Kawaguchi N, Yanagida T. Photoluminescence and X-ray-induced scintillation of BaO-TiO2-SiO2 glasses and the glass-ceramics. Journal of Non-Crystalline Solids. 2017. https://goo.gl/UDfK55
- Sánchez-Rodríguez D, Méndez Medrano MG, Remita H, Escobar-Barrios V. Photocatalytic properties of BiOCl-TiO2 composites for phenol photodegradation. Journal of Environmental Chemical Engineering. 2018; 6: 1601-1612. https://goo.gl/SQoavx
- Kato T, Okada G, Kawaguchi N, Masai H, Yanagida T. Scintillation properties of BaO-TiO2-GeO2-SiO2 glass-ceramics. Journal of Non-Crystalline Solids. 2018. https://goo.gl/Jf8gtS

Sign up for Article Alerts