Research on Three-Order Nonlinear Optical Properties of Polymers with Different Substituent Groups of Azobenzothiazole Structure on Side Chains?
Liang Zhang1* and Feng Zhang2
1School of Material Science and Engineering, Yancheng Institute of Technology
2Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province, Yancheng Institute of Technology
*Address for Correspondence: Liang Zhang, School of Material Science and Engineering, Yancheng Institute of Technology, Yancheng, China, Tel: +865-158-829-8872; ORCID ID: 0000-0002-3109-1940; E-mail: zhangliang@ycit.edu.cn / ycitzl@hotmail.com
Submitted: 21 June 2019; Approved: 04 July 2019; Published: 06 July 2019
Citation this article: Zhang L, Zhang F, Research on Three-Order Nonlinear Optical Properties of Polymers with Different Substituent Groups of Azobenzothiazole Structure on Side Chains. American J Mater Appl Sci. 2019;2(1): 001-004.
Copyright: © 2019 Zhang L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Azo benzothiazole; Third-order NLO property; Substituent group
Download Fulltext PDF
Allyl monomers with different substituent groups of azobenzothiazole chromophores were synthesized in this study. Through free radical polymerization of these synthesized allyl monomers, polymers containing chromophores with nonlinear optical properties on side chains were obtained. The three-order nonlinear optics of monomers and polymers were acquired through Z-scanning method. The electronic effect of substituent groups on azobenzothiazole groups may influence nonlinear optics significantly.
Introduction
Organic three-order nonlinear optical materials are widely used in the field of information technology [1-3]. Researchers have attempted to improve the nonlinear optical characteristics of organic materials in order to enhance the efficiency of photon equipment. Modifying and reconstructing the molecular structure of the organic conjugated system can improve the performance of nonlinear optical materials [4]. In fact, structures containing heterocycles, such as thiazole and benzothiazole have lower delocalization energy than benzene ring; thus, they can increase charge transfer characteristics and nonlinear optical responses effectively [5-8]. Hence, designing and synthesizing chromophores containing these heterocyclic structures are an effective way to obtain high nonlinear optical responses.
Previous studies have successfully synthesized polymers containing azobenzothiazole structures on side chains and discussed their three-order nonlinear optical performance [9]. In the present study, monomers containing azobenzothiazole structures with pushing and drawing electrons were designed and synthesized to further study the influences of electronic effect of substituent groups on three-order nonlinear optical properties of polymers with heterocyclic azo structures on side chains. Polymers with nonlinear optical chromophores on side chains were obtained through free radical polymerization.
Materials and Methods
Regents and solvents
6-Methoxy-Benzothiazole-2-Ylamine (99%; Ninghai Shangfeng Chemical Reagent Co., Ltd) were used as received. 6-Nitro-Benzothiazole-2-Ylamine and 4-(6-Nitro-Benzothiazole-2-Ylzao)-phenol were synthesized according to the reported procedure [10,11]. Methacryloyl chloride (≥ 98%, Haimen Best Fine Chemical Co. Ltd) was distilled under vacuum before using. Azobisisobutyronitrile (AIBN, CP; Shanghai Chemical Reagent Co., Ltd.) was purified by recrystallization from ethanol.
All other reagents and solvents were analytic pure and were used as received.
Measurements
1H NMR spectra were measured by INOVA 400 MHz NMR spectrometer, CDCl3 or DMSO-d6 as solvent and Tetramethylsilane (TMS) as the internal standard at ambient temperature. UV-vis absorption spectra of the polymers and initiator in DMF solutions were determined on a Shimadzu RF540 spectrophotometer. Third-order NLO response of monomers and polymers was measured according to the literature [9,12].
Synthesis of monomers
2.3.1. Synthesis of 4-(6-Methoxy-Benzothiazole-2-Ylzao)-phenol: 6-Methoxy-Benzothiazole-2-Ylamine (0.04 mol) was added to a mixture of distilled water (32 mL), concentrated H2SO4 (20 mL) and formic acid (8 mL). A solution of sodium nitrite (0.044mol) in distilled water (20 mL) was prepared in a test tube. Sodium nitrite solution was added dropwise to the acidic solution of amine over 1 hour at 0~2ºC. The mixture was stirred at 0~2ºC for 45 min. Phenol (0.04mol) was dissolved in ethanol (20 mL) and cooled to 0ºC. Phenol solution was added slowly to the diazonium salt solution at 0~5ºC. The resultant colored mixture was stirred for 24 hour at 0~5ºC. The solution was filtered and the obtained crude product was recrystallized for two times from ethanol.
Henna powder. Yield: 46%. 1H NMR (DMSO-d6, δ): 10.86 (s, 1H), 8.01-7.98 (d, 1H), 7.92-7.89 (d, 2H), 7.66 (s, 1H), 7.19-7.15 (d, 1H), 7.03-7.00 (d, 2H), 3.88 (s, 3H).
Synthesis of MBAMA and NBAMA:
• General procedure for synthesis of MBAMA and NBAMA: The phenol derivates (0.01 mol) was dissolved in a mixture of Et3N (0.02 mol) and freshly distilled THF (80 mL). This solution was then cooled in an ice-water bath with vigorous stirring. A solution containing methacryloyl chloride (0.01 mol) and freshly dried THF (20 mL) was added to the above mixture by a dropping funnel under a nitrogen atmosphere. After 1 hour, the ice-water bath was removed and the reaction was allowed to continue for 24 hours at room temperature figure 1. The solution was filtered and poured into a large amount of water. The precipitated product was washed by ethanol (95%) and recrystallized from trichloromethane and petroleum ether (60~90ºC).
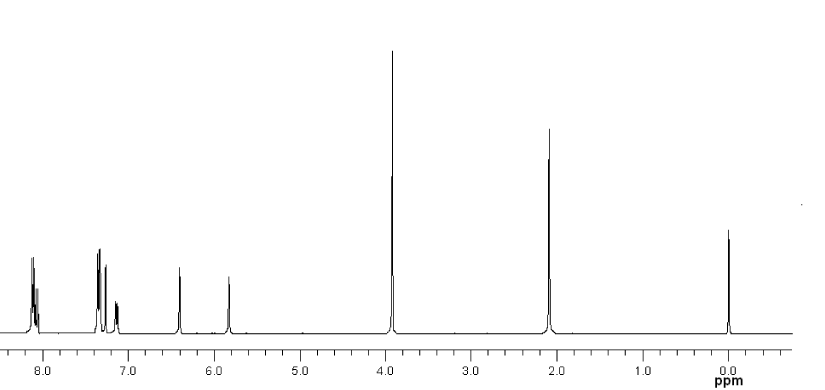
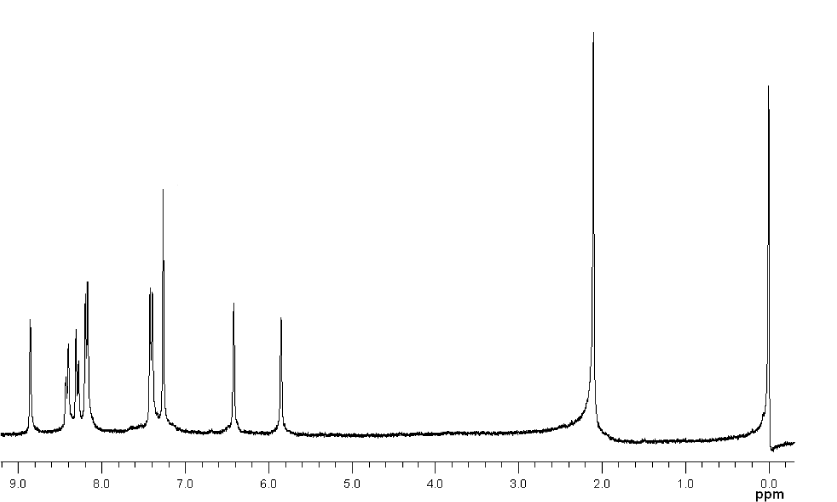
• MBAMA: Orange powder. Yield: 71%. 1H NMR (CDCl3, δ): 8.12-8.10 (d, 2H), 8.08-8.05 (d, 1H), 7.36-7.33 (d, 3H), 7.15-7.13 (d, 1H), 6.40 (s, 1H), 5.83 (s, 1H), 3.93 (s, 3H), 2.09 (s, 3H).
• NBAMA: Henna powder. Yield: 64%. 1H NMR (CDCl3, δ): 8.86 (s, 1H), 8.43-8.40 (d, 1H), 8.31-8.28 (d, 1H), 8.20-8.17 (d, 2H), 7.42-7.39 (d, 2H), 6.42 (s, 1H), 5.85 (s, 1H), 2.10 (s, 3H).
In a general procedure, cyclohexanone (monomer/cyclohexanone = 1: 5 g·mL-1), initiator, and monomer were mixed in a round-bottom flask. Polymerization was conducted at 70°C under N2 atmosphere for 10 h. Subsequently, the samples were diluted with THF and precipitated through the addition of a mixture of ethyl acetate and methanol (Vethyl acetate/Vmethanol/VTHF = 5/5/1). Reprecipitation was conducted three times and the samples were dried under vacuum at room temperature.
Results and Discussion
Linear optical properties
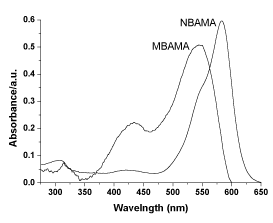
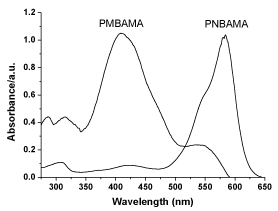
The UV-visible spectra of monomer and solution prepared by DMF are shown in figure 2. The monomer has strong absorption in the visible light zone, which is attributed to the π-π* electron transition of the azo conjugated structure. In addition, MBAMA has one shoulder peak at approximately 430 nm, which is produced by electron transition of methoxy group replacing the benzothiazole structures. The UV-visible spectra of polymers are shown in figure 3. Different from monomers, the polymer PMBAMA has a strong absorption peak at approximately 410 nm, which is produced by electron transition of methoxy group replacing benzothiazole structures. This phenomenon might be due to group arrangement caused by polymers with different azo structures. The ultraviolet absorptions of monomer NBAMA and polymer PNBAMA remain basically the same.
Three-order nonlinear optical properties of monomers and polymers
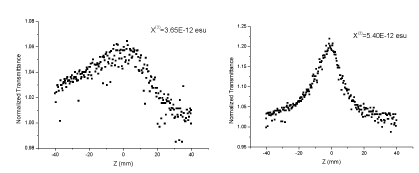
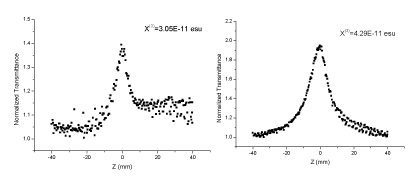
Nonlinear absorption and refraction coefficient of monomers and polymers were evaluated by Z-scanning method. In this experiment, samples moved back and forth close to the focus along the directions. The relationship between light intensity through small holes and sample position was determined through Z-scanning of open and closed holes, allowing us to obtain the nonlinear absorption and refractive index of samples. According to the Z-scanning experiment of closed holes, nonlinear refraction does not influence the three-order nonlinear performance of experimental samples. Hence, the three-order nonlinear optical coefficient (χ(3)) measured by this experiment was determined by nonlinear absorption of molecules completely. As shown in figures 4,5, all monomers and polymers are in the ground state absorption, and relatively low transition is observed at 532 nm. After the introduction of a 532 nm laser radiation, a thermal effect was produced, which affected the nonlinear test results. In this study, laser with low energy (< 20 μJ) and low frequency (2 Hz) were applied to reduce the thermal effect. DMF was used as the solvent, and 10-3 mol/L monomer was prepared. At the same time, polymer and corresponding monomers were prepared with the same mass fraction to assure the same chromophore concentrations. Z-scanning experimental results of open holes are shown in figures 6,7. The monomer structure of benzothiazole has different substituent groups, and the electrophilic nitro can make the system produce a pushing–drawing electronic effect. Given that the structure of benzothiazole is rich with electrons, the electron mobility of delocalization in the azobenzothiazole system is increased, which further intensifies polarization of azo molecules. Finally, the three-order nonlinear optical effect is strengthened. As a result, the three-order nonlinear optical coefficient of monomers and polymers with nitro is at the magnitude of 10-11, which is significantly higher than those of monomers and polymers in which chromophores have methoxy groups (10-12). Furthermore, the three-order nonlinear optical coefficient of polymers is higher than those of corresponding monomers. This can be interpreted by the ordered arrangement of elements on repeated azo chains of the polymer chain, thus increasing electronic delocalization of azo structures and enhancing three-order nonlinear optical effects after polymerization of monomers.
Conclusion
Monomers with different substituent groups of azobenzothiazole structures were synthesized through polymerization with free radical groups. Later, chromophores were introduced into the side chain of the polymer, which resulted in increased three-order nonlinear optical coefficients of monomers and polymers. After polymerization, the nonlinear optical performance of polymers was improved to some extent compared with that of monomers. Nitro-substitution of azobenzothiazole can improve the three-order nonlinear optical effects significantly.
Acknowledgement
This work was supported by “Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (No. PPZY2015A025)” and “Jiangsu Six Talent Peaks Program (No. XCL-087)”.
- Zhai GX, Wu XZ, Jin PC, Song TT, Xiao JC, Song YL. Synthesis, optoelectronic properties and third-order nonlinear optical behaviors of the functionalized acene derivatives. Dyes and Pigments. 2018; 155: 93-99. https://bit.ly/2LDSDxI
- Jia JH, Li T, Cui HH, Li YZ, Wang WB, Han L, et al. Study on the synthesis and third-order nonlinear optical properties of D-A poly-quinacridone optical materials. Dyes and Pigments. 2019; 162: 26-35. https://bit.ly/2RSm3cD
- Shetty TCS, Raghavendra S, Kumar CSC, Naveen S, Patil MSR, Chandraju PS, et al. Crystal structure, Hirshfeld and third-order nonlinear optical properties of 3-(4-Dimethylamino)Phenyl)-1-(4-Methoxyphenyl)Prop-2-En-1-One: A potential material for optical limiting applications. Optical Materials. 2018; 86: 138-147. https://bit.ly/2YBnoqO
- Bredas JL, Adant C, Tackx P, Persoons A, Pierce BM. Third-order nonlinear optical response in organic materials: theoretical and experimental aspects. Chem Rev. 1994; 94: 243-278. https://bit.ly/2xxONxM
- Qian Y. 3,6-Disubstituted carbazole chromophores containing thiazole and benzothiazole units: synthesis, characterization and first-order hyperpolarizabilities. Dyes and Pigments. 2008; 76: 277-281. https://bit.ly/2L18XJy
- Wang D, Sui Y, Yin J, Tan GZ, Zhu ZK, Wang ZG. Poling and orientational stability of a novel poly (urethane-imide) containing a diazo nonlinear optical chromophore in side-chain. Journal of Functional Polymers. 2001; 14: 35-38. https://bit.ly/2YzUAPc
- Wang J, Liang ZX. Second order nonlinear optical polymeric materials. Journal of Functional Polymers. 1999; 12: 479-485.
- Gao HF, Ren GM, Sun JY, Shi YF, Zhao MG. Synthesis and third-order nonlinear properties of a benzothiazole vinyl derivative. Journal of Shanxi University (Nat. Sci. Ed.). 2018; 39: 626-630.
- Zhang L, Ding L, Zhang F, Wu QS. Thirder-order nonlinearity optical property of azoheterocycle-containing side-chain polymer. Journal of Yangzhou University (Nat. Sci. Ed.). 2016; 19: 29-32.
- Xia XQ, Zhao SL, Jiang J, Wu ZT. Synthesis of nitrobenzothiazolydiazoaminoazobenzene and its colour reaction with acdmium. Chinese Journal of Analytical Chemistry. 1998; 26: 103-106.
- Goikhman MY, Subbotina LI, Gofman IV,Yakimanskii AV, Bursian AE, Lukoshkin VA, et al. Polyamidoimides with side chromophoric groups. Russian Chemical Bulletin. 2005; 54: 1481-1487. https://bit.ly/2RYQ36K
- Li NJ, Lu JM, Xia XW, Xu QF, Wang LH. Synthesis and the third-order nonlinear optical properties of soluble polymers with different substituted azobenzene side chains. Polymer. 2009; 50: 428-433. https://bit.ly/2Yymn2z
- Wu YL, Demachi Y, Tsutsumi O, Kanazawa A, Shiono T, Ikeda T. Photoinduced alignment of polymer liquid crystals containing azobenzene moieties in the side chain. 3. Effect of structure of photochromic moieties on alignment behavior. Macromolecules. 1998; 31: 4457-4463. https://bit.ly/327uMwl

Sign up for Article Alerts