Case Series Analysis of Necrotizing Fasciitis in a District Hospital
Vasarhelyi F*, Arany L, Kiraly Z, Naik K, Bott A R and Deo SD
Department of Trauma & Orthopaedics, Great Western Hospitals NHS Foundation Trust, Marlborough Rd Swindon, SN3 6BB, United Kingdom*Address for Correspondence: Vasarhelyi F, Department of Trauma & Orthopaedics, Great Western Hospitals NHS Foundation Trust, Marlborough Rd Swindon, SN3 6BB, United Kingdom, Tel: +441-793-604-020; E-mail: f.vasarhelyi@nhs.net
Submitted: 25 October 2020; Approved: 07 November 2020; Published: 09 November 2020
Citation this article: Vasarhelyi F, Arany L, Kiraly Z, Naik K, Bott AR, et al. Case Series Analysis of Necrotizing Fasciitis in a District Hospital. American J Current App Res Microbiol. 2020;2(1): 018-023.
Copyright: © 2020 Vasarhelyi F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Necrotizing; fasciitis; sepsis; severe infection; skin; musculoskeletal system; streptococcus A; synergistic infection
Download Fulltext PDF
Introduction: Necrotizing Fasciitis is the most feared soft tissue infection in surgery, due to rapid progression and high mortality. Diagnosis can be challenging in the initial phase. Early recognition, radical surgical intervention and multidisciplinary management will determine the prognosis. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) scoring system can be helpful in uncertain situations to differentiate Necrotizing Fasciitis from other musculoskeletal infections and conditions.
Patients and methods: This is a retrospective, case series. We obtained all Intensive Care Unit (ICU) admissions from our ICU & hospital coding database. We reviewed all patients with a diagnosis of Necrotizing Fasciitis in a 12-year period, 2003 to 2015. We went on to review case notes, serology, microbiology and radiology results.
Aims: Our aims were to define the populations most at risk of developing Necrotizing Fasciitis, detect epidemiologic trends, assess our performance with published series and validate the use of the LRINEC score.
Results: Our series comprises 32 patients, with 19 (60%) occurring in a 3 year time period. The mean age of patients was 55, median 59 (range 20 to 83), with almost equal male to female ratio. Almost 2/3 (62%) were admitted under surgical disciplines. Twenty nine (91%) underwent surgery and the overall mortality rate was 56%, within a mean of 3 days. No patients treated non-operatively survived. We applied the LRINEC criteria to the patients to assess its sensitivity and predictive value. We noted an increasing number of monomicrobial cases, mostly due to virulent, rapidly progressing Group A β-hemolytic Streptococcal infection.
Conclusion: A high index of suspicion and the use of the LRINEC score can help to diagnose Necrotizing Fasciitis in its early stages. However, decisions in terms of definitive management are led by clinical findings and must be achieved as rapidly as possible. Early aggressive surgical debridement, high dose antibiotics & multidisciplinary management is crucial for patient-survival. The prognosis is highly influenced by the age and medical co morbidities; therefore, the mortality remains high.
Introduction
The term Necrotizing Fasciitis (NF) or Necrotizing Soft Tissue Infection (NSTI) refers to severe inflammation of the muscle sheath that leads to necrosis of the subcutaneous tissue and adjacent fascia [1]. It is associated with rapid progression and a high risk of mortality despite advances in antibiotics, surgery, intensive care and interdisciplinary management. Rapid progression and necrosis leads to a severe Systemic Inflammatory Response Syndrome (SIRS), multi-system organ failure and death [2]. The condition is frequent enough that almost every hospital physician will at some point be involved in the care of a patient suffering with NF. However, due to its relative rarity, familiarity of the condition and management cannot be always guaranteed [3].
NF`s first description comes from Hippocrates from around 500 BC, as complication of erysipelas [4]. Claude Colles described a condition very similar to the modern definition of NF in 1783, but the first modern description of NF comes from the American Civil war, when J. Jones army surgeon reported more than 2000 gas gangrene cases with high mortality rate 46% [5,6]. Jean Alfred Fournier described a syndrome with necrosis of the perineum in 1883, this type of NF is known as Fournier’s gangrene [7]. In 1952, the term “Necrotizing Fasciitis” was proposed by Wilson, as a more accurate description of this disease [8]. The disease was popularized by the media as “flesh-eating bacteria syndrome” [9].
Epidemiology
The prevalence of NF reported to be around 0.24-0.4 cases per 100,000 population and a male-to-female ratio of 3:1 [10]. The disease can affect all age groups. The median mortality is around 34% [11]. However, its range in the literature is extensive, varying from 9 to 76% [1]. In regard to NF of the extremities, the mortality rate is slightly lower than that recorded for abdominal and perineal infections [12,13]. As a general rule: without treatment, the mortality rate approaches 100%.
Microbiology
There is wide spectrum of bacteria that can cause NF, these can be broadly classified into two groups (bear in mind that there are classifications mentioning 4 groups) [2].
Type I NF (synergistic NF)
Type I NF is a polymicrobial infection caused by aerobic, anaerobic and facultatively anaerobic bacteria (gram-negative and positive). Most of the bacteria are part of the bowel flora (E. coli, Bacteroides, Pseudomonas species). Recent surgery, abdominal malignancy and immune compromise are the most common risk factors [2,14].
Type II NF (monomicrobial)
Type II NF is usually caused by gram-positive organisms. The most common pathogen is Group A β-haemolytic Streptococcus (GAS) alone or in combination with other species such as Staphylococcus aureus. Risk factors in this group includes injuries that can cause breach to the dermis, recent surgery, varicella infection, Intravenous Drug Use (IVDU) or haematogenous spread. This type of NF has the highest mortality rate approaching 50-70%.
Pathophysiology
Infection begins in the hypodermis and superficial fascia and spreads along the muscle fascia and the overlying dermis can appear normal [15]. This makes NF very difficult to diagnose in the early stage. The overall pathophysiology is an exotoxin driven toxic shock syndrome with massive cytokine release (cytokine storm) and T cell proliferation due to the M protein of Group A β-hemolytic Streptococcal GAS. This protein acts as a virulence factor and also produces pyogenic exotoxins, well known as super-antigens. This combination leads to SIRS, which can progress to multi organ failure and death. It is the prevalence of this cascade occurring with Type II NF that generates the very high mortality rate of 40-67% [2]. Thrombosis of the nutrient arterioles in the hypodermis leads to necrosis. This can then affect nerve branches; causing pain which is usually described as out of proportion.
Risk Factors
As already mentioned above, recent surgery, immune compromise, malignancy, trauma can increase the risk of developing necrotising fasciitis. Age over 50 years, IVDU, obesity, peripherial vascular disease and diabetes are general risk factors [1].
Non-steroidal anti-inflammatory drugs (NSAIDs) are thought to mask early signs of NF, but they can also inhibit neutrophil function and facilitate cytokine release [2,14].
Clinical Diagnosis
Diagnosis of NF is often very difficult due to the lack of specific signs. High index of suspicion and the presenting pain, which is out of proportion, should guide the management. Clues and risk factors from the history (recent surgery, IVDU, diabetes, trauma) can help us to get closer to the diagnosis. Most common symptoms are pain, fever, tachycardia [2]. Skin changes early on may look like a rapidly progressing, florid cellulitis. Generalised cutaneous and sub-cutaneous oedema, haemorrhagic bullae and skin necrosis are late presentation of the infection and usually accompanied by symptoms of multi-organ failure (haemodynamic instability, low urine output, etc.) [1]. Crepitus (gas formation) can be found in some cases.
Differential diagnosis includes cellulitis, deep vein thrombosis, myonecrosis, allergic reaction, auto-immune vasculitis, or sunburn. Necrotizing Fasciitis is a surgical diagnosis in that the true definitive diagnosis is the visualisation of “dishwater fluid” in fascial planes and necrotic subcutaneous tissues. The diagnosis can however be accurately confirmed prior to this by appropriate ultrasound or MRI imaging.
Imaging such as X-ray, Computed Tomography (CT), Magnetic Resonance Imaging (MRI) should never delay surgical intervention, but those can be useful in diagnosing NF if there is subcutaneous gas present and the patient is stable.
Treatment
Definitive treatment of NF generally surgical debridement of all necrotic tissue, coupled with broad spectrum antibiotics and haemodynamic support. In our series no surviving patient avoided surgery. These treatments should be delivered promptly as a team involving intensive care physicians, surgeons and microbiologists.
Radical surgical debridement or occasionally amputation is necessary. Without surgery the mortality rate approaches 100%. This means NF is a surgical emergency. Patients will require further re-assessment in theatre after the first debridement, usually 12-24 hrs later. Any deterioration in the patient`s condition should facilitate prompt further, sooner surgical intervention as clinical deterioration indicates infective progression through remaining tissue planes, with escalating risk of SIRS & multi organ failure if left untreated.
High dose, intravenous antibiotics are essential in NF, and should be given as soon as the diagnosis is suspected. They may prevent progression of septic shock when commenced early. It is well known that early administration of antibiotics within the first hour of documented hypotension is associated with increased survival in patients with septic shock. Also, the delay in administering antibiotics was associated with decrease in survival.17 Appropriate broad-spectrum antibiotics should be used to cover against gram positive, negative and anaerobic organisms:
There are some pitfalls when we try to choose:
1. Penicillin sounds a good choice, but high concentration of GAS in the tissue results in most bacteria being in stationary growth phase where there is no cell wall synthesis - which is the target of penicillins [2].
2. It would be more useful to switch off the exotoxin synthesis as this will derive SIRS [2]
3. Should have cover for the synergistic gram negative and positive bacteria
4. Ideally should have cover for Methicillin-Resistant Staphylococcal (MRSA) infections until proven otherwise [22]Most hospitals now have published antibiotic guidelines for possible NF and other severe infections, which are regularly reviewed to account for local antibiotic prevalence, sub-types and resistance patterns. Common combinations include: the use of combinations of Clindamycin/Meropenem/Linezolid. Clindamycin can switch off exotoxin production even in the stationary growth phase [2]. Meropenem will cover for the synergistic gram negative and positive bacteria. Linezolid can be used if MRSA present [22].
After administering intravenous antibiotics and urgent surgical referral, every patient with the suspicion of possible NF should be transferred to intensive care unit where general supportive treatment, e.g. ventilation, inotropic, renal support and close monitoring are available. The use of intravenous immunoglobulins (IVIG) and hyperbaric oxygen are newer treatment options under evaluation. IVIG thought to be useful in GAS infection as they contain neutralising antibodies that act against streptococcal antigens [23]. Hyperbaric oxygen thought to increase the bactericidal effects of neutrophils, but the access to hyperbaric oxygen units are very limited worldwide [2].
Our Case Series
We describe a single institution, retrospective, case series. We reviewed all patients with a diagnosis of Necrotizing Fasciitis or necrotizing soft tissue infection, over a 12 year period from 2003 to 2015. We went on to review case notes, serology, microbiology and radiology results.
Our hospital is a 600 bed multi-specialty district general hospital, serving a population of approximately 350, 000 patients. Based on the past 3 years data, we estimate that in the 12 year study period we had over 850,000 Emergency Department attendances, 450,000 emergency (non-elective) admissions and there were 8,607 ICU admissions. We did not include neonates in this study. We obtained all Intensive Care Unit (ICU) admissions from our ICU and hospital coding databases.
In March 2015 we introduced the LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) scoring system to help distinguish from other types of soft tissue infections. This scoring system was first introduced in 2004 by Wong et al. and is based on laboratory investigations such as white cell count, CRP, haemoglobin, sodium, creatinine and glucose as these were found to be most statistically significant indicators for NF (Table 1). The original study by Wong, et al. included that a score of six or above should raise suspicion of NF. In this article, the positive predictive value of the LRINEC score was reported as 92% with a negative predictive value of 96%. We went on to apply this scoring system to our patients [16].
The key study aims were:
1. to define the populations most at risk of developing necrotising fasciitis,
2. to assess our performance with published series,
3. to validate the use of the LRINEC score.
Results
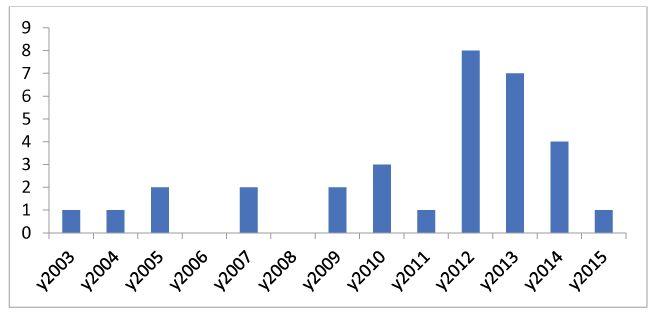
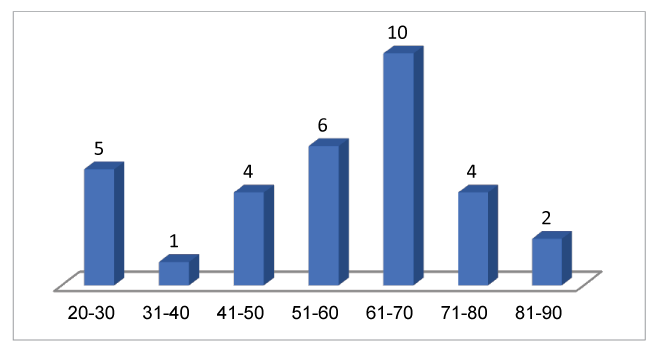
Following data received from our databases, our series comprises 32 patients, with 19 (60%) occurring in a 3 year period from 2012 to 2014. According to the literature and the population of GWH Swindon the predicted number of cases per year is around 2.6. However, between 2012 and 2014 we admitted almost three times more patients with the diagnosis of Necrotizing Fasciitis than predicted (Figure 1). The mean age of patients was 56, median 59, with age range 20 to 83. The age distribution is shown in Figure 2. There was an almost equal male to female ratio, 54% male.
One may observe varying incidences of NF, as seen in our case series-we observed a high incidence of NF in 2012 and 2013. There appears to be no causal link to this, but it highlights the fact that sporadic “runs” of such cases can and do occur. However, one should therefore maintain a high index of suspicion of NF, particularly in patients with the above mentioned risk factors.
Almost 2/3 of our patients (62%) were admitted initially under surgical disciplines and 29 patients (91%) underwent surgery and the overall mortality rate was 56% within an average time to death of 3 days. This mortality rate is slightly higher than the overall average in the literature although we found articles reporting mortality rate with 76% [1].
In our study period increasing number of monomicrobial cases were found, mostly representing as rapidly progressing Group A β-hemolytic Streptococcal (GAS) infection. 62% of all cases were found as Type I NF and 38% of cases presented as monomicrobial NF and affected the upper or lower extremities. The mortality rate in Type II group reached 75%. All cases admitted under orthopaedics were Type II NF.
In our Hospital, every patient will be assessed following the “Sepsis 6 pathway” with a clinical suspicion of infection on presentation or any unwell or deteriorating patient.
The Sepsis Six is an initial resuscitation bundle designed to offer basic intervention within the first hour. This pathway is developed to facilitate early diagnosis of sepsis and commencement of early aggressive treatment and referral to subspecialties and intensive care [17-21] To complete this pathway accurate recording of vital observations and basic urgent laboratory investigations are needed (Figure 3).
We went on to apply the LRINEC criteria (see Figure 2) to the patients in our study period to assess its sensitivity. We were able to calculate LRINEC score for 26 patients out of total 32 (81% of our cohort). We had 3 patients with a false negative LRINEC score (score < 6 and proven diagnosis of necrotising fasciitis). Therefore, in our series the sensitivity and positive predictive value of LRINEC was 88% compared to 92% reported by Wong et. al.
Departmental Policy Change
After valuation of the LRINEC scoring system, we introduced new guidance (Table 2), to be used in the Accident and Emergency Department (A&E) in suspicious cases, as we think there is a role for the LRINEC criteria in diagnosing NF in its early stage.
Summary
Necrotising fasciitis:
1. can kill, and delay to diagnosis and treatment is a key factor
2. is not common, but is not rare either
3. disease progression is often rapid
4. early identification or suspicion of this problem is essential
5. use of pathways such as Sepsis 6 and criteria such as LRINEC are useful
6. early broad spectrum high-dose IV antibiotics as recommended locally are mandatory
7. early surgery either in terms of radical debridement or high amputation may be necessary and can be life saving.
8. will occur sporadically and clusters of patients do occur
Authors’ contributions
All authors participated in the design of the paper, conceived the paper, and participated in drafting and critical revision for important intellectual content. All authors read and approved the final form of this manuscript.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007 Mar 1;44(5):705-10. doi: 10.1086/511638. Epub 2007 Jan 22. PMID: 17278065.
- Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010 Aug;75(4):249-57. doi: 10.1016/j.jhin.2010.01.028. Epub 2010 Jun 9. PMID: 20542593.
- Ellis Simonsen SM, van Orman ER, Hatch BE, Jones SS, Gren LH, Hegmann KT, Lyon JL. Cellulitis incidence in a defined population. Epidemiol Infect. 2006 Apr;134(2):293-9. doi: 10.1017/S095026880500484X. PMID: 16490133; PMCID: PMC2870381.
- Descamps V, Aitken J, Lee MG. Hippocrates on necrotising fasciitis. Lancet. 1994 Aug 20;344(8921):556. doi: 10.1016/s0140-6736(94)91956-9. PMID: 7914656.
- Pouteau C. Mémoire ou recherches sur les symptômes de la gangrene humide des Hôpitaux. editor. uvres posthumes. (Vol. 3), Paris, 1783; p. 239-268.
- Jones J. Surgical Memoirs of the War of the Rebellion: Investigation Upon the Nature, Causes and Treatment of Hospital Gangrene as Prevailed in the Confederate Armies 1861-1865. New York, NY: US Sanitary Commission. 1871.
- Fournier JA. Gangrene foudroyante de la verge. Med Pract. 1883; 4: 589-597.
- Wilson B. Necrotising fasciitis. Am Surg. 1952; 18: 416-431.
- Stevens DL, Tanner MH, Winship J. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989; 321: 17. doi: 10.1056/NEJM198907063210101.
- Kaul R, McGeer A, Low DE, Green K, Schwartz B. Population based surveillance for group A streptococcal necrotising fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am J Med. 1997; 103: 18-2410. doi: 1016/S0002-9343(97)00160-5.
- McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995 May;221(5):558-63; discussion 563-5. doi: 10.1097/00000658-199505000-00013. PMID: 7748037; PMCID: PMC1234638.
- Schecter W, Meyer A, Schecter G. Necrotising fasciitis of the upper extremity. J Hand Surg. 1982; 7:15–910. doi: 1016/S0363-5023(82)80006-3.
- Eke N. Fournier's gangrene: a review of 1726 cases. Br J Surg. 2000 Jun;87(6):718-28. doi: 10.1046/j.1365-2168.2000.01497.x. PMID: 10848848.
- Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52. doi: 10.1093/cid/ciu444. Erratum in: Clin Infect Dis. 2015 May 1;60(9):1448. Dosage error in article text. PMID: 24973422.
- Gozal D, Ziser A, Shupak A, Ariel A, Melamed Y. Necrotizing Fasciitis. Arch Surg. 1986 Feb;121(2):233-5. doi: 10.1001/archsurg.1986.01400020119015. PMID: 3947221.
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing Necrotizing Fasciitis from other soft tissue infections. Crit Care Med. 2004 Jul;32(7):1535-41. doi: 10.1097/01.ccm.0000129486.35458.7d. PMID: 15241098.
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589-96. doi: 10.1097/01.CCM.0000217961.75225.E9. PMID: 16625125.
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003 Apr;31(4):1250-6. doi: 10.1097/01.CCM.0000050454.01978.3B. PMID: 12682500.
- Trzeciak S, Chansky ME, Dellinger PR. Operationalizing the use of serum lactate measurement for identifying high risk of death in a clinical practice algorithm for suspected severe sepsis. Acad Emerg Med 2006; 13: 150-151.
- Daniels R, Nutbeam T, McNamara G, Galvin C. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J. 2011 Jun;28(6):507-12. doi: 10.1136/emj.2010.095067. Epub 2010 Oct 29. PMID: 21036796.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013 Feb;41(2):580-637. doi: 10.1097/CCM.0b013e31827e83af. PMID: 23353941.
- Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, Tang AW, Phung TO, Spellberg B. Necrotizing Fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005 Apr 7;352(14):1445-53. doi: 10.1056/NEJMoa042683. PMID: 15814880.
- Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O'Rourke K, Talbot J, Low DE. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome--a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis. 1999 Apr;28(4):800-7. doi: 10.1086/515199. PMID: 10825042.


Sign up for Article Alerts