Triple Drug Regimen as Induction Treatment of Lupus Nephritis: a Pilot Randomized Controlled Trial?
Arpita Roy Chowdhury*, Subho Banerjee, Rayees Yousuf, Dipankar Sircar and Rajendra Pandey
Department of Nephrology, Institute of Post Graduate Medical Education and Research, Kolkata, West Bengal, India
*Address for Correspondence: Arpita Roy Chowdhury, Department of Nephrology, Institute of Post Graduate Medical Education and Research, 244 A.J.C Bose Road, Kolkata, West Bengal, India, 700020, ORCID: orcid.org/0000-0002-3601-6579; E-mail: lahiri.arpi@gmail.com
Submitted: 27 January 2018; Approved: 16 February 2018; Published: 19 February 2018
Citation this article: Chowdhury AR, Banerjee S, Yousuf R, Sircar D, Pandey R. Triple Drug Regimen as Induction Treatment of Lupus Nephritis: a Pilot Randomized Controlled Trial. Int J Nephrol Ther. 2018;4(1): 007-011.
Copyright: © 2018 Chowdhury AR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Introduction
The management of Proliferative Lupus Nephritis (PLN), in spite of the progressive enlargement of the drug armamentarium, remains a challenge and is largely unsatisfactory. Balancing the immunosuppression and the risk of infection is like a tight rope walk. Although pulsed doses of cyclophosphamide in conjunction with steroids (the ‘NIH’ protocol) remains the standard of care in most centers, several newer options have become available to the treating physicians [1]. The low dose cyclophosphamide regimen, Mycophenolate Mofetyl (MMF), calcineurin inhibitors and more recently the multitarget therapy regimen have had reasonable success in induction of complete or partial remission in PLN [2-6]. The Induction therapy is particularly important for treating this severe disease because the patients with complete remission typically have a better prognosis, with fewer episodes of relapse, than the patients who do not achieve remission. (Chen ye, Korbet SM et al: Value of complete or partial remission in severe lupus nephritis CJASN 2008 and Moroni G et al: the long term outcome of 93 patients with proliferative lupus nephritis. NDT 2007). We are yet to achieve satisfactory remission rate with the induction regimes available. The study was done with multiple immunosuppressive agents so that various Tcell and B cell dependent pathogenic mechanisms of Lupus nephritis may be blocked simultaneously using minimum possible dose of each drug so that toxicity of the individual drug can be avoided and at the same time net immunosuppression remains high enough to tackle the disease. Multitarget therapy with similar hypothesis worked out well in the Chinese population [5,6].
We present a pilot study that combined tacrolimus with azathioprine along with steroids (Triple Drug Regimen) in the induction phase of treatment of PLN. The transplant experience with MMF and azathioprine makes us believe that azathioprine is a slightly less potent immunosuppressive agent compared to MMF [7]. Although the replacement of MMF with azathioprine doesn’t clearly affect graft survival the incidence of opportunistic infections is reduced. Most of the patients at our center hail from a low socioeconomic background where immunosuppression related sepsis remain a major concern. A previous attempt to replicate the early success of the multitarget therapy at our center had to be terminated prematurely due to unacceptable rates of severe sepsis. The hypothesis behind the present study was to offer the benefit of tacrolimus for both immunological and nonimmunological action targeting podocytes. Azathioprine was added to act synergistically with tacrolimus increasing immunosuppressive efficacy, to reduce the burden of infection and for being economically more affordable, making the regime suitable for better compliance.
Randomized trials have shown activity of tacrolimus as a single agent in the induction therapy of PLN [4]. Tacrolimus, in a manner similar to cyclosporine, is postulated to have direct stabilizing effect on the podocytes [8]. This effect may be independent of the immunosuppressive effect of the calcineurin inhibitors and possibly related to the stabilization of the actin cytoskeleton and other proteins that maintain the integrity of the podocytes [9]. Azathioprine, on the other hand, has largely been rejected as a single agent in induction therapy in PLN [10]. However it continues to be an acceptable choice in the maintenance phase. A combination of tacrolimus and azathioprine may have complementary effect and may induce remission in PLN.
Methods
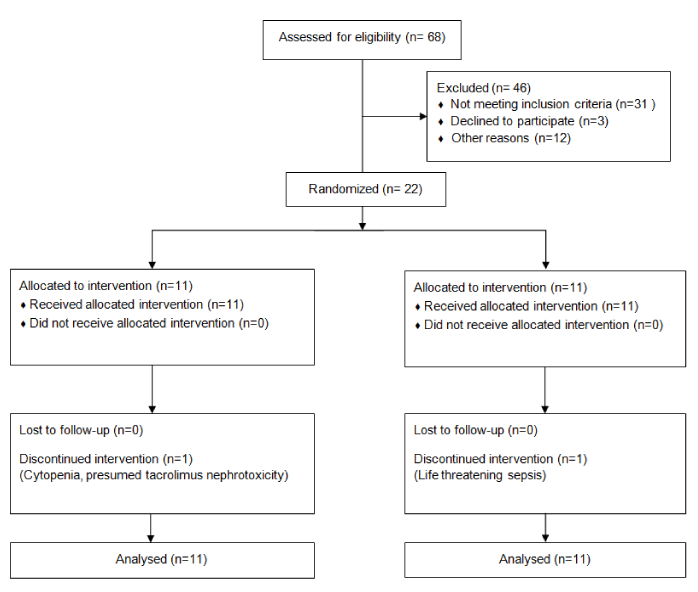
Design: The study was conducted in the Department of Nephrology in a tertiary hospital in Eastern India between June 2014 and June 2015. The study was designed as a single center randomized open label clinical trial with an active control group. Consecutive patients admitted with a diagnosis of SLE as per SLICC classification criteria and a histopathological diagnosis of Proliferative Lupus Nephritis (ISN/RPS class III and IV, alone or in combination with class V) on renal biopsy, were randomly assigned to either the tacrolimus/azathioprine group or the intravenous cyclophosphamide group, which was the active control group. The patients or the treating physicians were not blinded to the treatment protocols. The Institutional Ethics Committee had approved the study design and all participants were required to provide written informed consent before inclusion (Figure 1).
Settings and participants: Patients between the ages of 15 to 60 years with biopsy proven proliferative lupus nephritis (International Society of Nephrology/Renal Pathology Society classes III, IV, V+III or V+IV) were considered for inclusion. Exclusion criteria included patients with biopsy proven class IIIC/IV C lupus nephritis, an estimated Glomerular Filtration rate (eGFR) of <30 ml/min/1.73 m2, life threatening complication of SLE (including specifically but not limited to cerebral lupus), contraindication to any of the therapy protocols or known hypersensitivity to treatment drugs, pregnancy or lactation and those with active life threatening infection/sepsis at onset, active viral hepatitis, HIV, any malignancy or any contraindication for immunosuppression.
Randomization: After inclusion, patients were randomized using a computer generated random number sequence in a 1:1 ratio to the two intervention groups. Concealment was done using sequentially numbered sealed opaque envelopes.
Interventions: Common intervention in both groups included 3 pulsed doses of methylprednisolone 500mg each given on days 1 through 3. Subsequently, prednisolone was given at doses of 0.5 mg/kg/day as a single daily dose for the next 1 month and then tapered as tolerated to 10mg or less by 3 months. All patients received hydroxychloroquine at doses of 6 mg/kg rounded to the nearest 50 mg multiple. Angiotensin Converting Enzyme (ACE) inhibitors or Angiotensin Receptor Blockers were used in all patients with hypertension as the preferred agents. The choice was limited to Ramipril and Telmisartan. Statins were used a fixed dose of 10 mg/day in all patients who tolerated the drug. The Triple Drug regimen group received tacrolimus (0.075 mg/kg, rounded to the nearest 0.5 mg given as twice a day) and azathioprine (2 mg/kg, rounded to the nearest 25 mg given as a single dose). The trough tacrolimus levels were maintained between 5-10 ng/ml. The control group received intravenous cyclophosphamide, at a dose of 500 mg/m2 once a month as an infusion over 4 hours, with premedication with intravenous Mercaptoethane Sulphonate Sodium (MESNA, 400mg). Dose adjustments were done for renal dysfunction and the nadir leukocyte counts.
Follow up and outcomes
The patients on the triple drug regimen were followed weekly till their tacrolimus trough levels were adjusted to the therapeutic levels; subsequently they were followed once a month till three months and then at the end of 6 months. The patients on the cyclophosphamide were followed up every month on the day of their scheduled dose, which were given as a day care procedure. A complete blood count was done every month 10-14 days after the previous pulsed dose to check the nadir leukocyte counts. A complete response was defined as return of the serum creatinine to baseline or within lab references (when baseline was not known) plus decrease in proteinuria to less than 500 mg/day. A partial response was defined as a decrease of serum creatinine but not reaching baseline (or normal reference) and a 50% or more decrease in proteinuria but not less than 500 mg/day (essentially less than 3 g/day). Those with deterioration or not meeting either of these criteria at three months of therapy were regarded as failure. However those who were clinically stable but some improvement were allowed to stay on in the respective therapeutic arms and evaluated at the end of 6 months.
Statistical Analysis
As the trial was designed as a pilot study, the sample size was restricted to 11 patients per arm, in view of the absence of efficacy and safety data of the triple drug regimen. Categorical variables (for example, complete remission, overall response, and adverse experiences) were analyzed by using the Fisher exact tests. Continuous variables were analyzed by using a t-test, or Wilcoxon signed-rank test, if the data were skewed. Kaplan–Meier estimates of the cumulative probability of complete remission and overall response and median time to overall response were calculated; the between-group difference was compared by using the log-rank test.
Results
Between June 2014 and June 2015, 68 patients were diagnosed with lupus nephritis were screened for inclusion into the study; 22 patients were included after the exclusion and consent process. The demographic determinants and the baseline characteristics are presented in table 1.
A total of 20 patients completed the study. The primary outcome measures were similar in both groups. Complete remission occurred in 7 patients on the triple drug regimen and in 6 patients in the cyclophosphamide arm (63.6 % vs 54.5 %, p = 1.00). Partial remission occurred in 2 patients in the triple drug group and 4 patients in the cyclophosphamide group (18.2% vs. 36.3%, p = 0.64). Overall the composite of any response (complete or partial) occurred in 9 and 10 patients respectively (81.8% vs 90.9%, p = 1.00). There was a trend towards more rapid remission in the triple drug regimen, which was most apparent in the 3rd month. However the numbers were not statistically different at any time during follow up.
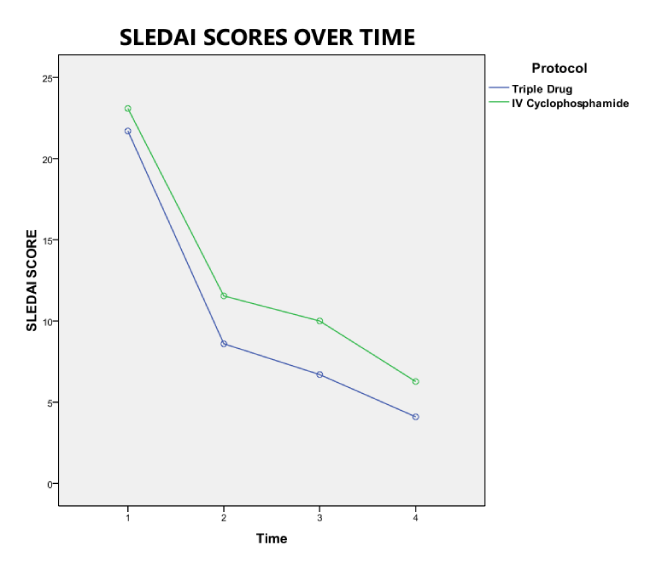
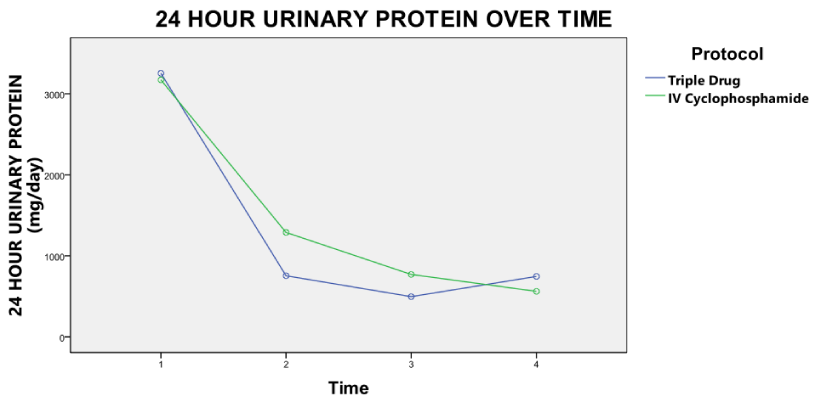
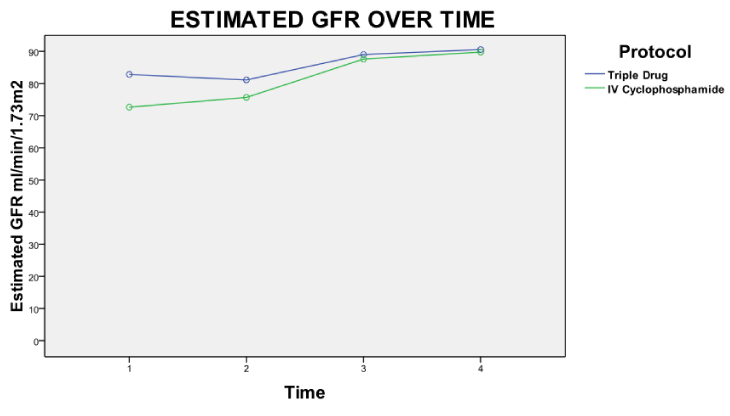
At the end of the study, there was no difference in the clinical and laboratory parameters between the two groups (Table 2). The SLEDAI activity improved more rapidly in the triple drug group, (Figure 2) riding mostly on the improvement of proteinuria but at the end of the study, differences were not statistically significant. A clear trend towards early remission of proteinuria in the triple drug regimen was appreciated (Figure 3) and the median proteinuria in the triple drug group was lower at 1 month (700 mg/d vs 1258 mg/d), 3 months (360 mg/day vs. 723 mg/d) and 6 months (184 mg/d vs 315 mg/d). However, the differences were not statistically significant. Comparison of eEGFR improvement was comparable in both groups (Figure 4).
The median cumulative dose of cyclophosphamide given was 3250 (first and third quartiles 3000 and 3750 mg, respectively). Two patients on cyclophosphamide developed sepsis requiring in-patient management. Significant leucopenia occurred in one patient for whom the subsequent dose was delayed and dose reduction done. One patient discontinued therapy after the episode of sepsis. Both the episodes of sepsis occurred in the first 3 months of therapy, when doses of prednisolone were relatively higher. At the end on the six month duration, ovarian suppression was documented in one patient (Age at presentation 32 years, total dose of cyclophosphamide received 3700 mg; presented with menstrual irregularity after completion of therapy and was documented to have elevated gonadotropins after 3 months of completion of therapy).
The mean tacrolimus trough level in the patient on triple drug regimen was 7.25 ± 1.6 ng/ml and the mean daily dose of azathioprine used was 84.09 ± 12.6 mg. Two patients developed reversible elevation of serum creatinine (one of the patients had a high trough level); of these one patient had to discontinue therapy and the other was managed with dose reduction alone. The patient who had to discontinue the drug also developed leucopenia and hence a decision to change therapy was taken by the treating physician at 3 months. One patient in this group developed varicella infection for which drugs were discontinued for 1 month. No patient developed hyperglycemia.
Discussion
In the present study, a combination of tacrolimus and azathioprine, together with steroids (named the Triple Drug Regimen) proved to be at least as effective as monthly cyclophosphamide and quite safe in the induction of remission of proliferative lupus nephritis. To the best of our knowledge, this is the first report of such a regimen.
Most of the knowledge regarding the modes of action of tacrolimus in immunosuppressive regimen stems from bench-side studies in organ transplant. Tacrolimus mainly suppresses IL-2 transcription, but also inhibits T cell activation and decreases TNF-α, IFN-γ and inhibit IL-10 production [11-14]. How exactly this effect is translated into clinical benefit remains elusive but tacrolimus monotherapy has been found to be effective in small open label trials of PLN [4]. Azathioprine, an antimetabolite, acts by incorporation into the cellular DNA and inhibition of gene replication, ultimately preventing T-cell activation [15]. Tacrolimus and azathioprine combination remains the standard of care in renal transplant patients who are unable to tolerate the adverse effects or costs of mycophenolate mofetyl. Acting through different mechanisms their immunosuppressive effects are additive or synergistic, and do not share a common side effect profile.
It is obvious that in essence, the theme is the same as the multi-target regimen used successfully in two Chinese cohorts [5,6]. However the safety aspect of the regimen was a matter of greater concern for us. From indirect observations that MMF affords more protection from graft loss, is associated with higher incidence of malignancy and certain infections in post-transplant patients, an inference that MMF has higher immunosuppressive potency compared to azathioprine seems justified; such a correlation has never been proven. A previous study in our institute using the combination of tacrolimus and MMF had to be terminated prematurely due to unacceptable incidence of sepsis. The rates of complete and partial remission in our study are similar to previous reports.
Tacrolimus related nephrotoxicity is a major limitation in various settings where the drug is used. It can cause both acute and long term deterioration in renal function. In the Chinese cohort of tacrolimus induction in lupus nephritis, incidence of transient rise of creatinine was reported to be 8.15% [5]. We also found a similar incidence (10% when the trough levels were within therapeutic range). Although biopsy was not done at the end of induction phase, it is reasonable to assume that significant chronic toxicity is unlikely to occur with 6 months of therapy with tacrolimus as calcineurin inhibitor toxicity is cumulative over time. Also the toxicity is related to the dose of tacrolimus used and in the present study we have opted for a relatively lower dose than is usually used in the setting of transplantation.
The burden of sepsis was lesser in the triple drug group. Sepsis remains the Achilles heel of treatment with immunosuppressive in most settings.
We have not re-biopsied the patients to look for histologic remission, but triple drug regimen with tacrolimus, MMF and steroids showed that the multi target therapy induces histological remission as well.
Conclusion
The pilot study has documented that induction treatment of lupus with Tac-Aza-Pred triple drug regimen is non inferior to IV CYC steroid standard therapy with documented less sepsis related adverse events in triple drug arm. The earlier response of proteinuria and SLEDAI score further strengthens the probability of achieving complete remission faster with this modality. The strength of the study lies in its randomized design and limitation is its small number and reported from a single centre.so this triple drug therapy can be accepted as an alternative to IV CYC regime to apply in larger number of patients.
- Austin III HA, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986; 314: 614-619. https://goo.gl/J6UZjN
- Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido Ed Ede R, Danieli MG, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002; 46: 2121-2131s. https://goo.gl/xrg7Ma
- Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009; 20: 1103-1112. https://goo.gl/F2i2CD
- Mok CC, Tong KH, To CH, Siu YP, Au TC. Tacrolimus for induction therapy of diffuse proliferative lupus nephritis: an open-labeled pilot study. Kidney international. 2005; 68: 813-817. https://goo.gl/cmWzvH
- Bao H, Liu ZH, Xie HL, Hu WX, Zhang HT, Li LS. Successful treatment of class V+ IV lupus nephritis with multitarget therapy. J Am Soc Nephrol. 2008; 19: 2001-2010. https://goo.gl/Rxr9yn
- Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z, et al. Multitarget therapy for induction treatment of lupus nephritisa randomized trial. Ann Intern Med. 2015; 162: 18-26. https://goo.gl/7q4ebR
- Wagner M, Earley AK, Webster AC, Schmid CH, Balk EM, Uhlig K. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2015; 3. https://goo.gl/9By16z
- Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008; 14: 931-938. https://goo.gl/qVdmd8
- Schonenberger E, Ehrich JH, Haller H, Schiffer M. The podocyte as a direct target of immunosuppressive agents. Nephrology Dialysis Transplantation. 2011; 26: 18-24. https://goo.gl/EHPJFo
- Grootscholten C, Bajema IM, Florquin S, Steenbergen EJ, Peutz‐Kootstra CJ, Goldschmeding R, et al. Treatment with cyclophosphamide delays the progression of chronic lesions more effectively than does treatment with azathioprine plus methylprednisolone in patients with proliferative lupus nephritis. Arthritis Rheum. 2007; 56: 924-937. https://goo.gl/9SbBSo
- Andersson J, Nagy S, Groth CG, Andersson U: Effects of FK506 and cyclosporin A on cytokine production studied in vitro at a single-cell level. Immunology.1992; 75: 136-142. https://goo.gl/KHdU7c
- Jiang H, Wynn C, Pan F, Ebbs A, Erickson LM, Kobayashi M. Tacrolimus and cyclosporine differ in their capacity to overcome ongoing allograft rejection as a result of their differential abilities to inhibit interleukin-10 production. Transplantation.2002; 73: 1808-1817. https://goo.gl/z5kLtN
- Holdsworth SR, Kitching AR, Tipping PG: Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int.1999; 55: 1198-1216. https://goo.gl/FGVAMu
- Llorente L, Richaud-Patin Y: The role of interleukin-10 in systemic lupus erythematosus. J Autoimmun.2003; 20: 287-289. https://goo.gl/JrG4SD
- Danovitch GM. Immunosuppressive medications and protocols for kidney transplantation. In: Danovitch GM, editor. Handbook of kidney transplantation. 5th ed. Lippincott William& Wilkins.10: 77-126. https://goo.gl/h9jbPU

Sign up for Article Alerts