Lysosomal Storage Disorders (LSDs): The Prevalence in the Eastern Province of Saudi Arabia?
Nouriya A. Al-Sannaa*, Hind Y. Al-Abdulwahed and Mohammed S. Al-Ghamdi
Pediatrics Services Division Johns Hopkins Aramco Healthcare, Dhahran, Saudi Arabia
*Address for Correspondence: Nouriya Abbas Al-Sannaa, Clinical Geneticist, Johns Hopkins Aramco Healthcare, Pediatrics Services Division, Dhahran, Building 61/Room D-269, Saudi Arabia, Tel: +966-13877-8290/877-7442; Fax-966-13877-3792; E-mail: [email protected]
Submitted: 14 September 2017; Approved: 03 October 2017; Published: 05 October 2017
Citation this article: Al-Sannaa NA, Al-Abdulwahed HY, Al-Ghamdi MS. Lysosomal Storage Disorders (LSDs): The Prevalence in the Eastern Province of Saudi Arabia. Int J Neurol Dis. 2017;1(2): 038-043.
Copyright: © 2017 Al-Sannaa NA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
The aim of this hospital-based retrospective analysis is to estimate the prevalence of Lysosomal Storage Disease (LSDs) in the Eastern Province of Saudi Arabia between 1983 and 2016. A total number of 89 Saudi patients from 43 families were diagnosed with different types of LSDs within this time period. Genotypes were available for 24 families (55 %). The overall prevalence for LSDs was estimated to be 42.2 per 100,000 live birth. This figure is significantly higher than that previously reported for other countries. This high prevalence was anticipated in such communities with an increased degree of consanguinity. In our studied population, Lipidosis was found to be the most common diagnosed subtype (40 % of LSDs) followed by Mucopolysaccharidosis (37%). A single common genotype was identified for Mucopolysaccharidosis type VI, ARSB (c.753C > GY251X), and Neuronal Ceroid Lipofuscinosis type 5, CLN5 (c.595C > Tp.R199X) in geographically isolated population at the Eastern Province explaining the relatively high prevalence for these two disorders (8/100,000, & 4.74/100,000 respectively). This information will justify evaluating the LSDs as candidates for the national newborn screening program. Estimating the birth prevalence will provide the opportunity to increase the awareness among the healthcare providers for these inherited live-threatening disorders.
Introduction
Lysosomal Storage Disorders (LSDs) comprise a group of at least 50 distinct genetic diseases, each one resulting from a deficiency of a particular lysosomal protein activity, or in a few cases from non-lysosomal activities that are involved in lysosomal biogenesis or protein maturation. All share a common biochemical characteristic in that they result in accumulation of normally degraded substrates within lysosomes. This eventually leads to an irreversible cell damage, and ultimately multi-organs dysfunction. The substrates stored and site of storages vary, leading to a wide spectrum of clinical manifestations [1-3].
The majority of LSDs are inherited in an autosomal recessive manner, with the exception of few disorders which follow the X-Linked mode of inheritance. The overall frequency of LSDs varies according to the populations studied, time of data collection, method of diagnosis and calculation used to estimate the prevalence [4-10]. The collective prevalence was estimated to be 13/100,000 live births in Australia [4] and up to 26/100,000 live births in United Arab of Emirates [10]. However, newborn screening a high prevalence for LSDs due to the detection of a later onset of forms of the disease [11-14]. Since the recognition by Chamoles and his coworkers [15] that lysosomal enzymes retain activity in dried blood spots on filter paper, novel substrates were designed to be used by diagnostic and screening laboratories for detection of LSDs. This ultimately had facilitated the introduction of few LSDs for routine Newborn screening utilizing mass spectrometry and recently fluorimetry for enzyme activity assay [16]. Interest in newborn screening for additional LSDs continues to expand as new technologies, second tier tests and treatments become available.
The aim of our study is to calculate the prevalence of LSDs among the Saudi population in The Eastern Province of Saudi Arabia from January, 1st, 1983 to December, 31st, 2013. To the best of our knowledge, there is no reported data on the prevalence of LSDs in Saudi Arabia. A former published data on the incidence of the inborn errors of metabolic diseases in our population showed that LSDs represented one third of all the disorders [17].
Method
Saudi Aramco provides a comprehensive healthcare for a population of 363,973 including the employees and their dependents at several medical facilities and a main tertiary hospital in Dhahran. This review covers a 33 years from January 31st 1983 to December 31st 2016. The study was approved by the institutional review board.
The medical records of all the patients diagnosed with LSDs born from January 1st 1983 to December 31st 2016 were reviewed. During the study period 210,897 live births were registered. Those children followed within our medical facility from the time of their birth till their father retirement from the company. The diagnosis was suspected due to the presence of typical manifestation, or history of previous affected family members. The confirmation was established by measuring the enzyme activity in either the skin cultured fibroblasts, or leukocytes of the index case. Genetic study was available for only 24 of the 43 (55 %) studied families due to the early death of the affected individuals. DNAs were extracted from the index patients after obtaining the consents. The samples used to be sent for analysis to different internationally accredited diagnostic Labs (Mayo Clinic, Willink Royal Manchester, Toronto molecular Lab, and others). Recently, all the samples are directed to Cento gene, Rostock, Germany. The methodology included PCR and sequencing of the entire coding region and the highly conserved exon-intron splice junctions. Parents of the deceased patient with Wolman disease were tested for a previously identified genotype c.260G > Tp.G87V in the LIPA gene within their tribe. Both were found to be heterozygous for the same variant. The prevalence was calculated as the number of the patients diagnosed with particular LSDs divided by the total number of live birth during the same period (the interval between the year of the birth of the eldest patient and the year of the birth of the youngest patient). Patients born prior to 1983 were excluded from the study. Birth prevalence was expressed as number patients per 100,000 live births.
Results
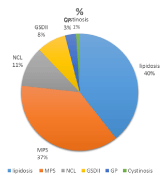
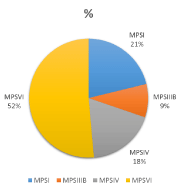
A total number of 89 patients from 43 families were diagnosed with different types of LSDs over the last three decade. All except one family were consanguineous (first cousins marriage). The combined birth prevalence of all LSDs is 42 per 100,000 live births (Table 1). More than one third of these patients had lipidosis and the second one third had Mucopolysaccharidosis (MPSs). MPS VI represented the largest subtype where a single genotype was identified in all except one of the tested families [18]. The remaining one third of patients were distributed between different types including NCL, glycogenosis II, mucolipidosis, glycoprotienosis and cystinosis (Figure 1). Another common genotype was also identified among families with NCL5. Ten of the twenty four identified mutations were novels (Table 2).
Discussion
Saudi Arabia is the second largest Arab state situated at southwest of Asia and occupies almost 80 % of the Arabian Peninsula (map) with an estimated population of approximately 22,000,000 people. It is characterized by a rapid growth, large family size with a high rate of consanguineous marriages (first or second cousin). This had resulted in the appearance of certain genetic disorders among some extended families and tribes [19].
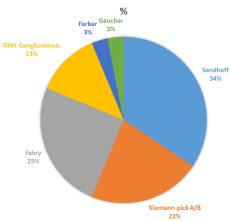
Given the progressive industrial development at the Eastern province, people from the rest of the country started migrating to where a good job opportunity and better standard of living. This is a hospital-based study with its limitation to estimate the exact prevalence of the LSDs in this part of the word. However, it provides a good overview of the phenotype and genotypes spectrum for these disorders among the Saudi population. To the best of our knowledge, there had been no reported data on the prevalence of LSDs in Saudi Arabia. Ozand et al 1990 [20] had reported 125 cases in three years reflecting the high prevalence of LSDs in the Saudi population. His data suggested that MPS IVA (Morquio disease), multiple sulfatase deficiency, Niemann-Pick disease type B, Sandhooff disease, GM2, and ceroid Neuronal lipofuscinosis are encountered frequently in Saudi Arabia, as compared to other storage diseases. The reported incidence of inborn error of metabolism in our population showed that LSDs represented 30 % of all the disorders [17]. The collective prevalence of LSDs in the Saudi population at the Eastern Province of Saudi Arabia was estimated to be 42.2 per 100,000 live births. This is significantly higher than former reported prevalence for other countries [4-10]. In the other hand, this not the case when our results were compared with data obtained in recent screening studies (Table 3). This is due to missing of patients with late onset presentation in the old studies. In our group of patients lipidosis was the most frequently diagnosed LSD (20 per 100,000 live births), followed by MPS (18 per 100,000 live births), and then NCL (5.5 per 100,000 live birth). Sandhoff disease was the most common lipidosis subtype (6 per 100,000 live births). Interestingly, Tay-Sachs disease was not detected in our population. The high frequencies for MPSVI, and NCL5 reflect the presence of gene founder for these two disorder in a geographically isolated community. Gaucher disease which is the most prevalent LSD in other population [4,5,7,8] was diagnosed only in one patient presented with an early onset non-neuropathic phenotype. This patient was found to be homozygous for c.1448T > C (p.Leu483Pro) mutation which is known to be associated with a severe phenotype [21]. Fabry disease is the second most LSDs disorder was encountered in one family with 9 affected family members including the mother (three males and six females). Ten novel mutations were identified for CLN5, CNL8, GALNS, NAGLU, GAA, IUDA, and GLB1 genes resulting in severe phenotypes and significant decreased enzyme activity. For patients with NCL, the diagnosis was supported by the presence of the typical fingerprint intra cytoplasmic inclusions on either white blood cells or cultured skin fibroblasts. LSDs are considered to be rare disorders. However, as a group they are not uncommon as seen from the published data. Unfortunately, the information on the birth prevalence is still missing for most populations due to the lack of clinical experience and accessibility to the diagnostic tool. This information has become essential for genetic counseling and estimating the risk for those disorders. Given the availability of different therapeutic options for these progressive and their life-threatening nature, an accurate estimation of the birth prevalence is mandatory. This eventually will have a positive impact for an earlier diagnosis and treatment. The last but not the least, identifying the patient and family genotyping is necessary for providing asymptomatic carrier testing, selective screening for high risk population, and offering invasive prenatal genetic diagnosis for couple with an increased recurrence risk.
Acknowledgment
The authors thank the hospital management and in particular the pediatrics service division for providing the support to conduct the genetic and biochemical study for the patients, the lab for collecting the samples and coordination with other labs, the clinicians and supportive services involved in the management of the patients with lysosomal storage diseases.
- Fuller M, Meikle PJ, Hopwood JJ, Epidemiology of lysosomal storage diseases: an overview. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis; 2006. Chapter 2. https://goo.gl/V8jMwv
- Mehta A, Beck M, Linhart A, Sunder-Plassmann G, Widmer U. History of lysosomal storage diseases: an overview. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis; 2006. Chapter 1. https://goo.gl/uY8dVX
- Wang RY, Bodamer OA, Watson MS, Wilcox WR; ACMG work group on diagnostic confirmation of lysosomal storage diseases. Lysosomal storage diseases: diagnostic confirmation and management of presymptomatic individuals. Genet Med. 2011; 13: 457-484. https://goo.gl/sDwJhA
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999; 281: 249-54. https://goo.gl/VcWxuZ
- Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999; 105: 151-156. https://goo.gl/M5rVNi
- Dionisi-Vici C, Rizzo C, Burlina AB, Caruso U, Sabetta G, Uziel G, et al. Inborn errors of metabolism in the Italian pediatric population: a national retrospective survey. J Pediatr. 2002; 140: 321-327. https://goo.gl/sMxY7G
- Pinto R, Caseiro C, Lemos M, Lopes L, Fontes A, Ribeiro H, et al. Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genet. 2004; 12: 87-92. https://goo.gl/sNddZs
- Poupetova H, Ledvinova J, Berna L, Dvorakova L, Kozich V, Elleder M. The birth prevalence of lysosomal storage disorders in the Czech Republic: comparison with data in different populations. J Inherit Metab Dis. 2010; 33: 387-396. https://goo.gl/KY59GL
- Ozkara HA, Topçu M. Sphingolipidoses in Turkey. Brain Dev. 2004; 26: 363-6. https://goo.gl/DTQHi7
- Al-Jasmi FA, Tawfig N, Berniah A, Ali BR, Taleb M, Hertecant JL, et al. Prevalence and novel mutations of lysosomal storage disorders in United Arab Emirates: LSD in UAE. JIMD Rep. 2013; 10: 1-9. https://goo.gl/JQ7wf5
- Peter C J I Schielen, Evelien A K, Michael H G. Newborn screening for lysosomal storage diseases: a concise review of the literature on screening methods, therapeutic possibilities and regional programs. Int J Neonatal Screen. 2017; 3. https://goo.gl/ja3QaA
- Scott CR, Elliott S, Buroker N, Thomas LI, Keutzer J, Glass M, et al. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J Pediatr. 2013; 163: 498-503. https://goo.gl/YQdsya
- Hopkins PV, Campbell C, Klug T, Rogers S, Raburn-Miller J, Kiesling J. Lysosomal storage disorder screening implementation: findings from the first six months of full population pilot testing in missouri. J Pediatr 2015; 166: 172-177. https://goo.gl/xnsULT
- Orsini JJ, Kay DM, Saavedra-Matiz CA, Wenger DA, Duffner PK, Erbe RW, et al. Newborn screening for Krabbe disease in New York State: the first eight years' experience. Genet Med. 2016; 18: 239-248. https://goo.gl/9LmcNt
- Chamoles NA, Blanco M, Gaggioli D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001; 308: 195-196.
- Yijun Li, C. Ronald Scott, Nestor A. Chamoles, Ahmad Ghavami, B. Mario Pinto, et al. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004; 50: 1785–1796. https://goo.gl/TDJMnG
- Moammar H, Cheriyan G, Mathew R, Al-Sannaa N. Incidence and patterns of inborn errors of metabolism in the Eastern Province of Saudi Arabia, 1983-2008. Ann Saudi Med. 2010; 30: 271-277. https://goo.gl/VkQc27
- Al-Sannaa NA, Al-Abdulwahed HY, Al-Majed SI, Bouholaigah IH. The clinical and genetic Spectrum of Maroteaux-Lamy syndrome (Mucopolysaccharidosis VI) in the Eastern Province of Saudi Arabia. J Community Genet. 2017. https://goo.gl/H737hS
- AS Warsy, MH Al-Jaser, A Albdass, S Al-Daihan, M Alanazi. Is consanguinity prevalence decreasing in Saudis? : A study in two generations. African Health Sciences, 2014; 14: 314-321. https://goo.gl/Et3AYk
- Ozand PT, Gascon G, al Aqeel A, Roberts G, Dhalla M, Subramanyam SB. Prevalence of different types of lysosomal storage diseases in Saudi Arabia. J Inherit Metab Dis. 1990; 13: 849-861. https://goo.gl/3rcjTU
- Bendikov-Bar I, Ron I, Filocamo M, Horowitz M. Characterization of the ERAD process of the L444P mutant glucocerebrosidase variant. Blood Cells Mol Dis. 2011; 46: 4-10. https://goo.gl/2VNviN

Sign up for Article Alerts