Angong Niuhuang Pill Attenuates Blood-Brain Barrier Dysfunction through Inhibiting Lipopolysaccharide Translocation in Intracerebral Hemorrhagic Mice?
Wang Tian#, Meng Xiaoyu#, Wang Wenqian, Li Ting, Fan Yiqian, Yue Shumin, Yang Yunqi and Fu Fenghua*
School of Pharmacy, Yantai University, Yantai, Shandong 264005, PR China
#These authors are contributed equally
*Address for Correspondence: Fu Fenghua, School of Pharmacy, Yantai University, Yantai, Shandong 264005, PR China, Tel: +86 -138-063-856-90; Fax: +865-356-706-066; E-mail: fufenghua@sohu.com/fufh@ytu.edu.cn
Submitted: 26 December 2019; Approved: 09 January 2020; Published: 11 January 2020
Citation this article: Tian W, Xiaoyu M, Wenqian W, Ting L, Yiqian F, et al. Angong Niuhuang Pill Attenuates Blood-Brain Barrier Dysfunction through Inhibiting Lipopolysaccharide Translocation in Intracerebral Hemorrhagic Mice. Int J Neurol Dis. 2020;4(1): 005-011.
Copyright: © 2020 Tian W, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Angong niuhuang pill; Intracerebral hemorrhage; Blood-brain barrier; Lipopolysaccharide
Download Fulltext PDF
Objective: Intracerebral Hemorrhage (ICH) induces Blood-Brain Barrier (BBB) dysfunction. The purpose of this study was to investigate whether Angong Niuhuang Pill (ANP) attenuates blood-brain barrier dysfunction by inhibiting lipopolysaccharide translocation in ICH mice.
Methods: Male CD-1 mice were randomly divided into sham, ICH, and ANP-treated groups. The mice were subjected to intrastriatal injection of bacterial collagenase or PBS (Sham group). Then they were administered intragastrically with vehicle (for the sham and ICH group) or ANP (0.1, 0.2, or 0.4 g/kg) respectively.
Results: The results showed that treatment with ANP significantly promoted functional recovery in ICH mice compared with the ICH group (p < 0.05). Brain water content was significantly decreased in the ANP group when compared with that of ICH group (p < 0.05). The intestinal permeability to FITC-dextran in ICH group was higher compared to the Sham group, and the increase of intestinal permeability was abated partially by ANP treatment (p < 0.01). ANP attenuated the pathological changes of the jejunal villi and the ileal villi in ICH mice. In line with above results, ANP inhibited the increase of Lipopolysaccharide (LPS) level in serum (p < 0.05). ANP administration significantly decreased ICH-induced Evans blue extravasation (p < 0.01). In the ICH group, the levels of medullary differentiation factor 88 (MyD88) and matrix metalloprotein-9 (MMP-9) were increased while the expression of claudin-5 were reduced (p < 0.05). However, ANP not only significantly reduced the expression of MyD88 and MMP-9 but also increased the expression of claudin-5 (p < 0.05). ANP treatment had no effect on the Toll-Like Receptor 4 (TLR4) expression.
Conclusion: These findings indicate that ANP attenuates BBB dysfunction by inhibiting lipopolysaccharide translocation in ICH mice.
Introduction
Intracerebral Hemorrhage (ICH) is a stroke subtype resulting from bleeding within the brain parenchyma without trauma. Approximately 10-15% of strokes are ICH and ICH accounts for an even greater percentage of strokes in Asian patients [1]. Brain edema is a critical factor in the subsequent development of neurologic deficits by increasing intracranial pressure or by causing a substantial midline shift. While multiple forms of edema develop after ICH, the primary type is vasogenic cerebral edema resulting from an increase in permeability of Blood-Brain Barrier (BBB) [2].
Thrombin, fibrin and erythrocyte components are involved in BBB disruption and brain injury after ICH [1]. Blood components also induce an inflammatory response in the perihematomal area [3]. There is evidence that these elements of the inflammatory response can alter BBB function in ICH [4]. Significant upregulation of TLR4 expression occurs at approximately 2 to 6 hours after ICH [5-7], accompanied by the appearance of infiltrating neutrophils in the hematoma at 4 h after ICH [8]. TLR4 signaling in the neutrophils further mediate a release of proinflammatory cytokines, which contributes to the detrimental inflammatory response [9,10]. In the Central Nervous System (CNS), microglia and neurons express Toll-Like Receptor 4 (TLR4). TLR4 is stimulated by increases of cytokine and chemokine, which will lead to a sustained CNS inflammation independent of blood-derived leukocytes [11].
Notably, intestinal barrier dysfunction is a common complication after stroke that leads to malabsorption, malnutrition, hypoimmunity, and poor prognosis in patients [12,13]. Increased intestinal permeability caused by stroke lead to the Lipopolysaccharide (LPS) of the intestine translocation into the blood circulation, triggering a systemic inflammatory response and a release of various inflammatory mediators [14]. Blood LPS then cross the BBB and exacerbate a neuroinflammation in the brain, in turn worsening the prognosis of patients [15,16]. TLR4 recognizes LPS and produce an inflammatory response [17]. Previous study showed that agent which protects intestinal barrier alleviates ICH-induced neurological impairment [18].
Angong Niuhuang Pill (ANP), a well-known traditional Chinese medicine, has been widely used to treat stroke in China. It contains buffalo horn, calculus bovis, radix curcumae, radix scutellariae, and so on. ANP is considered as an adjunct therapy with conventional medicine for ischemic stroke patients and hemorrhagic stroke patients [19-21]. Recent findings demonstrate that ANP attenuates the infarction sizes, the BBB permeability and neurological deficit in the MCAO rats with 2 h ischemia plus 22 h reperfusion [22]. Meanwhile, clinical studies suggest that ANP ameliorates the neurological impairment in ischemic stroke patients [23,24].
Up to date, almost all the researches hypothesize that ANP can cross the BBB. A good many of studies focus on elucidating the mechanism of action of ANP on the cells in brain. However, we think that not all components of ANP penetrate the BBB. Therefore, whether ANP exhibit a peripheral action and then improve neurological function of ICH mice? This study aims to investigate whether ANP attenuate BBB dysfunction through inhibiting lipopolysaccharide translocation in ICH mice.
Materials and Methods
Chemicals and reagents
Angong Niuhuang Pill were purchased from Beijing Tong Ren Tang Chinese Medicine Co. Ltd. (Beijing, China). Ketamine, xylazine, collagenase VII, Fluorescein Isothiocyanate-Dextran (FITC-D), Evans blue, trichloracetic acid were purchased from Sigma-Aldrich (Shanghai, China). LPS ELISA kit were purchased from Cloud-Clone CORP (CCC, USA). Rabbit anti-TLR4, rabbit anti-MyD88, rabbit anti-MMP-9, and rabbit anti-claudin-5 were purchased from Abcam (Cambridge, UK). RIPA lysis buffer, 10% SDS-PAGE, horseradish peroxidase-labeled secondary antibody, ECL kit, β-actin were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Image Quant LAS 4000 were purchased from GE Healthcare Bio-Sciences (Tokyo, Japan).
Drugs
ANP was suspended in 0.5% sodium carboxymethyl cellulose (CMC-Na).
Animals
Male CD-1 mice weighing 22 g to 25 g were purchased from Jinan Pengyue Experimental Animal Breeding Co. Ltd. (License number: SCXK (Lu) 20140007). The mice were placed in an appropriate environment at 22-23°C and 60% humidity under a 12 h light/dark cycle, with freely available water and food. The use of experimental animals and the experimental design complied with the National Institutes of Health Guidelines for the Use of Laboratory Animals and were approved by the Ethics Committee of Yantai University (approval number, YTU20180124).
ICH model
Mice were anesthetized with ketamine-xylazine (80 mg/kg ketamine hydrochloride and 10 mg/kg xylazine, intraperitoneal injection). They were fixed in a stereotactic frame after anesthetization, and a hole was drilled into the right side of the head (coordinates 0.2 mm anterior, 3.5 mm ventral, and 2.2 mm lateral to the bregma). Then, 500 nL of phosphate-buffered saline containing 0.075 U collagenase VII was injected using a micro-infusion pump. The needle was then maintained in place for 5 min after injection. The needle was then withdrawn, and the hole was sealed with bone wax and sutured.
Grouping and treatment
Mice were randomly assigned to five experimental groups: sham group (Sham), ICH group (ICH), ANP at dose of 0.1 g/kg group (ANP 0.1), ANP at dose of 0.2 g/kg group (ANP 0.2), and ANP at dose of 0.4 g/kg group (ANP 0.4). At 0.5 h after ICH, mice in ANP groups were intragastrically administered with ANP at dose of 0.1, 0.2, 0.4 g/kg. The mice in the sham and model groups were subjected to 0.5% CMC-Na.
Evaluation of neurological function
Garcia test was performed to assess neurological function at 48 h after ICH. Garcia test includes spontaneous activity, side stroking, vibrissae touch, limb symmetry, lateral turning, forelimb walking, and climbing.
Measurement of brain water content
After Garcia test, the brains of the anesthetized mice were dissected through the midline. A total of 5 parts from each brain were harvested: the cerebellum, the ipsilateral cortex, the contralateral cortex, the ipsilateral striatum, and the contralateral striatum. The five parts of samples were weighed on an electronic analytical balance (Mettler-Toledo, Shanghai, China). The samples were dried at 100°C for 24 h before determining the dry weight. Brain water content (%) was calculated as (wet weight-dry weight)/wet weight × 100%.
Evaluation of FITC-D concentration
Intestinal mucosal permeability was evaluated by assaying the FITC-D concentration in blood, which reflected the level of intestinal barrier dysfunction. Briefly, food and water were withdrawn for 12 h. Then the mice were gavaged with FITC-D at dose of 300 mg/kg. After 3 h, the serum of animals was collected. FITC-dextran concentrations were determined using a fluorescence spectrophotometer (Molecular Devices, Shanghai, China) at an excitation wavelength of 485 nm and an emission wavelength of 525 nm.
Histopathological examination of intestine
The jejunum and ileum of mice were fixed in 10% formaldehyde and then were sliced into 5 μm thick sections with a microtome. Hematoxylin-Eosin staining was performed for the histopathological examination of intestine.
Evaluation of evans blue concentration
BBB permeability was assessed with Evans blue method. Evans blue was dissolved in normal saline. The mice were intraperitoneally injected with Evans blue at a volume of 0.01 ml/g. Three hours after, the mice were transcardially perfused with cold PBS. Right cortex and right striatum were harvested and homogenized with 50% trichloracetic acid. The supernatants were collected and were analyzed at 610 nm by spectrophotometry. Quantitative evaluation of BBB permeability was performed by calculating the amount of Evans Blue deposition per gram tissue.
Determination of LPS levels in serum
Serum LPS levels were quantified using the LPS ELISA kit following the manufacturer’s instructions.
Western blot analysis
The right cortex and right striatum were lysed with RIPA lysis buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) and centrifuged at 12,000 g for 20 min at 4°C. Total protein (20 μg) was separated on 10% SDS-PAGE. The membranes were incubated overnight at 4°C with the primary antibodies: rabbit anti-TLR4 (1:1000), rabbit anti-MyD88 (1:1000), rabbit anti-MMP-9 (1:1000), and rabbit anti-claudin-5 (1:1000). The membranes were processed with the corresponding horseradish peroxidase-labeled secondary antibody (1:1000). Bands were visualized using the ECL kit and then the bands were quantified using Image Quant LAS 4000. β-actin (1:1000) was served as the loading control.
Statistical analysis
All data were analyzed using GraphPad Prism 6.0 and are expressed as mean ± SD. Statistical significance was determined by one-way ANOVA followed by Kruskal-Wallis H test or Least Significant Difference (LSD). p < 0.05 were considered significant.
Results
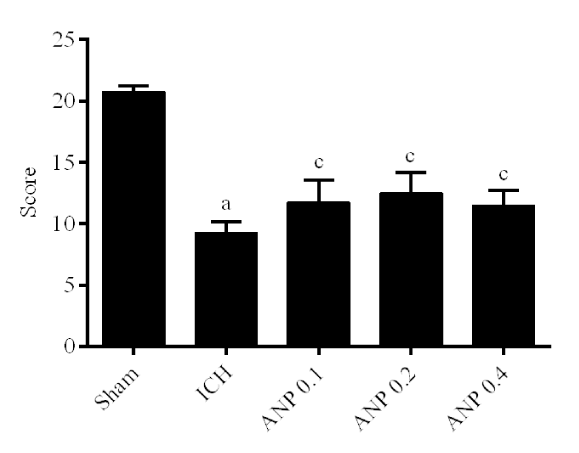
ANP improved neurological function in ICH mice
Compared to sham group, ICH induced a severe neurobehavioral deficit (p < 0.01). In contrast, treatment with ANP attenuated (p < 0.01) significantly the neurobehavioral function deficit induced by ICH (Figure 1).
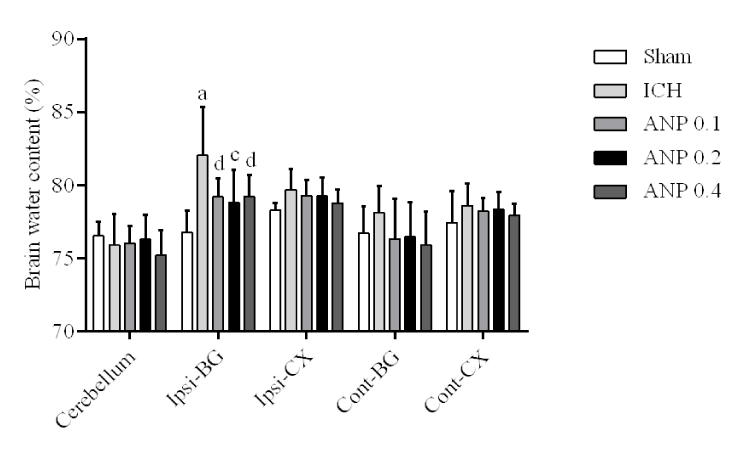
ANP decreased brain edema in ICH mice
In the ipsilateral basal ganglia, brain water content was significantly increased in the ICH group compared with that of the sham group (p < 0.01). Compared with the ICH group, ANP treatment reduced (p < 0.05) brain water content (Figure 2).
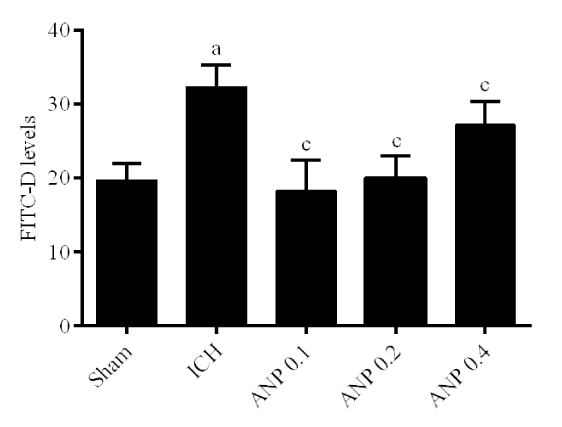
ANP attenuated intestinal permeability in ICH mice
Low FITC-D levels was detected in the sham group. ICH caused a significant increase in the level of serum FITC-D compared to the sham group (p < 0.01). However, ANP administration markedly decreased (p < 0.01) FITC-D levels in ICH mice(Figure 3).
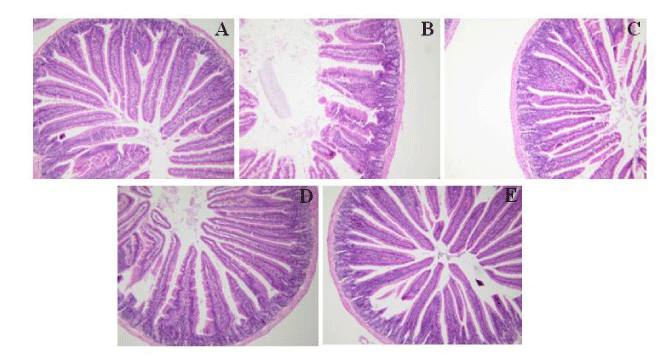
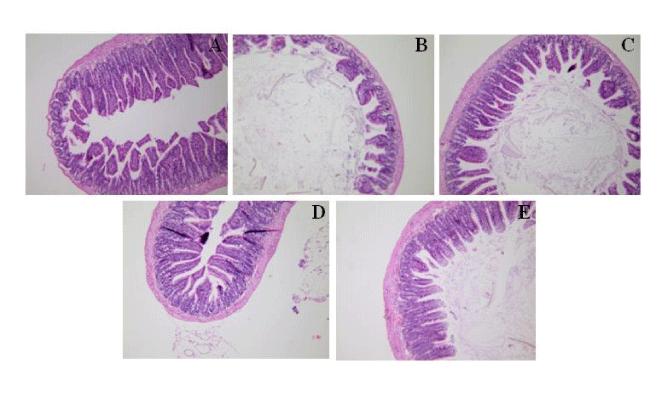
ANP ameliorated the injuries of jejunum and ileum in ICH mice
In the sham group, the intestinal jejunal and ileal villi were intact and slender, and the epithelial cells were arranged neatly. The jejunal villi and the ileal villi were shed and the epithelial cells were disordered in ICH mice. The pathological changes of the jejunal villi and the ileal villi were attenuated after treatment with ANP (Figure 4 and 5).
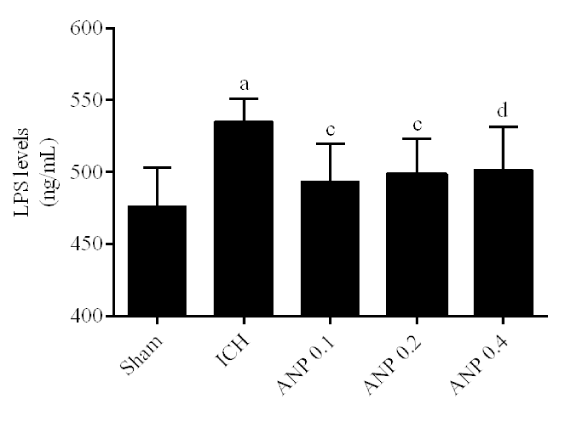
ANP decreased LPS levels in ICH mice
ICH led to a significant increase in the level of serum LPS compared to that of the sham group (p < 0.01). Compared with the ICH group, ANP treatment significantly decreased (p < 0.05) the LPS levels in ICH mice (Figure 6).
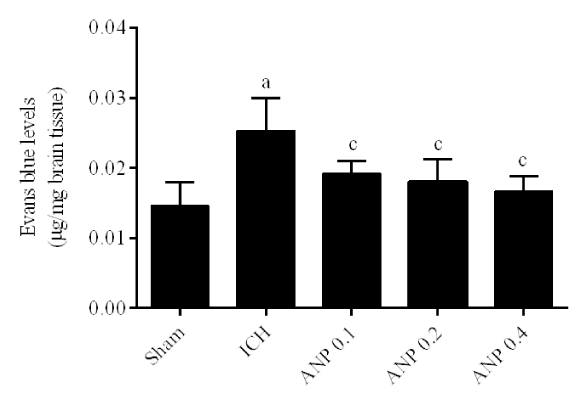
ANP attenuated BBB permeability in ICH mice
Compared with the sham group, the content of Evans blue in the right hemisphere exhibited a significant increase in the ICH group (p < 0.01). ANP administration significantly decreased (p < 0.01) the Evans blue levels in ICH mice (Figure 7).
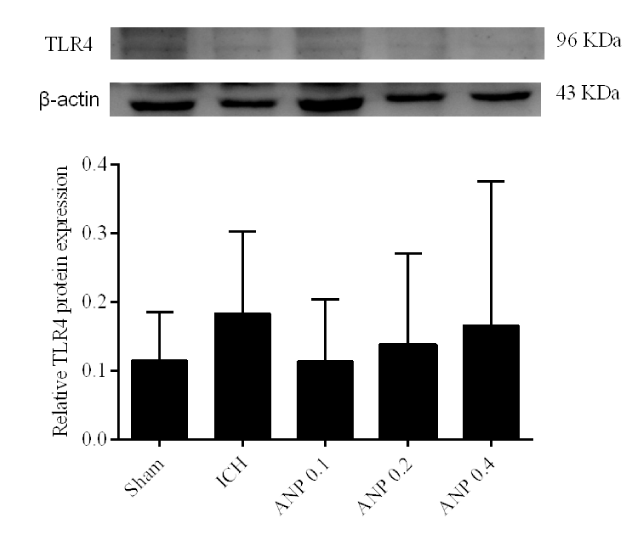
ANP has no effect of TLR4 expression in ICH mice
Compared with the sham group, the TLR4 expression in ICH group did not show any change (p > 0.05). Furthermore, treatment with ANP also did not influence (p > 0.05) TLR4 expression in ICH mice (Figure 8).
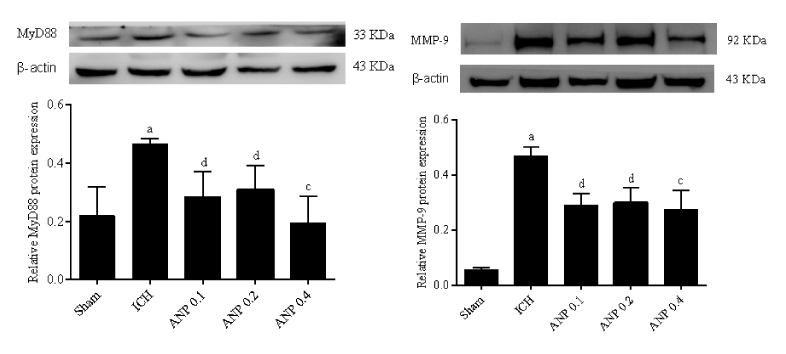
ANP downregulated MyD88 and MMP-9 expression in ICH mice
MyD88 and MMP-9 expression were significantly increased (p < 0.01) in the ICH group in compared with the sham group. However, treatment with ANP significantly decreased (p < 0.05) MyD88 and MMP-9 expression in ICH mice (Figure 9).
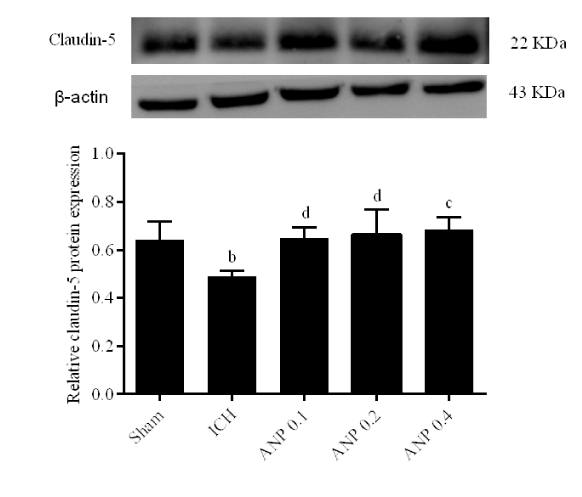
ANP upregulated Claudin-5 expression in ICH mice
The results showed that there was a significant decrease (p < 0.05) in claudin-5 expression in ICH mice. Compared with the ICH group, treatment with ANP significantly increase (p < 0.05) the expression of claudin-5 in ICH mice (Figure 10).
Discussion
In this study, we found that ANP not only improved neurological function of ICH mice but also reduced brain water content and Evans blue dyes extravasation, ANP also attenuated injured intestinal mucosa permeability in ICH mice. Furthermore, ANP treatment decreased LPS levels, the expression of MyD88, MMP-9, and enhanced claudin-5 expression.
ICH caused neurological function dysfunction [25], disruption of BBB integrity and subsequent increased permeability are the major aspects of brain injury after ICH. BBB injury results in the formation of brain edema and swelling, the increased intracranial pressure, and the shift of the midline, which aggravates the outcomes of ICH [3].
This study suggestes that ANP treatment improves neurological function and decreases BBB permeability, then results in a significant reduction of brain edema.
ICH can cause intestinal mucosal barrier damage, and LPS translocates into the blood circulation. These findings indicate that treatment with ANP attenuates the the intestine injury and the intestinal mucosa permeability and then decreases LPS levels in blood.
TLR4 plays a key role for innate immune responses and LPS can binds to TLR4 triggering the TLR4/MyD88/MMP-9 signaling pathway. In our study, ANP did not affect the TLR4 levels in ICH mice. MyD88, a critical adapter protein for TLR4, leads to the activation of downstream NF-κB and the subsequent production of pro-inflammatory cytokines [26,27]. Previous studies have shown that NF-κB triggers the production of MMP-9 [28]. MMP-9 is implicated as a pathological mediator during the acute phase of stroke. Putative mechanisms include the proteolysis of neurovascular substrates such as tight junction proteins essential for BBB integrity and components of the extracellular matrix integral to cerebrovascular and neuronal viability [29]. BBB integrity is compromised during CNS infection, increasing permeability and strongly contributing to secondary brain edema, which directly influences patient prognosis. MMP-9 plays an important role in regulating TJ protein expression [30,31]. Claudin-5 is the essential integral proteins in tight junction, and its levels have been considered as indicators of normal and dysfunctional states of the BBB. Our study showed that ANP regulates the TLR4/MyD88/MMP-9 signaling pathway and then enhances claudin-5 levels.
There is a limitation in the present study. This experiment did not detect inflammatory factors in the brain. Therefore, whether ANP play a role in regulating the central inflammation in ICH mice should be elucidated in the further study.
In summary, ANP improves the neurological function of ICH mice. ANP attenuates the blood-brain barrier dysfunction by inhibiting lipopolysaccharide translocation in ICH mice. This study suggests ANP attenuate blood-brain barrier dysfunction by inhibiting lipopolysaccharide translocation in ICH mice. Further, ANP regulates the TLR4/MyD88/MMP-9 signaling pathway and enhances the expression of claudin-5.
Acknowledgement
Supported by the National Natural Science Foundation of China (81873039).
- Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014; 11: 1-13. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25120903
- Wang G, Li Z, Li S, Ren J, Suresh V, Xu D, et al. Minocycline preserves the integrity and permeability of BBB by altering the activity of DKK1-Wnt signaling in ICH model. Neuroscience. 2019; 415: 135-146. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31344398
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006; 5: 53-63. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16361023
- Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Leucocyte-endothelial cell crosstalk at the blood-brain barrier: A prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011; 37: 24-39. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20946472
- Lin S, Yin Q, Zhong Q, Lv FL, Zhou Y, Li JQ, et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation. 2012; 9: 46. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22394415
- Teng W1, Wang L, Xue W, Guan C. Activation of TLR4-mediated NF-κB signaling in hemorrhagic brain in rats. Mediators Inflamm. 2009; 2009: 473276. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20150961
- Ma CX, Yin WN, Cai BW, Wu J, Wang JY, He M, et al. Toll-like receptor 4/nuclear factor-κ B signaling detected in brain after early subarachnoid hemorrhage. Chin Med J (Engl). 2009; 122: 1575-1581. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19719951
- Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain 2007; 130: 1643-1652. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17525142
- Gautier S, Ouk T, Petrault O, Caron J, Bordet R. Neutrophils contribute to intracerebral haemorrhages after treatment with recombinant tissue plasminogen activator following cerebral ischaemia. Br J Pharmacol. 2009; 156: 673-679. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19210512
- Moxon Emre I, Schlichter LC. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol. 2011; 70: 218-235. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21293296
- Sansing LH, Harris TH, Welsh FA, Kasner SE, Hunter CA, Kariko K. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann Neurol. 2011; 70: 646-656. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22028224
- Yang TC, Li JG, Shi HM, Yu DM, Shan K, Li LX, et al. Gastrointestinal bleeding after intracerebral hemorrhage: a retrospective review of 808 cases. Am J Med Sci. 2013; 346: 279-282. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23221511
- Jin W, Wang H, Ji Y, Hu Q, Yan W, Chen G, et al. Increased intestinal inflammatory response and gut barrier dysfunction in Nrf2-deficient mice after traumatic brain injury. Cytokine. 2008; 44: 135-140. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18722136
- Ahmed SH, He YY, Nassief A, Xu J, Xu XM, Hsu CY, et al. Effects of lipopolysaccharide priming on acute ischemic brain injury. Stroke. 2000; 31: 193-199. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10625737
- Morganti Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Curr Opin Crit Care. 2002; 8: 101-105. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12386508
- Pu X, Dong C, Zhu W, Li W, Jiang H. Silencing stomatin-like protein 2 attenuates tumor progression and inflammatory response through repressing CD14 in liver cancer. Onco Targets Ther. 2019; 12: 7361-7373. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31571899
- Wu S, Lv HY, Wang WQ, Wang T. Effect of AngongNiuhuang Pill on neurological function and intestinal mucosal barrier in intracerebral hemorrhage mice. Pharmacology and Clinics of Chinese Materia Medica. 2018; 34: 6-10.
- Wang Z, Wen X. Observation on 60 cases of acute cerebral infarction treated by combination of traditional Chinese medicine and Western medicine. J Pract Tradit Chin Med. 2010; 6: 397-398.
- Wu YF, Luo C, F LH. Effects of Angong Niuhuang Pills on plasma BNP and CRP and neurological functional defect in patients with acute cerebral infarction. Chinese Journal of Modern Applied Pharmacy. 2012; 29: 550-553.
- Zhang L, Li L. 90 Cases of cerebral hemorrhage disease using traditional Chinese medicine and western medicine treatment. China Pract Med. 2013; 1: 151-152.
- Tsoi B, Wang S, Gao C, Luo Y, Li W, Yang D, et al. Realgar and cinnabar are essential components contributing to neuroprotection of Angong Niuhuang Wan with no hepatorenal toxicity in transient ischemic brain injury. Toxicol Appl Pharmacol. 2019; 377: 114613. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31207256
- Xing FL, Li Q, Zhang W. Therapeutic effects of Angongniuhuang pill on 34 patients with cerebral stroke. Hebei Journal of Traditional Chinese Medicine. 2005; 27: 13-14. http://bit.ly/35yJ35C
- Luo Y. Angongniuhuang pill treatment of acute stroke clinical observation of 64 cases. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2009; 10: 45-46.
- Yu L, Lu Z, Burchell S, Nowrangi D, Manaenko A, Li X, et al. Adropin preserves the blood-brain barrier through a Notch1/Hes1 pathway after intracerebral hemorrhage in mice. J Neurochem. 2017; 143: 750-760. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29030969
- Vaure C, Liu Y. A comparative review of Toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014; 5: 316. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25071777
- Jing N, Li X. Dihydromyricetin attenuates inflammation through TLR4/NF-kappaB pathway. Open Med (Wars). 2019; 14: 719-725. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31572805
- Chen JK, Peng SF, Lai KC, Liu HC, Huang YP, Lin CC, et al. Fistein suppresses human osteosarcoma U-2 OS cell migration and invasion via affecting FAK, uPA and NF-ĸB signaling pathway in vitro. In Vivo. 2019; 33: 801-810. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31028200
- Raz L, Yang Y, Thompson J, Hobson S, Pesko J, Mobashery S, et al. MMP-9 inhibitors impair learning in spontaneously hypertensive rats. PLoS One. 2018; 13: e0208357. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30533010
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2006; 27: 697-709. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16850029
- Jin Z, Liang J, Li J, Kolattukudy PE. Absence of MCP-induced protein 1 enhances blood-brain barrier breakdown after experimental stroke in mice. Int J Mol Sci 2019; 20: e3214. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31261992

Sign up for Article Alerts