A Double Blind Case Control Study of Hypertensive Stroke Patients Prognosis after Amlodipine Treatment in Comparison to Captopril?
Bahareh Bazooyar*
Department of Neurology, Babol University of Medical Science, Babol, Iran
*Address for Correspondence: Department of Neurology, Babol University of Medical Science, 26, Afsson, Parking 1 Ave, Babol, Iran, Tel: +989-133-059-366; E-mail: bazooyar.bahareh@gmail.com
Submitted: 27 October 2020; Approved: 07 November 2020; Published: 09 November 2020
Citation this article: Bazooyar B. A Double Blind Case Control Study of Hypertensive Stroke Patients Prognosis after Amlodipine Treatment in Comparison to Captopril. Int J Neurol Dis. 2020;4(1): 046-049. https://dx.doi.org/10.37871/ijnd.id39
Copyright: © 2020 Bazooyar B. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Ischemic stroke; Stroke prognosis; Amlodipine; Captopril; Neuroprotection
Download Fulltext PDF
Background: Stroke is a major cause of death and disability. Current studies show that increased cellular calcium concentrations cause neuronal death in ischemia. Calcium channel blockers such as amlodipine inhibit cellular oxidative stress and may be effective in the treatment of cerebrovascular stroke and decrease excessive apoptosis.
Objective: This study tests the hypothesis that amlodipine is superior to captopril in reducing neurological deficit and improving functional outcome in patients with acute cerebral infarction of an intermediate severity.
Methods: This multicenter, double blind, case controlled trial randomized 124 stroke patients with just hypertension (mean arterial pressure: 140-180 mmHg) as a risk factor (cases mean age: 66.20 ± 8.78 and controls mean age: 67.19 ± 9.04) in Babol with a National Institutes of Health Stroke Scale score (NIHH) 6 to 14. 63 cases treated with amlodipine (5-10 mg daily) and the other 61 controls were treated with captopril (25-75 mg daily) after 72 hours of the onset of symptoms of an acute ischemic stroke for 3 months.
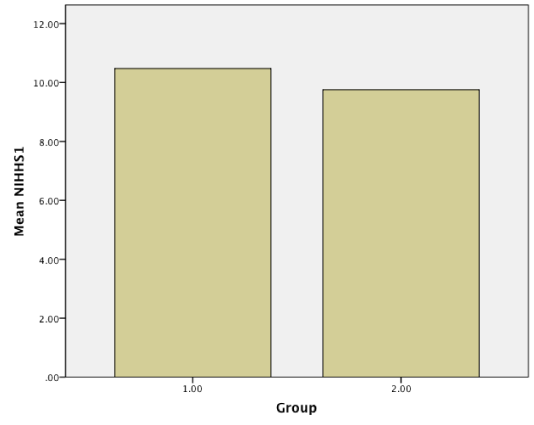
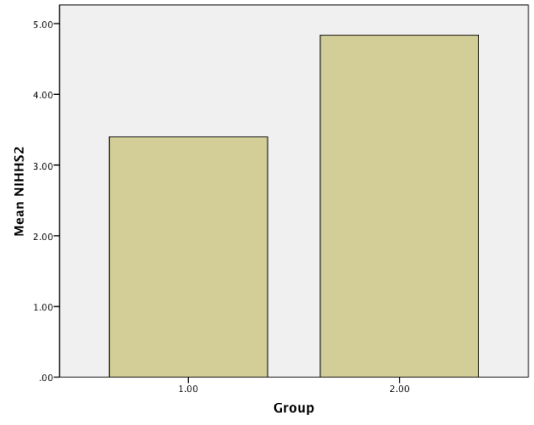
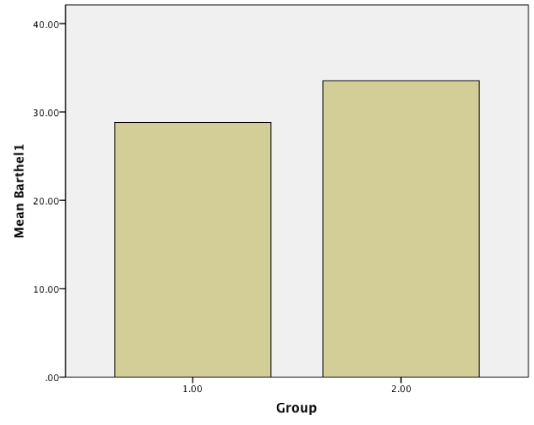
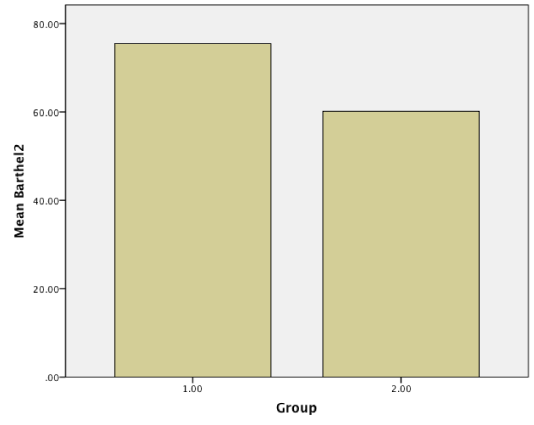
Results: Baseline NIHHS and Barthel index were measured and compared with these scores after 3-month treatment. Although there were no statistical significant differences in primary scores in two groups(NIHH; 10.47 vs 9.75, p = 0.422 > 0.05, CI: 95%, Barthel; 28.80 vs 33.52, p = 0.126 > 0.05, CI = 95%), Amlodipine produced statistically significant mean improvements in NIHH(3.39 vs 4.83, p = 0.016 < 0.05, CI = 95%) and Barthel index (75.47 vs 60.16, p = 0.02 < 0.05, CI = 95%) compared with captopril after 3 month treatment.
Conclusion: This study mention that treatment with amlodipine may improve prognosis of ischemic stroke patients.
Introduction
Stroke is a major cause of death and disability in many countries of the world, placing a heavy burden on patients, families, healthcare systems, and economies [1]. Only a few therapies have consistently reduced death or disability. There is thus a real need for better treatments to enhance poststroke recovery [2].
Current studies show that increased cellular calcium concentrations cause neuronal death in ischemia. In the brain, lipid peroxidation and neuronal apoptosis are associated with aging and age-related disorders, including cerebrovascular stroke [3-7]. Amlodipine reduces oxidative stress in brain and decreases blood pressure without sympathetic nerve stimulation.8Amlodipine has also anti-inflammatory-antiapoptotic effect [9,10]. Amlodipine can prevent cell death during reoxygenation after cortical ischemia [11].
Although most patients experience some spontaneous recovery in the months after a stroke, the degree and timing of recovery are variable. Improving functional outcomes through specific interventions is a relatively unexplored area of great public health potential. Furthermore, the first 3 months after stroke may offer the most significant window of opportunity for recovery of function and give the demonstration of both neuroprotective and neuroregenerative properties in models of focal and global brain ischemia [12-14].
Methods
This randomised, multicenter, double-blinded study assessed physical examination, CT or MRI, neurological signs with the use of the National Institutes of Health Stroke Scale (NIHSS) and Barthel index. Patients were also assessed on days 1-3 after stroke onset, and then after 3 months. Patients were eligible for enrolment if they were 50-80 years old with good compliance and had an acute ischemic stroke referable with confirmed neuroimaging and onset of symptoms within the previous 3 days. We chose patients with NIHSS score of 6 to 14. They didn’t have any cardiovascular risk factors (diabetes, hyperlipidemia, coagulopathy, atrial fibrillation, heart failure) except systemic hypertension (mean systolic blood pressure: 14 to 18). All the patients had hypertension before stroke onset. All of our patients had normal renal, pulmonary and hepatic function. Patients were randomly assigned to receive amlodipine or captopril for a period of 3 months. Amlodipine 5-10 mg a day was given to patients in the active treatment group and control groups were treated with captopril 25-75 mg daily. We adjusted drug doses due to primary mean arterial pressure of our patients. All our patients were treated with atorvastatin 20mg daily. Vital signs were closely monitored for the first 72 h and then at each assessment time. Routine lab data’s (CBC-diff, Bun, Cr, Na, K, LDL, HDL, TG, Total Chol, PT, PTT, INR), Echocardiography and neuroimaging were done. To establish whether amlodipine had any effect on functional outcome after ischemic stroke, The NIHSS and Barthel indexes were assessed and compared at the 1-3td day of stroke onset and after 3 months in two groups. This data set consists of data recorded or carried forward from the most recent visit and after 3 months treatment. Patients who had died (one patient died suddenly without any reason in captopril group) were excluded. Data were analysed by SPSS. Independent t-test was done to compare mean NIHHS and Barthel indexes. p < 0.05 was statistically significant (CI; 95%).
Results
Patients were studied from May, 2014, to April, 2015 in Babol. All our patients referred to Rohani hospital and neurology clinic. Of the 124 patients who gave informed consent, 63 were assigned to amlodipine and 61 to captopril. The demographic and clinical characteristics of the 124 ischemic stroke patients who were received amlodipine or captopril are shown in (Table 1). No differences in demographic characteristics or underlying risk factors (blood pressure and age) were observed between the two groups. The mean age of the trial population were 66.20 ± 8.78(case group) and 67.19 ± 9.04 (control group). Baseline NIHH and Barthel index were measured and compared with these scores after 3 month treatment. Although there were no statistical significant differences in primary scores in two groups (NIHH; 10.47 vs 9.75, p = 0.422 > 0.05, CI: 95%, Barthel; 28.80 vs 33.52, p = 0.126 > 0.05, CI = 95%), Amlodipine produced statistically significant improvements in NIHH (3.39 vs 4.83, p = 0.016 < 0.05, CI = 95%) and Barthel index (75.47 vs 60.16, p = 0.02 < 0.05, CI = 95%) compared with captopril after 3 month treatment (Figure1-4).
Discussion
Acute ischemic stroke is a major cause of death and disability. Treatment methods for decreasing neuronal injury after a stroke are difficult to develop, because the pathophysiology is not yet well understood [15]. Several mechanisms of neuronal injury in strokes have been shown, including excitotoxicity, increased calcium and free radicals. T-type calcium channels are expressed in many diverse tissues, including neuronal, cardiovascular, and endocrine. Aside from their established clinical applications, recent studies have suggested neuroprotective effects of T-type calcium channel blockers. Current reports mentioned neuroprotective effect of Ca+ channel antagonists by improving of endothelial function and decreasing in size of ischemic region [11].
Numerous clinical trials have established the similar benefits of amlodipine treatment in patients with ischemic stroke. Our study is a planned follow-up of the randomized case-controlled clinical trial of traditional medicine in ischemic stroke. In this study, an initial 3-month treatment with amlodipine demonstrated a statistically significant benefit at 3 months. Moderate-to-severe acute ischemic stroke patients were studied.
Similar our results one study found that in comparison to Angiotensin Receptor Blockers (ABRs), amlodipine provides better quality of life over the 5 year time period projected. From a Chinese payer perspective, amlodipine is a cost-saving therapy when compared with ARBs by lowering the costs needed to manage acute stroke and MI episodes and related long-term recovery [16].
In other results, treatment with amlodipine in hypertensive rats preserved cerebral perfusion at a lower blood pressure [9]. Long treatment with amlodipine in hypertensive rats, caused to reduce the oxidative stress in brain10. In another study amlodipine reduced the size of the ischemic lesion and improved the neurological score in apolipoprotein E-deficient mice [17]. In one study amlodipine and valsartan improved stroke outcome as monotherapy in patients with high cardiovascular risk [18]. Similar our results, several previous randomised trials with amlodipine showed promising results, with an increased probability of global recovery at day 90. Furthermore, results were also positive in the individual Barthel index scale [16-18].
Conclusion
This trial showed that amlodipine is effective in treatment of moderate-to-severe acute ischemic stroke patients. Hence, 3 months of follow up, as is the case in many, if not all, previous trials, may be of insufficient length to detect a treatment effect. There are not enough studies in order to evaluate amlodipine effect in stroke prognosis in human beings, most of previous surveys studied animal models.
- European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25(5):457-507. doi: 10.1159/000131083. Epub 2008 May 6. PMID: 18477843.
- Myron D Ginsberg. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40:S111–S114. https://doi.org/10.1161/STROKEAHA.108.528877
- Harman D. The free radical theory of aging, in Free Radicals in Biology (Pryor WA, ed). 1982; pp. 255-275. Academic Press, New York.
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10540-3. doi: 10.1073/pnas.88.23.10540. PMID: 1683703; PMCID: PMC52964.
- Cotman CW, Pike CJ, Copani A. beta-Amyloid neurotoxicity: a discussion of in vitro findings. Neurobiol Aging. 1992 Sep-Oct;13(5):587-90. doi: 10.1016/0197-4580(92)90060-b. PMID: 1461347.
- Choi JH, Yu BP. Brain synaptosomal aging: free radicals and membrane fluidity. Free Radic Biol Med. 1995 Feb;18(2):133-9. doi: 10.1016/0891-5849(94)00106-t. PMID: 7744296.
- Johnson EM Jr, Greenlund LJ, Akins PT, Hsu CY. Neuronal apoptosis: current understanding of molecular mechanisms and potential role in ischemic brain injury. J Neurotrauma. 1995 Oct;12(5):843-52. doi: 10.1089/neu.1995.12.843. PMID: 8594212.
- Mason RP, Leeds PR, Jacob RF, Hough CJ, Zhang KG, Mason PE, Chuang DM. Inhibition of excessive neuronal apoptosis by the calcium antagonist amlodipine and antioxidants in cerebellar granule cells. J Neurochem. 1999 Apr;72(4):1448-56. doi: 10.1046/j.1471-4159.1999.721448.x. PMID: 10098848.
- Cai H, Yao H, Ibayashi S, Takaba H, Fujishima M. Amlodipine, a Ca2+ channel antagonist, modifies cerebral blood flow autoregulation in hypertensive rats. Eur J Pharmacol. 1996 Oct 10;313(1-2):103-6. doi: 10.1016/0014-2999(96)00618-8. PMID: 8905335.
- Hirooka Y, Kimura Y, Nozoe M, Sagara Y, Ito K, Sunagawa K. Amlodipine-induced reduction of oxidative stress in the brain is associated with sympatho-inhibitory effects in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2006 Jan;29(1):49-56. doi: 10.1291/hypres.29.49. PMID: 16715653.
- Kopecky BJ, Liang R, Bao J. T-type calcium channel blockers as neuroprotective agents. Pflugers Arch. 2014 Apr;466(4):757-65. doi: 10.1007/s00424-014-1454-x. Epub 2014 Feb 25. PMID: 24563219; PMCID: PMC4005039.
- Heurteaux C, Gandin C, Borsotto M, Widmann C, Brau F, Lhuillier M, Onteniente B, Lazdunski M. Neuroprotective and neuroproliferative activities of NeuroAid (MLC601, MLC901), a Chinese medicine, in vitro and in vivo. Neuropharmacology. 2010 Jun;58(7):987-1001. doi: 10.1016/j.neuropharm.2010.01.001. Epub 2010 Jan 11. PMID: 20064536.
- Quintard H, Borsotto M, Veyssiere J, Gandin C, Labbal F, Widmann C, Lazdunski M, Heurteaux C. MLC901, a traditional Chinese medicine protects the brain against global ischemia. Neuropharmacology. 2011 Sep;61(4):622-31. doi: 10.1016/j.neuropharm.2011.05.003. Epub 2011 May 14. PMID: 21605573.
- Moha Ou Maati H, Borsotto M, Chatelain F, Widmann C, Lazdunski M, Heurteaux C. Activation of ATP-sensitive potassium channels as an element of the neuroprotective effects of the Traditional Chinese Medicine MLC901 against oxygen glucose deprivation. Neuropharmacology. 2012 Sep;63(4):692-700. doi: 10.1016/j.neuropharm.2012.05.035. Epub 2012 May 30. PMID: 22659084.
- Read SJ, Hirano T, Davis SM, Donnan GA. Limiting neurological damage after stroke: a review of pharmacological treatment options. Drugs Aging. 1999 Jan;14(1):11-39. doi: 10.2165/00002512-199914010-00002. PMID: 10069406.
- Wu Y, Zhou Q, Xuan J, Li M, Zelt S, Huang Y, Yin H, Huang M. A Cost-Effectiveness Analysis between Amlodipine and Angiotensin II Receptor Blockers in Stroke and Myocardial Infarction Prevention among Hypertension Patients in China. Value Health Reg Issues. 2013 May;2(1):75-80. doi: 10.1016/j.vhri.2013.01.005. Epub 2013 Mar 13. PMID: 29702856.
- Mogi M, Iwai M, Chen R, Iwanami J, Ide A, Tsukuda K, Yoshii T, Horiuchi M. Amlodipine treatment reduces stroke size in apolipoprotein E-deficient mice. Am J Hypertens. 2006 Nov;19(11):1144-9. doi: 10.1016/j.amjhyper.2006.04.009. PMID: 17070425.
- Atsuhiro Ichihara, Kanako Bokuda. Valsartan and Amlodipine: Safety and Efficacy in Stroke Prevention. Clinical Medicine Reviews in Vascular Health. 2010; 2 :151. doi: 10.4137/CMRVH.S5204

Sign up for Article Alerts