Unmasking Intracranial Atherosclerotic Disease: Experience from an Outpatient Neurosonology Lab?
Anzola Gian Paolo1* and Bartolaminelli Clara2
1Neurosonology lab Hospital Piccole Figlie, Parma, Italy
2Emergency Department Presidio Ospedaliero di Montichiari, Spedali Civili, Brescia, Italy
*Address for Correspondence: G. Paolo Anzola, Consultant Neurologist, Neurosonology Lab Hospital Piccole Figlie HPF, Via Po, 2-43100 Parma, Italy, Mob: 0039-333-6410034; E-mail: gpanzola@gmail.com
Submitted: 01 May 2017; Approved: 11 May 2017; Published: 18 May 2017
Citation this article: Anzola GP, Bartolaminelli C. Unmasking Intracranial Atherosclerotic Disease: Experience from an Outpatient Neurosonology Lab. Int J Neurol Dis. 2017;1(1): 013-017.
Copyright: © 2017 Anzola GP, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Background and Purpose: Intracranial Atherosclerotic Disease (ICAD) is a malignant affection which carries a high risk of first or recurrent stroke if unnoticed, but the consequences of which may be drastically improved by early appropriate medical treatment. Early diagnosis is therefore advisable. We analyzed the 12-year database of consecutive patients studied in a single neurosonology lab to assess ICAD prevalence and predictors
Material and Methods: From 18702 examinations we extracted 622 records of patients (M/F ratio 337/ 285, age 61 + 16), who had undergone a complete extra and intracranial assessment with ultrasound. By using a computer based program we recorded all variables in a standardized manner.
Results: ICAD was detected in 62 (M/F = 36/ 26) patients (9.9%), in 45 (7.2%) in the anterior circulation only , in 13 (2.1%) in the posterior circulation only, in 4 (0.6%) in both anterior and posterior circulations. Independent predictors were hypertension, extracranial carotid disease and family history for stroke.
Conclusions: Unmasking ICAD is feasible with TCCD. The likelihood of a positive result increases from one every10-20 examination to one every 1-4 if hypertension, carotid disease and family history of stroke are present in isolation and more so if they are associated.
Introduction
Intracranial Atherosclerotic Disease (ICAD) is estimated to carry a 1-year risk rate of stroke of 3.5%, as calculated from the outcome of asymptomatic lesions found in the Warfarin versus Aspirin Symptomatic Intracranial Disease Study for Stroke WASID [1] and is burdened with a 2 year recurrence rate ranging from 19.7% in aspirin and 17.2% in warfarin-treated patients in WASID, to 38.2% in the GESICA study [1,2].
Since aggressive and timely treatment of ICAD may improve the outcome of affected patients [3] it would be desirable to discover it as soon as possible, but in the real life this is seldom done despite the fact that non-invasive neuroradiological investigations such as CT angiography and MR angiography are highly reliable in comparison with the gold standard of intra-arterial angiography [4]. The reasons for this relative neglect of ICAD are multiple: availability of radiological expertise, costs, radiation exposure and in part the absence of a clear-cut clinical profile that helps to select those individuals most likely to carry this pathology.
It is thus likely that ICAD is largely underestimated in the general population [5].
Transcranial Doppler or Transcranial Color-Coded Doppler (TCCD) sonography may non-invasively detect focal narrowing of intracranial vessels with a negative predictive value close to 90%, which reliably allows the exclusion of intracranial stenosis [6], it is free of untoward effects, inexpensive and relatively widely available in vascular labs. Hence it appears the ideal screening tool to exclude intracranial stenosis when combined with the more conventional ultrasound assessment of supra-aortic vessels.
The scope of this study was to report the incidence of ICAD as resulting from the 12-year experience of a neurosonology lab and its relationship with extracranial atherosclerotic disease and with other vascular risk factors with the aim of deriving predictive rules able to maximize the yield of transcranial Doppler examination for ICAD detection.
Material and Methods
We analyzed the database of the Neurosonology labs of S. Orsola Hospital –Brescia (period 2003 Jan 1st - 2011 Dec 31st) and of the Hospital Piccole Figlie – Parma (period 2012 Jan 1st - 2014 Dec 31st), ruled by one of us (GPA).
Out of 18702 examinations of supra aortic vessels performed in the study period we were able to extract 622 records of patients in whom both extracranial and intracranial vessels were examined.
The acquisition of data occurred in every patient in a standardized way through multiple choice computerized pop-up windows for each item. For extracranial Internal Carotid Arteries (ICAs) the percent luminal narrowing was ranked as absent, < 30, 30-50, 50-70,70-85, near-occlusion and occlusion based on the combined results of peak systolic velocity measured in the Doppler modality and cross section imaging in B-mode according to the ECST criteria [7,8]. Plaque texture was graded according to published criteria from hypoechoic to hyperechoic in four steps [9]. For the purpose of this study ICA pathology was re-categorized in NO ATHERO, MILD ATHERO and SEVERE ATHERO according to whether both ICAs were normal or at least one showed a < 50% or > 50% stenosis respectively. Occluded ICAs were classified as SEVERE ATHERO.
The evaluation of intracranial vessels at the supra tentorial level was restricted to Middle Cerebral Artery (MCA) and Terminal ICA (TICA), at a depth ranging from 45 to 65 mm from the temporal bone, as MCA-TICA assessment is easy and highly reproducible and MCA or TICA are most often involved in ICAD [2,10,11]. TICA and MCA (both M1 and M2 segments) were visually identified and thereafter insonated with angle correction. The result was deemed abnormal if in at least one MCA or TICA mean flow velocity exceeded 80 cm/sec, implying a stenosis of at least 50%, or side to side velocity asymmetry was greater than 20%, implying distal MCA or branch occlusion [12,13]. In cases of ICA stenosis greater than 85%, ipsilateral ICAD in the anterior circulation was diagnosed by means of the normal-to-stenotic ratio as described by Felberg, et al. [12], as absolute velocity values may be dampened. In the basilar or intracranial vertebral arteries, insonated through the occipital window, we defined as abnormal any focal velocity increase exceeding 80 cm/ sec peak systolic velocity or the inability to detect the artery [14]. The vast majority of tests were performed with a Philips IU 21 machine.
We deemed ICAD present when at least one vessel either in the anterior or in the posterior circulation was abnormal. In doubtful cases, a second examination was performed and a consensus reached. When ICAD was diagnosed a confirmation was looked for with either MR or CT angiography. Interrater correlation was assessed randomly throughout the study period and found > 85%.
Personal history of hypertension, diabetes, dyslipidemia, smoking habit, peripheral vein disease, stroke or TIA and heart disease were systematically recorded. Moreover, family history for either cerebrovascular or cardiovascular diseases was also noticed. Statistical comparisons were performed with Chi-square tests, independent sample t-test and logistic regression analysis when appropriate (SPSS version 22).
Results
Overall data was available for 622 patients (M/F ratio 337/285, age 61 + 16). Reasons for performing the neurosonological investigations were: screening in subjects with risk factors for 138 (22.2%), previous stroke or TIA in 136 (21.9%), follow-up of known atherosclerotic disease of supra-aortic vessels in 131 (21.1%), headache in 55 (8.8%), asymptomatic brain vascular disease in 38 (6.1%), vertigo or tinnitus in 32 (5.2%), visual troubles in 17 (2.7%), syncope in 16 (2.6%), TGA in 11 (1.8%), dementia in 8 (1.3%), miscellanea in the remaining 34 (5.5%).
Prevalence of vascular risk factors is shown in (Table 1).
Females were significantly younger than males (58.5 vs. 63.6, p < 0.0001), less frequently affected by diabetes (7% vs. 16%, p = 0.003), coronary artery disease (6.5% vs. 17.5%, p = 0.026) and severe atherosclerotic carotid disease (11% vs. 21.4, p < 0.0001), but more often by migraine (42.5% vs. 19%, p < 0.0001).
Overall ICAD was detected in 62 (M/F= 36/26) patients (9.9%), in 45 (7.2%) in the anterior circulation only, in 13 (2.1%) in the posterior circulation only, in 4 (0.6%) in both anterior and posterior circulations.
Compared with subjects without, patients with ICAD were overall older (68.2 vs. 60.4, p < 0.001) and showed a higher prevalence of hypertension (84.3% vs. 50%, p < 0.0001), diabetes (26.5% vs. 10.2%, p=0.001), dyslipidemia (61.7% vs. 43%, p = 0.016), family history positive for cerebrovascular disease (27.8% vs. 12.6, p = 0.038) and atherosclerosis in carotid arteries (72.6% vs. 42%, p < 0.0001).
In females, only age (69.5 vs. 57.3, p < 0.0001), hypertension (81.8% vs. 45.9%, p = 0.001) and carotid atherosclerotic disease (60.2% vs. 30.8%, p=0.001) were significantly associated with ICAD.
The prevalence of ICAD in patients with and without significant risk factors is shown in table 2.
Factors significantly associated with ICAD in both anterior and posterior circulation were entered as predictors in a logistic regression analysis. Only hypertension was significant (Table 3).
A second logistic regression was performed taking ICAD in anterior circulation as the dependent variable: this time hypertension, carotid atherosclerotic disease and family history of CVD turned significant (Table 4).
Discussion
Intracranial atherosclerotic disease has been estimated to account for 5-10% of strokes in white people, 15-29% of black people and up to 30-50% in Asian people the differences being probably due to both genetic factors and lifestyle habits, and it is considered the main cause of stroke worldwide [3-5]. Asymptomatic ICAD carries a 3.5% yearly risk of first in a lifetime stroke, a 2-year risk of recurrent stroke of 17.2 - 38.2 % [1,2], and a 31.2% risk of recurrent stroke at one year when combined with extracranial carotid disease [15]. This malignant outcome may be abated to a 12.2% risk of recurrence at one year with an aggressive medical treatment, as has been convincingly shown by the SAMMPRIS study [16], which makes early ICAD detection mandatory, especially so in the asymptomatic population.
Transcranial Color-Coded Doppler sonography has proved sufficiently accurate for being used as a screening tool: although its positive predictive value is quite low (55% in the SONIA study), the negative predictive value permits the exclusion of ICAD when findings are negative [6,12]. In recent years, TCCD facilities have become widespread in stroke units and neurosonology labs, but TCCD is still underused on an ambulatory basis partly because there are no clear indications on which patients are to be investigated because of a high probability of being affected. To overcome these drawbacks we tried to capitalize the results of all complete investigations performed over a 12 year period including extra and intracranial circulation in the neurosonology lab ruled by one of us taking advantage of the fact that the reporting system in use allows only standardized inputting of data and the collection of past history data and recording of risk factors has been systematical since 2002.
We were thus able to extract a cohort of 622 patients who had undergone a complete assessment of extra and intracranial vessels and who showed an epidemiological profile consistent with the one expected in the general population, thus excluding selection bias [17].
In this cohort, ICAD turned out to be present overall in 9.9% of patients, with a skewed distribution towards a higher prevalence of the anterior (7.2%) as compared to the posterior (2.1%) circulation and with a strict minority of double occurrence (0.6%). The absolute prevalence is in nice agreement with the AsIA, a population-based study on asymptomatic subjects with vascular risk factors, which reported a prevalence of 8.6% in European patients [18], whereas in Hong Kong it has been reported to be 12.6% [19] and in USA 13% [20].
ICAD prevalence did not differ in symptomatic compared with asymptomatic patients (9.7% vs 11.5% respectively, p = 0.608), which fits in well with the overall prevalence of 9.2% and 8.9% recently reported by Tsivgoulis, et al. and Baracchini, et al. in two series of 467 and 1134 acute stroke caucasian patients respectively [11,21], and was the same in male and female patients (10.5% vs. 9.3% respectively, p = 0.626).
In agreement with most reports, ICAD was significantly associated with hypertension, diabetes and increasing age [1,2,3,4], but also significant were dyslipidemia, family history of CVD and carotid atherosclerotic disease on univariate analysis table 2 reports the increase in ICAD prevalence brought about by each risk factor: from the table, it is clear that in our cohort the two most important factors were hypertension and severe carotid disease both of which almost quadruplicated the probability of ICAD.
This is confirmed by logistic regression analysis which confirmed hypertension as the most powerful single independent predictor with an odds ratio of 4.6, while for anterior circulation carotid disease and family history of CVD added to the list of independent predictors, however with a much smaller odds ratio of 1.9.
It is interesting that the two latter predictors emerged as such only for the anterior circulation. The reasons for this discrepancy are not entirely clear, but we would speculate that this resulted from the inability of TCCD to discriminate in the posterior circulation true stenosis from focal accelerations in tortuous vessels, although hypertension may be a risk factor for the elongation of the artery that takes place with advancing age.
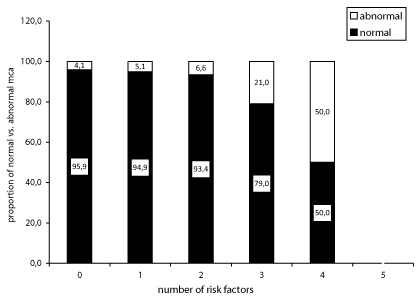
The different risk factors tended to have an additive effect, as is shown in figure 1, where it appears that with 0 risk factors the low prevalence of ICAD most closely reflects the genetically determined individual susceptibility. With the accumulation of risk factors, the process of atherosclerosis steeply worsens, hence increasing ICAD prevalence to 21% with three and up to 50% with four coexistent RF. A similar effect had been noticed by Wong and colleagues [19].
In conclusion, our findings, derived from a sample of individuals quite representative of the general population, indicate that the yield of ICAD detection with TCCD is related to the presence of different RF each exerting a multiplicative effect. The likelihood of discovering ICAD may thus rise from one every 10-20 to one every 2-4 examinations, mainly in the presence of hypertension, severe extracranial carotid disease and family history of CVD, in isolation and more so when these factors are associated (Table 2 and Figure 1).
Strengths of the study are the standardized collection of data, the consecutive assessment of patients and the sample size.
Limitations are mainly due to the single centre experience and to the fact that the cohort of patients was drawn from a neurosonology lab rather than from the general population.
- Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Warfarin–Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005; 352: 1305-1316. https://goo.gl/rijYJS
- Mazighi M, Tanasescu R, Ducrocq X, Vicaut E, Bracard S, Houdart E, et al. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology. 2006; 66: 1187-1191. https://goo.gl/hWNafe
- Qureshi AI, Feldmann E, Gomez CR, Johnston SC, Kasner SE, Quick DC, et al. Intracranial Atherosclerotic Disease: An Update. Ann Neurol. 2009; 66: 730-738. https://goo.gl/tACIL7
- Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013; 12: 1106-1114. https://goo.gl/Hd9DIo
- Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large Artery Intracranial Occlusive Disease A Large Worldwide Burden but a Relatively Neglected Frontier. Stroke. 2008; 39: 2396-2399. https://goo.gl/llCJ8u
- Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, et al. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007; 68: 2099-2106. https://goo.gl/nnYqnl
- Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth I, et al. Carotid Artery Stenosis: Gray-Scale and Doppler US Diagnosis-Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003; 229: 340-346. https://goo.gl/gKx8rf
- von Reutern GM, Goertler MW, Bornstein NM, Del Sette M, Evans DH, Hetzel A, et al. Neurosonology Research Group of the World Federation of Neurology. Grading Carotid Stenosis Using Ultrasonic Methods. Stroke. 2012; 43: 916-921. https://goo.gl/rxCjDh
- De Bray JM, Baud JM, Dauzat M. Consensus Concerning the Morphology and the Risk of Carotid Plaques. Cerebrovasc Dis. 1997; 7: 289-296. https://goo.gl/i1gRy7
- Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008; 39: 1142-1147. https://goo.gl/sIDbHf
- Baracchini C, Anzola GP, Cenciarelli S, Diomedi M, Bella R, Tonon A, et al. Italian symptomatic intracranial atherosclerosis study (ISIDE). A multicenter transcranial ultrasound evaluation. Neurol Sci. 2016; 37: 1645-1651. https://goo.gl/rmmD0B
- Felberg RA, Christou I, Demchuk AM, Malkoff M, Alexandrov AV. Screening for intracranial stenosis with transcranial doppler: the accuracy of mean flow velocity thresholds. J Neuroimaging. 2002; 12: 9-14. https://goo.gl/puJtjN
- Zanette EM, Fieschi C, Bozzao L, Roberti C, Toni D, Argentino C, et al. Comparison of cerebral angiography and transcranial Doppler sonography in acute stroke. Stroke. 1989; 20; 899-903. https://goo.gl/Xk5oJL
- Baumgartner RW, Mattle HP, Schroth G. Assessment of >/= 50% and < 50% Intracranial Stenoses by Transcranial Color-Coded Duplex Sonography. Stroke. 1999; 30: 87-92. https://goo.gl/46UFkx
- Weber R, Kraywinkel K, Diener HC, Weimar C; German Stroke Study Collaboration. Symptomatic Intracranial Atherosclerotic Stenoses: Prevalence and Prognosis in Patients with Acute Cerebral Ischemia. Cerebrovasc Dis. 2010; 30: 188-193. https://goo.gl/umlS4y
- Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011; 365: 993-1003. https://goo.gl/DEKVUl
- Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T, et al. Association between advanced age and vascular disease in different arterial terrioties. A population database of over 3. 6 million subjects. J Am Coll Cardiol. 2013; 61: 1736-1743. https://goo.gl/GEBXmE
- Lopez-Cancio E, Dorado L, Millan M, Reverte S, Sunol A, Massuet A, et al. The Barcelona-Asymptomatic Intracranial Atherosclerosis (AsIA) study: prevalence and risk factors. Atherosclerosis 2012; 221: 221-225. https://goo.gl/NWkA1j
- Wong KS, Ng PW, Tang A, Liu R, Yeung V, Tomlinson B. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology. 2007; 68: 2035-2038. https://goo.gl/nxNhac
- Elmore EM, Mosquera A, Weinberger J. The prevalence of asymptomatic intracranial large-vessel occlusive disease: the role of diabetes. J Neuroimaging. 2003; 13: 224-227. https://goo.gl/pcBmsD
- Tsivgoulis G, Vadikolias K, Heliopoulos I, Katsibari C, Voumvourakis K, Tsakaldimi S, et al. Prevalence of symptomatic intracranial atherosclerosis in Caucasians: a prospective, multicenter, transcranial Doppler study. J Neuroimaging. 2014; 24: 11-17. https://goo.gl/pcGW5B

Sign up for Article Alerts