Intradural Intramedullary Primary Spinal Melanoma: A Case Report and Review of the Literature?
Kashif Majeed1, Ibrahim Hussain1, David J. Pisapia2, Wei Song2, Rajiv Magge3, Ali A. Baaj1 and Philip E. Stieg1*
1Department of Neurological Surgery, Weill Cornell Brain and Spine Center, New York Presbyterian Hospital, New York
2Department of Pathology and Laboratory Medicine; Weill Cornell Medicine, New York Presbyterian Hospital, New York
3Department of Neurology; Weill Cornell Medicine, New York Presbyterian Hospital, New York
*Address for Correspondence: Philip E. Stieg, Department of Neurological Surgery, 525 E 68th St. Starr 651-Box 99, New York, Tel: +212-746-9883; Fax: +212-746-6607; E-mail: pes2008@med.cornell.edu
Submitted: 17 December 2019; Approved: 26 December 2019; Published: 27 December 2019
Citation this article: Majeed K, Hussain I, Pisapia DJ, Song W, Magge R, et al. Intradural Intramedullary Primary Spinal Melanoma: A Case Report and Review of the Literature. Sci J Neurol Neurosurg. 2019;5(1): 012-018.
Copyright: © 2019 Majeed K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Primary melanoma; Spinal cord; Thoracic; Cervical; Gross total resection; Radiotherapy; Chemotherapy
Download Fulltext PDF
Background & Importance: Primary CNS melanoma of the spinal cord are rare neoplasms. They represent a spectrum of benign melanocytomas to malignant melanomas.
Clinical Presentation: A 53-year-old female had presented with several months of right thigh pain and paresthesia’s. There was also midthoracic pain. MRI revealed a 1.5 cm contrast enhancing mass at T10-T11 level. It was exophytic, intradural and intramedullary. Patient underwent laminectomy, gross total excision and received radiotherapy post-operatively. Histopathology analysis showed features of malignant melanoma and molecular study revealed a GNAQ mutation. Full body exam and scanning did not reveal any other primary malignancy. Literature was reviewed and correlated to the current case with regards to symptoms, surgical management, adjuvant therapy, follow up and outcomes.
Conclusion: Most cases do remarkably well with gross total resection only. Post-op adjuvant therapy and long term follow up is advised in cases where gross total resection is not possible.
Background and Importance
Melanomas can arise wherever melanocytes exist [1]. Primary melanocytic tumors of the Central Nervous System (CNS) present along a spectrum from benign melanocytomas to malignant melanoma [2]. Primary Central Nervous System Melanoma (PCNSM) arise from leptomeningeal melanocytes [3,4]. PCNSM are rare, representing only 1% of total body melanomas and 0.07% of all brain neoplasms [1,3,5,6]. Hayward proposed the first diagnostic criteria to differentiate PCNSM from metastatic tumors [7,8].
Technological advances since then have enhanced our understanding of the genetic and molecular aberrations driving their development. PCNSM frequently harbor one of the two mutually exclusive mutations, GNAQ or GNA11 affecting codon 183 on exon 4 or codon 209 of exon 5, respectively, impairing enzymatic function that constitutively activates GTP-signaling cascades promoting oncogenesis [9,10].
Within PCNSM, Primary Spinal Melanoma (PSM) are even rarer with only 64 reported cases [11-16] (Supplementary Table 1). Here a unique case of a thoracic Intradural Intramedullary (IDIM) PSM, its histopathologic and genetic analyses and review of literature is presented. Whereas previous cases have reported GNAQ mutations in PCNSM, this is the first case to demonstrate this mutation in a solitary IDIM PSM.
Clinical Presentation
A 53-year-old female with no known family history presented with 6 months of progressive numbness, and right anterior thigh and mid-thoracic pain. There were no other symptoms including bowel or bladder incontinence. On examination her only deficit was in thigh sensation and no upper motor neuron signs elsewhere.
Preoperative imaging
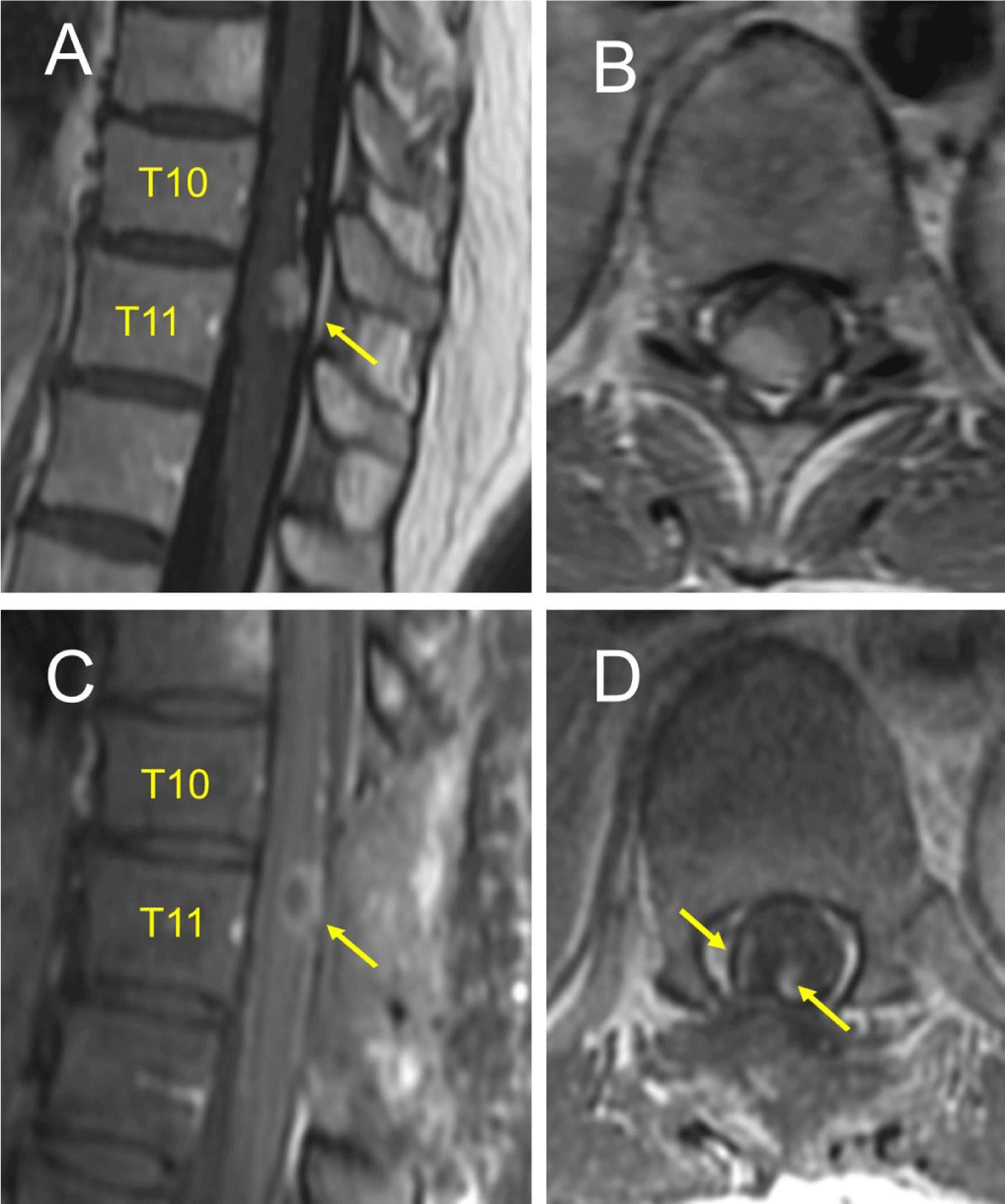
An MRI of the thoracic spine demonstrated a 14mm x 6mm gadolinium enhancing mass at the T10-T11 level (Figure 1A). There was mild cord effacement without intramedullary T2 hyperintensity (Figure 1B). There was a questionable pial enhancement versus an engorged vasculature extending superoinferior to the lesion (Figure 1A). MRI of the brain and cervical spine and preoperative angiography for tumor blush were negative. Considering an ependymoma, an astrocytoma or a hemangioblastoma surgery was recommended for decompression and definitive diagnosis.
Intraoperative findings
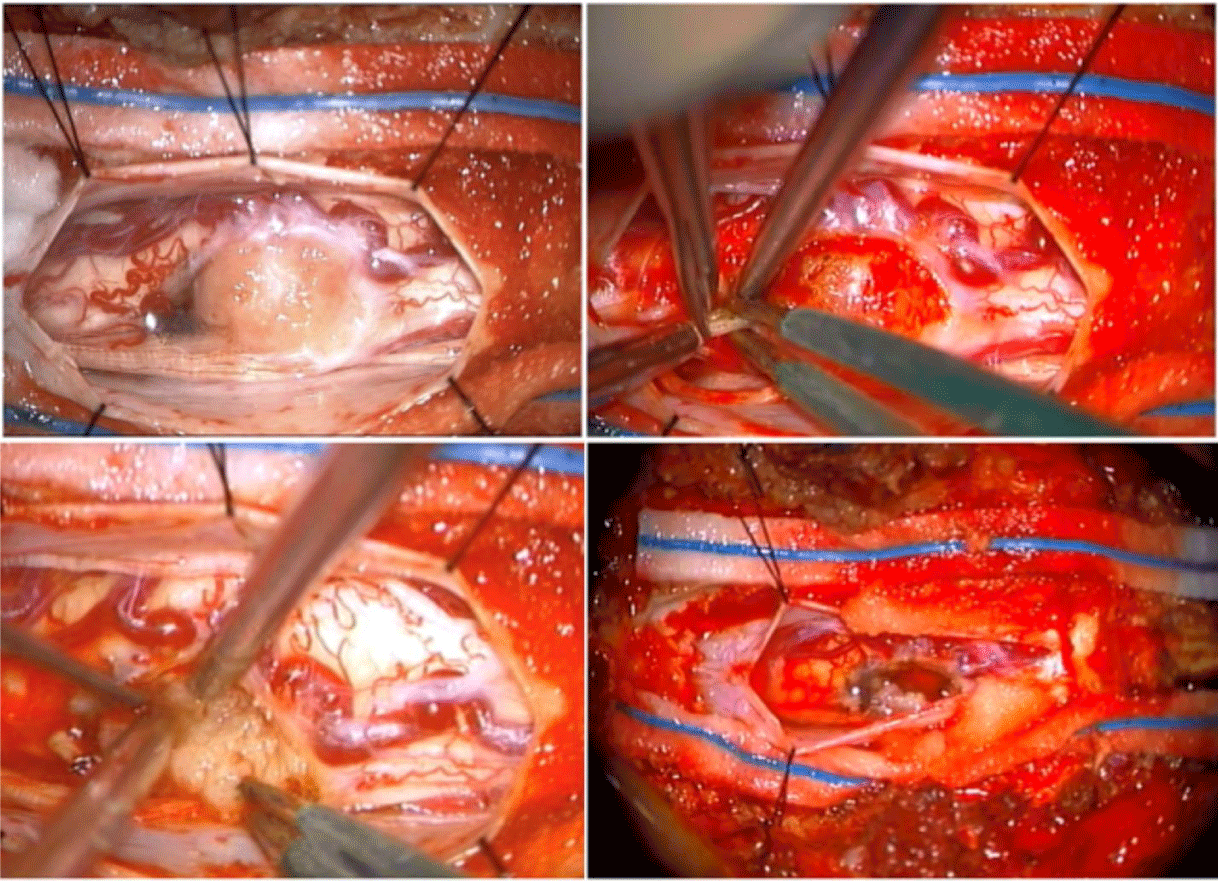
She underwent a T10-T11 laminectomy for tumor resection (Figure 1C). Intraoperative Somatosensory Evoked Potentials (SSEP) and Motor Evoked Potentials (MEP) were monitored. The tumor extent was determined using an intraoperative ultrasound. The mass lacked clear planes, appeared intramedullary, exophytic and invading the adjacent peripheral nerves. Laterally, the capsule was cauterized, incised, and tumor was debulked significantly but not completely due to over 50% drop in MEPs bilaterally. SSEPs remained stable though. Frozen section revealed a highly-cellular spindle cell neoplasm. A small capsule adherent to the cord was left behind (Figure 1D), hemostasis was secured and the dura, closed (Figure 2).
Postoperative course
On her immediate post-op exam, there was anterior thigh sensory loss and decreased right foot proprioception. Motor exam remained normal. In ICU she was on high dose steroids. On the floor on postoperative day 2, she had difficulty ambulating requiring assistance. She was discharged to an acute rehabilitation center. On one-month follow-up, her only deficit was sensory. Final pathology confirmed a PCNSM. A full body workup and an FDG-PET ruled out another primary, a cutaneous or a uveal melanoma.
Histopathology and genetic analysis
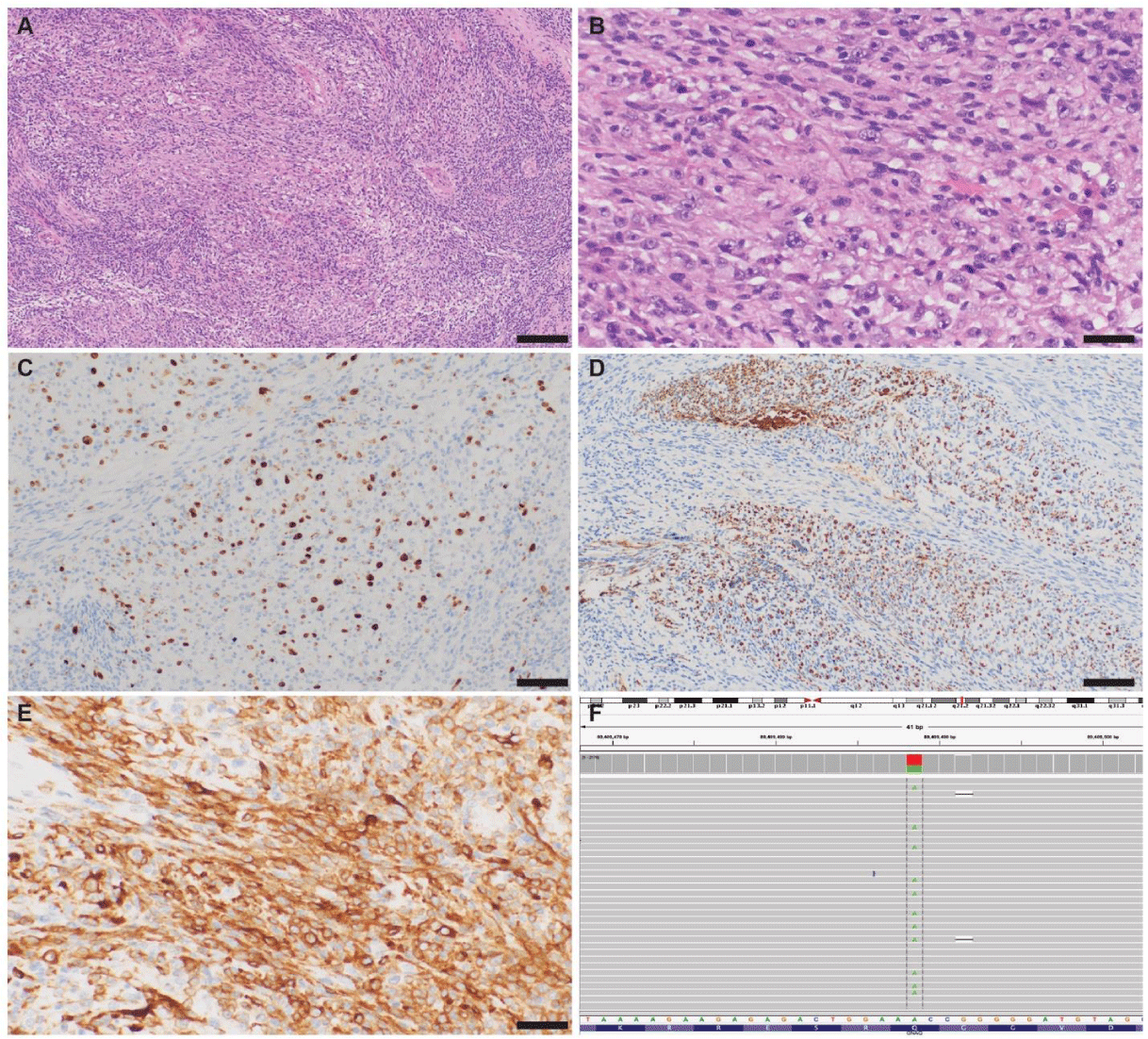
Histopathologically, the tumor cells were predominantly spindled demonstrating a fascicular growth pattern (Figure 3A). A nodular area of epithelioid features, increased nuclear atypia and prominent nucleoli (Figure 3B) revealed mitotic activity (2 mitotic figures in a single 600X field) and increased MIB-1 (Ki67) index of about 10 to 15% (Figure 3C). The neoplasm involved peripheral nerve axons splaying them apart as apparent on neurofilament staining (Figure 3D). Melanin containing cells were rare. Immunostaining was diffusely and strongly positive for SOX10 and A103 along with strong GFAP, S-100, and CK labelling, and diffuse yet weak labeling for S-100 and HMB45 in some foci (Figure 3E). Stains for EMA, PR, ERG, and CD45 were only focally weak or negative, and H3K27-me3 expression was preserved. PLD1 levels were 0%.
Therefore, melanotic schwannoma with focal atypical features, metastatic melanoma and PMM of the CNS were considered. Only upon 143 gene targeted next generation sequencing assay, Oncomine v2 (ThermoFisher) [17], a GNAQ Q209L mutation was confirmed (Figure 3F) without any other variants. GNAQ-Q209L is characteristic of PCNSM as well as uveal melanomas thereby ruling out metastatic cutaneous melanoma or a melanotic schwannoma.
Treatment
At 2-month follow-up, MRI revealed stable residual disease and no postoperative complication. With negative PDL1, a GNAQ mutation, and elevated MIB index, ipilimumab plus nivolumab combination toxicity appeared to outweigh benefits, like uveal melanomas where immunotherapy fails [18]. Therefore, only radiotherapy, of 5400 cGY over 30 fractions was given without any adverse sequalae. One-month post-radiation MRI failed to discern radiation effects from persistent tumor. Her surveillance MRIs every 3 months are pending. On her last 6 months follow-up, she remained clinically stable.
Literature review
Methods: Literature was searched for “primary CNS melanoma”, “primary melanoma of the spinal cord” and “primary malignant melanomas of the spinal cord.”
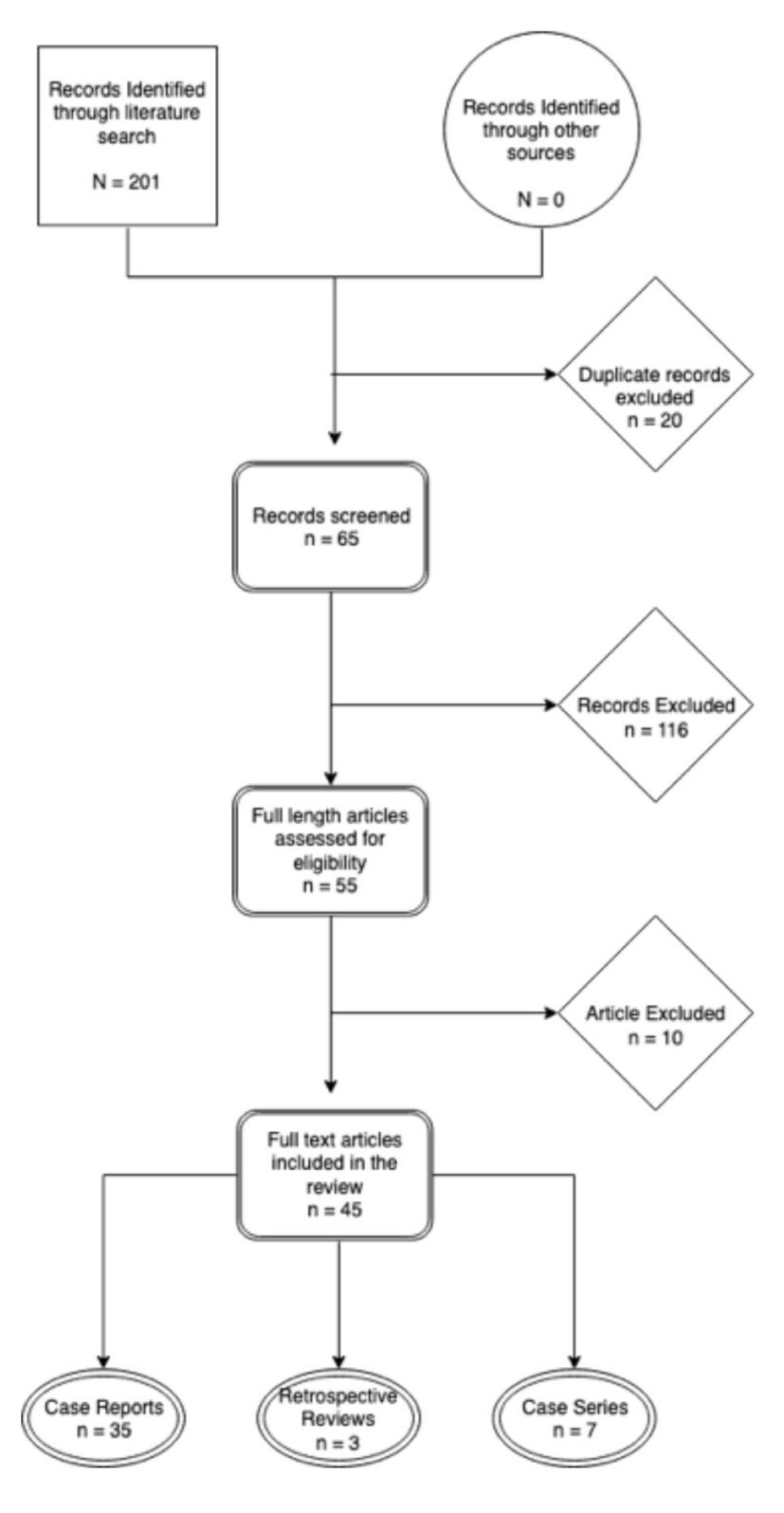
Results: The search yielded 201 articles in total. After removing 20 duplicates, metastatic and non-spinal cord melanomas, 65 articles remained. Fifty-five of these were case reports, 7, case series and 3, retrospective reviews. Four non-English articles were further excluded. Forty-five articles were available for final review (Figure 4).
Discussion
Melanoma represents the 3rd most common metastatic tumor to the CNS, behind breast and lung [6]. In contrast, the incidence of PCNSM is only 0.005 cases per 100,000 [7]. PSM is even rarer, with only 64 reported cases (Supplementary table 1). While many studies have reported solitary IDEM tumors in the cervical or thoracic spine [5,19,20], the incidence of IDIM PSM is far rarer [21], as seen in this case.
The peak incidence of PSM is in the fifth decade of life (52.4 15.5) with ages ranging from 15 to 80 years (Supplementary table 1) [5,20,22]. Motor weakness along with dysesthesias, abnormal reflexes, loss of bowel and bladder function, or pain are the most common presenting signs and symptoms [19,23,24]. MRI is the imaging modality for visualization [3,8,25], with tumors appearing hyperintense on T1 weighted imaging and hypo- to isointense on T2 weighted imaging with uniform contrast enhancement [1,5,8,25,26]. Confirmatory diagnosis with histology is required with genetic features aiding in ambiguous situations. Immunostaining characteristically demonstrates S-100 and HMB-45 immunoreactivity, markers of melanogenesis [8]. Additionally, GNAQ Q209L mutation is characteristic of PCNSM as well as uveal melanoma [9,10,27].
PSM must be differentiated from the metastatic cutaneous melanoma [1,19]. The 5-year survival rate for local melanoma is 98%, however it drops to 23% with widespread metastases [28]. The preferred treatment for PSM is complete surgical resection whenever possible followed by radiotherapy [1,5,20]. In cases where Gross Total Resection (GTR) was achieved, survival reached around 88%, compared to 78% in cases of Subtotal Resection (STR). Adjuvant radiation with 4700 cGy in subtotal resections has been reported, however, given the relatively small number of these cases, its effectiveness remains unclear [5,25]. The efficacy of adjuvant chemotherapeutic agents, used for PSM, like dacarbazine, vincristine, bleomycin, and cisplatin also needs to be ascertained [15,21,22,26,29,30] (Table 1).
- Farrokh D, Fransen P, Faverly D. MR findings of a primary intramedullary malignant melanoma: Case report and literature review. AJNR Am J Neuroradiol. 2001; 22: 1864-1866. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11733317
- Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol. 1999; 23: 745-754. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10403296
- Lee NK, Lee BH, Hwang YJ, Sohn MJ, Chang S, Kim YH, et al. Findings from CT, MRI, and PET/CT of a primary malignant melanoma arising in a spinal nerve root. Eur Spine J. 2010; 19: 174-178. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20127497
- Pappenheim E, Bhattacharji SK. Primary melanoma of the central nervous system. Clinical-pathological report of a case, with survey and discussion of the literature. Arch Neurol. 1962; 7: 101-113. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14483757
- Kim MS, Yoon DH, Shin DA. Primary spinal cord melanoma. J Korean Neurosurg Soc. 2010; 48: 157-161. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20856666/
- Kounin GK, Romansky KV, Traykov LD, Shotekov PM, Stoilova DZ. Primary spinal melanoma with bilateral papilledema. Clin Neurol Neurosurg. 2005; 107: 525-527. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16202828
- Hayward RD. Malignant melanoma and the central nervous system. A guide for classification based on the clinical findings. J Neurol Neurosurg Psychiatry. 1976; 39: 526-530. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/950562/
- Wadasadawala T, Trivedi S, Gupta T, Epari S, Jalali R. The diagnostic dilemma of primary central nervous system melanoma. J Clin Neurosci. 2010; 17: 1014-1017. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20627582
- Van Raamsdonk CD, Barsh GS, Wakamatsu K, Ito S. Independent regulation of hair and skin color by two G protein-coupled pathways. Pigment Cell Melanoma Res. 2009; 22: 819-826. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19627560
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010; 363: 2191-2199. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21083380
- Chatterjee R, Nascimento FA, Heck KA, Ropper AE, Sabichi AL. Primary spinal cord melanoma - an uncommon entity. Can J Neurol Sci. 2019; 46: 348-350. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31084668
- Hering K, Bresch A, Lobsien D, Mueller W, Kortmann RD, Seidel C. Primary intradural extramedullary spinal melanoma in the lower thoracic spine. Case Rep Oncol Med. 2016; 2016: 3815280. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27127667
- Iga T, Iwanami A, Funakoshi T, Mikami S, Tsuji O, Nagoshi N, et al. Multifocal primary melanoma of the cervical spinal cord successfully treated by tumorectomy: A case report. Spinal Cord Ser Cases. 2018; 4: 24. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29581891
- Wu L, Xu Y. Primary spinal intramedullary malignant melanoma involving the medulla oblongata. Spine J. 2016; 16: 499-500. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26806813
- Zhang M, Liu R, Xiang Y, Mao J, Li G, Ma R, et al. Primary spinal cord melanoma: a case report and a systemic review of overall survival. World Neurosurg. 2018; 114: 408-420. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29614357
- Zhang Z, Gong H, Zhao C, Wang D, Qian M, Wu Z, et al. Prognostic factors of patients with spinal malignant melanoma after surgical intervention: a case series of 21 patients and literature review. J Neurooncol. 2019; 142: 119-127. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30607707
- Robinson JT, Thorvaldsdottir H, Wenger AM, Zehir A, Mesirov JP. Variant review with the integrative genomics viewer. Cancer Res. 2017; 77: 31-34. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29092934
- Karivedu V, Eldessouki I, Taftaf A, Zhu Z, Makramalla A, Karim NA. Nivolumab and ipilimumab in the treatment of metastatic uveal melanoma: A single-center experience. Case Rep Oncol Med. 2019; 2019: 3560640. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31179139
- Fuld AD, Speck ME, Harris BT, Simmons NE, Corless CL, Tsongalis GJ, et al. Primary melanoma of the spinal cord: A case report, molecular footprint, and review of the literature. J Clin Oncol. 2011; 29: 499-502. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21444862
- Liubinas SV, Maartens N, Drummond KJ. Primary melanocytic neoplasms of the central nervous system. J Clin Neurosci. 2010; 17: 1227-1232. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20558070
- Wuerdeman M, Douglass S, Abda RB, Krasnokutsky M. A rare case of primary spinal cord melanoma. Radiol Case Rep. 2018; 13: 424-426. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29904488
- Mallick S, Roy S, Joshi NP, Julka PK. Primary spinal melanoma treated with adjuvant radiotherapy and concurrent temozolomide: A case report and review of literature. J Cancer Res Ther. 2015; 11: 1027. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26881599
- Salame K, Merimsky O, Yosipov J, Reider Groswasser I, Chaitchik S, Ouaknine GE. Primary intramedullary spinal melanoma: diagnostic and treatment problems. J Neurooncol. 1998; 36: 79-83. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9525829
- Theodore C Larson III, O Wayne Houser, Burton M Onofrio, David G Piepgras. Primary spinal melanoma. J Neurosurg. 1987; 66: 47-49. https://bit.ly/2ZuIQyI
- Somers KE, Almast J, Biemiller RA, Silberstein HJ, Johnson MD, Mohile NA. Diagnosis of primary CNS melanoma with neuroimaging. J Clin Oncol. 2013; 31: 9-11. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23169522
- Yamasaki T, Kikuchi H, Yamashita J, Asato R, Fujita M. Primary spinal intramedullary malignant melanoma: case report. Neurosurgery. 1989; 25: 117-121. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2755570
- Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009; 457: 599-602. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19078957
- Alliance MR. Melanoma Survival Rates. 2019.
- Lee CH, Moon KY, Chung CK, Kim HJ, Chang KH, Park SH, et al. Primary intradural extramedullary melanoma of the cervical spinal cord: Case report. Spine (Phila Pa 1976). 2010; 35: 303-307. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20308942
- Nishihara M, Sasayama T, Kondoh T, Tanaka K, Kohmura E, Kudo H. Long-term survival after surgical resection of primary spinal malignant melanoma. Neurol Med Chir (Tokyo). 2009; 49: 546-548. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19940408

Sign up for Article Alerts