Introduction
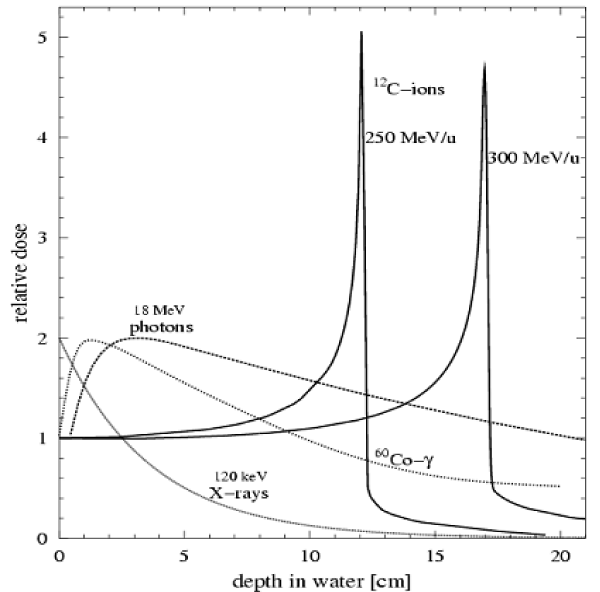
Heavy ions are positive ions with atomic numbers greater than protons, including 4He, 12C, 16O, 20Ne, 40Ca, 56Fe, 63Cu, 92Mo, 107Ag, 142Nd, 172Hf, 184Os, 197Au, 209Bi, 238U, 236Np and so on. Conventional radiation (X-ray, gamma ray, electron beam, neutron beam) has a natural defect in the process of tumor irradiation, such as the depth dose distribution exponentially decaying and the great damage of shallow epidermis and deeper health of the organization while conventional radiation reaching the tumor cells. So people are actively looking for a more superior radiotherapy with radiation - heavy ion beam. As shown in figure.1, when the heavy ion beam penetrates the material, the charged heavy ions lose energy by colliding with the electrons outside the target nucleus and the ion velocity slows down. Meanwhile, the contact time of the heavy ion beam with the local tissue is then extended and will release 80% of the energy in the region of several millimeters to form the Bragg peak. After the peak area, the beam energy drops to zero, and the peak area belongs to the lower energy dosage area before the peak area. This phenomenon was discovered by William Henry Bragg in 1903, hence the name of the Bragg peak. In addition, due to the large mass and inertia of heavy ions and the small influence of the Coulomb interaction between the nuclei, the lateral scattering is small at the time of advance. This can reduce the radiation on the surrounding healthy tissue damage. In most of the heavy ion beam, C beam treatment is the best and the side effects is the smallest, so most of the treatment of cancer patients are using C ion beam [1]. At present, there are more than 30 large-scale heavy ion accelerators in the operation or construction, including the United States’ BNL-RHIC and MSU-FRIB, Germany’s FAIR, Japan’s RIKEN-RIBF, China’s BRIF and HIRFL and France’s GANIL-SPIRAL2 [2].
Figure 1: Bundles of X, γ, photons and heavy ion C penetrate the depth dose distribution in the water target [34].
Since April 12, 1961, the Soviet Union launched the world’s first manned spacecraft Vostok 1, manned space flight space radiation has been a great concern of space medical experts [3-7]. Unlike X-rays and gamma-rays that are exponentially decreasing with increasing depth of radiation, charged heavy ions are characterized by high energy density (LET) and sharp Bragg peak and the energy will be in a specific range of acute release. Although charged heavy ions account for only 1% of the space flux in space, there is a huge threat to the health of astronauts and their radiation damage capacity cannot be ignored. First, astronauts appear to have a functional change in the central nervous system (eg, fatigue, memory loss, mood changes, immediate glimpse, etc.) because of large extent by radiation after long flights [6,8-10]; Second, the astronaut’s vagus nerve in the space flight in a state of inhibition and Eckberg et al. found that the average range of R-R interval responses to neck pressure changes declined from preflight levels by 37% on flight day 8 (P =0.051), maximum R-R intervals declined by 14% (P =0.003), and vagal barorflex gain by 9%(P =0.009) after two, 9- and 10-day space shuttlemissions, with graded neck pressure and suction, to elicit sigmoid, vagally mediated carotid baroreflex R-R interval responses, thus may including inordinate tachycardia, orthostatic hypotension, and uncommonly, syncope [11], thus affecting the basic function; Third, space heavy ion radiation can cause damage to the immune system, including the reduction of lymphocytes in peripheral blood, atrophy of thymus and spleen in peripheral immune organs and immunosuppression [12,13], resulting in decreased ability to resist infection [14]; In addition, space heavy ion radiation exists extensive side effects, and the side effects of injury targets are mainly concentrated in the circulatory system, respiratory system, digestive system and immune system [15]. Besides, the substantive organs have induced apoptosis and these injuries are a potential threat to the health of astronauts in long flight and deep space exploration activities. Therefore, in the study of damage to the body by space radiation, heavy ion radiation damage has become the focus of research [16]. Due to the complexity of the spatial environment, it is necessary to determine whether heavy ions radiate the biological sample and hit the specific parts of the sample by studying the effect of space heavy ion radiation on the organism. ESR’s MATROSHKA human simulation model is a useful method for studying heavy ion radiation in space. MATROSHKA is a simulated human body model with more than 6,000 radiation detectors. In 2004, it was successfully measured and compared the radiation absorption of human head and torso inside and outside the International Space Station [17-22]. The study found that high-energy heavy ions are mainly induced mutations [23], induced tumor [24,25], dysplasia [26,27], growth stagnation [28] and multiple chromosomal aberrations and other biological effects [29,30]. The energy of heavy ion radiation transfer to the body’s molecules, cells, tissues and organs, resulting in target organs and non-target organ morphology and function changes [31]. The mechanism of damage mechanism shows that DNA is one of the most important target molecules of ionizing radiation, and its structure is affected by ionizing radiation, such as base damage, sugar damage, DNA double strand breaks and cross-linking and all or part of advanced structural change. Through transcription or post-transcriptional regulatory mechanisms, a series of gene expression changes and biochemical cascade reactions can be induced, ultimately leading to changes in the structure and function of cell growth, proliferation and differentiation [32,33].
Research Status of Heavy Ion Radiotherapy
Through the Pub Med database, heavy ion radiotherapy [Abstract] was used to obtain the literature on heavy ion radiation. At the same time, BICOMS (Bibliographic Item Co-Occurrence Mining System) is used to extract and organize the author, country, city, publication date and periodical name. Afterwards, the data extraction table was created with Excel software, and the publication time and publication period were extracted. A total of 973 articles on heavy ion studies were searched. In addition, as of July 17, 2017, we searched the country and city of the first author of the 973 articles, and we retrieved 929 effective articles.
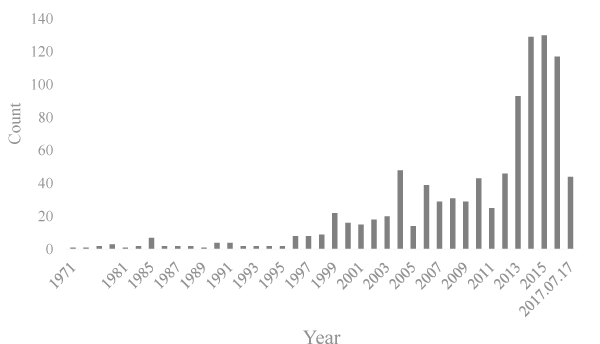
The trend of heavy ion research published in time
As shown in figure. 2, in addition to seven papers about heavy ions published in 1985, 0-4 papers were published from 1971 to 1995. The study of heavy ions has been increasing and peaked in 2013 after 1995. From 2013 to July 17, 2017, there were 442 research papers about heavy ions radiotherapy, accounting for 52.72% of heavy ion radiotherapy research papers.
Figure 2: The trend of heavy ion radiotherapy research published in time.
Information of heavy ion radiotherapy about countries, cities, authors and journals
Through Pub, 198 cities in 29 countries were involved in the research or writing of heavy ion radiation. Table 1 shows the top ten countries that have published heavy ion radiation therapy research papers. In addition, other countries (the number of published papers) including Sweden (7), Denmark (6), The Netherlands (6), Poland (5), Canada, (5), Switzerland (5), Argentina (3), Czech Republic (2), India (2), Australia (2), Norway (1), Singapore (1), Spain (1), Thailand (1), Russia (1), Lebanon (1), South Africa (1), Slovenia 1), Iran (1) also contributed to the study of heavy ions. The continents that publish heavy ion studies include Asia, Europe and North America. Europe has many countries involved in heavy ion research, including Germany, Italy, France, Austria, Belgium, UK and so on. The top three countries in Asia are Japan, China and South Korea, and North America is mainly the USA. Scientific research workers of Japan and Germany have achieved good results. Japan’s research is mainly concentrated in Chiba and Maebashi, Germany’s research is mainly concentrated in Heidelberg and Darmstadt, while China’s research focused on Lanzhou and Shanghai. Japan’s Chiba published the most research papers on heavy ions, reaching 221 (23.79%). The information of the 3054 authors retrieved was sorted out and the main participants were found to be Japanese, German and Chinese scholars. Debus J and Kamada T writed most papers, reaching 84 (8.63%). The research papers on heavy ions are mainly published in Phys Med Biol, Radiother Oncol and so on.
| Table 1: List of countries, cities, authors and periodicals of heavy ion, stop 10. |
| No. |
Country |
N (%) |
City (Country) |
N (%) |
Author |
N (%) |
Journal |
N (%) |
| 1 |
Japan |
385 (41.44) |
Chiba (Japan) |
221 (23.79) |
Debus J |
84 (8.63) |
Phys Med Biol |
105 (10.79) |
| 2 |
Germany |
228 (24.54) |
Heidelberg (Germany) |
110 (11.84) |
Kamada T |
84 (8.63) |
Radiother Oncol |
75 (7.71) |
| 3 |
China |
91 (9.80) |
Lanzhou (China) |
57 (6.14) |
Tsujii H |
81 (8.32) |
J Radiat Res |
68 (6.99) |
| 4 |
USA |
79 (8.50) |
Darmstadt (Germany) |
56 (6.03) |
Jäkel O |
73 (7.50) |
Med Phys |
64 (6.58) |
| 5 |
Italy |
32 (3.44) |
Maebashi (Japan) |
49 (5.27) |
Nakano T |
67 (6.89) |
Int J Radiat Oncol Biol Phys |
60 (6.17) |
| 6 |
France |
18 (1.94) |
Shanghai (China) |
24 (2.58) |
Kanai T |
64 (6.58) |
Radiat oncol |
38 (3.91) |
| 7 |
Korea |
12 (1.29) |
Tokyo (Japan) |
21 (2.26) |
Ohno T |
59 (6.06) |
Phys Med |
26 (2.67) |
| 8 |
Austria |
12 (1.29) |
Milan (Italy) |
11 (1.18) |
Tsuji H |
56 (5.76) |
Radiat Prot Dosimetry |
23 (2.36) |
| 9 |
Belgium |
12 (1.29) |
Seoul (Korea) |
10 (1.08) |
Yamada S |
47 (4.83) |
Gan to kagaku ryoho |
23 (2.36) |
| 10 |
UK |
8 (0.86) |
Tsukuba (Japan)
Munich (Germany) |
10 (1.08)
10 (1.08) |
Durante M |
45 (4.62) |
Strahlenther Onkol |
21 (2.16) |
Heavy Ion Radiation Reduces Central Nervous System Metabolic Levels and Triggers Neurological Lesions and Bystander Effects
After the nervous system irradiated by high-dose heavy ion, brain tissue can be expressed as edema, inflammation and chromatin dissolved in the early time and occur apoptosis, vacuolar degeneration, nerve fibers swelling and demyelination [35], severe drowsiness, distraction, and short-term memory loss in the later time. Late symptoms also include posterior micro vascular circulatory disorders, the appearance of white matter necrotic spots and cognitive dysfunction [16,36]. At the same time, degenerative changes occur in glial cells, including endochylema catched slightly colored and nuclear enrichment; astrocytes appear swelling, hypertrophy, nucleus light staining and nucleolus hypertrophy; oligodendrocytes are swelling or shrink. In addition, the cerebrovascular can be seen congestion, edema, endothelial cell shedding and nucleus swelling and these phenomena are closely related to the nerve inflammation,
In severe cases, cerebral hemorrhage occurs due to blood-brain barrier and increased vascular permeability and apoptosis and necrosis may occur in the hippocampus [37]. Molecular and cellular biology studies have found that larger doses of heavy ion radiation can cause neuronal disease [38]. Although mature nerve cells and glial cells are not sensitive to radiation, radiation-induced microcirculation disturbance [39,40], increased neurotoxin secretion [8], ischemia and hypoxia [41], increased oxidative stress [42] and metabolic disorders [43] can reduce the metabolic level of the nervous system, damage the cognitive ability of astronauts [44] and increase the neurodegenerative diseases [45,46]. The degree of radiation damage in the central nervous system is related to the time, dose, dose rate and region of irradiation. In addition, high doses of radiation damage can cause changes in body behaviors, including mental disorders (anxiety, panic, irritability, headache, insomnia, etc.) [47] and motor disorders [48]. At the same time, autonomic nerve function is also affected, including arrhythmia, blood pressure, digestive function inhibition, immune system dysfunction and so on. Sensors throughout the body can also occur obvious dysfunction, including visual, listening, smell and touch after radiation damage.
The central nervous system has a regulatory effect on peripheral organs. The injury of nervous system by heavy ion can cause metabolic changes and lesions of nervous system and affect the peripheral organs. This damage is manifested in multiple organ dysfunction [38] and plays a regulatory role in systemic organ dysfunction caused by radiation [49,50]. In addition, radiotherapy for central nervous system disorders often causes other effects, such as cognitive disorders [44], dyskinesia [48] and so on.
Heavy Ion Radiation Reduces Immune Organ Function and Triggers Immunosuppression
It can cause immune organ dysfunction and reduce the immune function whether systemic irradiation or regional cerebral irradiation [51]. The primary evaluation criteria for the peripheral immune system were changes in peripheral blood lymphocyte ratio, bone marrow, thymus and spleen. The blood and hematopoietic cells of the body are active and are highly sensitive to radiation damage. According to the degree of sensitivity, lymphocytes are the most sensitive, followed by young red blood cells, monocytes and promyelocytes [52]. As the most radiation-sensitive cells, lymphocytes show apoptosis and rapid decline of cell number after damage by radiation and generally the damage can be reduced to the lowest value after 3 days later. The degree of decline of lymphocytes can be used to determine the severity of radiation damage [53]. As an important central immune organ, bone marrow is also a highly sensitive tissue of radiation. Once causing bone marrow radiation damage, the body often appear anemia, bleeding and infection and life-threatening. In recent years, the study on the cell cycle distribution of bone marrow on radiation was focused on the cells or whole body irradiation. In fact, the mice were irradiated with the head to study the effect of bone marrow cell cycle distribution, which was more suitable for clinical practice. This is of great significance for conducting biomedical and ion radiation protection studies [54].
At present, there are many studies on the molecular mechanism of radiation damage caused by radiation injury, which is one of the hotspots in this field. Some studies have shown that heavy ion radiation damage can not only cause damage to the central nervous system, resulting in neuroendocrine system disorders, but also can cause immune system response, show “side effect”, and then cause changes in cell cycle distribution [55]. These changes may affect the secretion of colony-stimulating factors and other cytokines, which affect the normal function of the body. Radiation damage can also induce a number of signal molecules involved in the cell signal transduction pathway including phosphatidylinositol pathway, cAMP-PKA pathway, MAPK pathway and ATR pathway to affect the body’s immune function [56]. When the radiation damage cause immune dysfunction, it will cause a series of radiation damage complications, including their own infection mainly caused by the pathogens of the body and exogenous infections mainly caused by the pathogens invasion of the body.
Heavy Ion Radiation Affects the Interaction of the Nervous System and the Immune System
There is a bi-directional feedback regulation between the neuroendocrine system and the immune system. On the one hand, when excitatory stimulation are transmitted to the brain, neurotransmitter transmits the information to the hypothalamus, causing The Hypothalamus-Pituitary-Adrenal Gland axis (HPA axis) excited, inhibiting the immune system by the secretion of adrenaline, and inhibiting the hypothalamus and pituitary by feedback mechanism. In addition, this excitatory stimulus also makes the brain dry blue spot excited, followed by the excitement of the sympathetic nervous system to secret norepinephrine and inhibit the immune system. On the other hand, IL-1 and IL-6 secreted by the immune system can also excite the HPA axis, sympathetic nervous system, Hypothalamic-Pituitary-Gonad axis (HPG axis), Hypothalamic-Pituitary- Thyroid axis (HPT axis) and so on. This feedback regulation system under normal circumstances can regulate the cyclical changes of lymphocytes, function of Natural Killer Cells (NK) and secretion of cytokine [40]. Central nervous system, especially the hypothalamus, is the central control of immune and hypothalamic neuronal activity can directly affect the immune function. Pre-hypothalamic damage can lead to the reduction of nucleated spleen cells and thymocytes, resulting in a decrease in T cell proliferation and a decrease in NK cell activity of mitogen-derived Concanavalin A (Con A). Damage of the middle of the hypothalamus can lead to a decrease in the number of T and B lymphocytes. Recent studies have shown that, in addition to the biological effects of radiation of various organs or systems, the interaction between the systems can lead to damage caused by non-target or side effects after radiation. This will be a new challenge for radiation biology.
Some studies show that the heavy ion radiation mainly damage the central nervous system of the endocrine system of the mouse head. The hypothalamus is a brain region with high sensitivity to ionizing radiation. When the hypothalamic - pituitary neuroendocrine system occur complex changes, the two-way feedback between the neuroendocrine system and the immune system will be broken, resulting in spleen cell cycle distribution changes [57]. The possible mechanism is that when the brain is irradiated, the hypothalamus - pituitary system enhance the secretion of adrenal hormones and inhibit the immune system, thereby inhibiting the spleen cell division. It is also shown that changes in the cell cycle distribution of the immune system caused by ionizing radiation in mice are not only related to the direct damage of the heavy ion radiation, but may also be related to the indirect effects of the central nervous system. The function of the spleen is closely related to the function of the nervous system, endocrine system and immune system, so that the change of the spleen cell cycle will also affect the function of the nervous system, endocrine system and immune system. In recent years, our task force has focused on the study of neurological and immunological damage and protective in radiation biological effects [58-60]. All the genomic analysis of neurons has found important molecular information related to apoptosis and radiation tolerance after heavy ion radiation [61]. In addition, we also found that SSAO interacts with CCL20 [62]. SSAO which is associated with many diseases and widely distributed in the human body is a metabolic enzyme of monoamine neurotransmitters, but so far it’s physiological functions are not yet clear.
To sum up, as the body defense system, the immune system and the nervous system are closely linked. The immune system and the nervous system are interrelated and regulated in the body, neurotransmitters and endocrine hormones released by the nervous system can regulating the immune response of the body. The immune cells have neurotransmitters and hormonal receptors. When the neurotransmitters act on the immune cells, the receptor concentration on the surface of the immune cells increases, thus increasing the sensitivity to the corresponding hormones and affecting some of the immune cells link. The immune system also synthesizes and releases neuroendocrine polypeptides that affect the function of the nervous system.
Effects of Heavy Ion Radiation on Digestive System
In 1969, Jervis et al. thought that heavy ion radiation and photon radiation related to the same cytokines and have similar pathologic mechanisms in tissue fibrosis [63]. Fatemi et al. irradiated mice with 10 Gy of 12C heavy ion beam. It was observed that there were vesicles with granules or fibrous material in Paneth cells; obvious vacuoles appeared in the epidermal cells of jejunum and ileum, while the small intestine epidermal cells appearing protrusion and mesenchymal cells connected through the basement membrane; small intestinal vascular endothelial cells, smooth muscle cells and nerve fiber get injury; small intestinal artery basement membrane, venous basement membrane and nerve fiber base membrane get thickening phenomenon [64]. Carr used accelerated protons, neutrons, Fe ions and Si ions to irradiat mice and found that the crypt form was similar to the form of coeliac disease. The effect of Fe ion beam generation is most significant at the same dose because the probability of thickening of the crypt diameter is related to the linear energy transfer [65].
There are complex structure and a large number of microbial flora known as intestinal microbes settled in the mammalian intestine. The dynamic and relatively stable intestinal microecology includes not only the structure of intestinal flora, but also the position of the flora and the proportion of different bacteria. This balance is the guarantee and symbol of the health of the body. Once this balance is broken, it is easy to cause flora imbalance and related diseases. Digestive system irradiated by heavy will have a serious impact on the digestive system. Therefore, changes in the living environment will break this balance. Intestinal microbes are not only closely related to the host’s digestive system, but also to the immune system and the nervous system. In addition, the intestine is the largest lymphoid organ in the body. As the intestinal epithelial cells is in direct contact with the intestine bacteria, the immune system of the host and the bacteria must occur between the immune response regulation events. In the study of autistic children, it was found that children had almost all gastrointestinal disorders and there were significant differences between the clostridia [66,67], firmicutes and bacteroidetes [68]. The level of bifidobacteria in the intestine was lower and the level of Lactobacillus was significantly higher than normal children whether or not supplement probiotics to autistic or asperger children [69]. The study of patients with short bowel syndrome found that the number of intestinal lactobacillus increased significantly in the intestine because of these patients, intestinal pH value is lower than normal and suitable for the survival of eosinophilic bacteria. These bacteria mainly produced D-lactose through carbohydrate metabolites, increase blood lactose content, and then lead to lactose-type encephalopathy [70].
Summary and Prospect
Heavy ions play an increasingly important role in the treatment of tumors, while also posing a serious challenge for astronauts’ physical protection. Both whole body irradiation and local irradiation can damage the nervous system, immune system and digestive system of the body. There is still a long way to go on how to safely use heavy ions to treat tumors and reduce the radiation of heavy ions in spaceflight. Although high-energy heavy ion radiation is of great concern to the radiation damage of nerve, immune and digestive system, the number of existing relevant research papers is still less and the mechanism of heavy ions on the systems of the body is still not very clear because of the limitation of heavy ion accelerator resource conditions. In addition, the impact of heavy ion radiation on intestinal microbes will attract more and more researchers’ attention.
- Bevelacqua J J. Internal dosimetry primer. Radiat. Prot. Manag. 2005, 22: 7. https://goo.gl/1sswmf
- Xia J. W. HIRFL Research device for heavy ion accelerator in Lanzhou. Sci. Bull. 2016: 467-477. https://goo.gl/ZGsVVt
- Wang X, Key J R. Recent trends in Arctic surface, cloud, and radiation properties from space . Science. 2003; 299: 1725-1728. https://goo.gl/tyZbkK
- Dye D L, Wilkinson M. Radiation hazards in space [J]. Science. 1965; 147: 19-25.
- Fry R J M, Lett J T. Radiation hazards in space put in perspective [J]. Nature. 1988; 335: 305-306.
- Cucinotta F A. Space radiation risks for astronauts on multiple International Space Station missions. PloS one. 2014; 9: 96099. https://goo.gl/wQo4pn
- Cucinotta F A. Review of NASA approach to space radiation risk assessments for Mars exploration. Health Phys. 2015; 108: 131-42. https://goo.gl/mL5Xh8
- Chancellor J C, Scott G B I, Sutton J P. Space radiation: the number one risk to astronaut health beyond low earth orbit. Life (Basel). 2014; 4: 491-510. https://goo.gl/aKhDfe
- Huff J L. Space Radiation and Risks to Human Health. Aerospace Medical Association 2014 Conference. 2014; San Diego, CA; United States. https://goo.gl/HwR11x
- Cucinotta F A. Space radiation risks to the central nervous system. Life Sciences in Space Research. 2014; 2: 54-69. https://goo.gl/PYgyiV
- Dwain L Eckberg, John R Halliwill, Larry A Beightol, Troy E Brown, J Andrew Taylor, Ross Goble. Human vagal baroreflex mechanisms in space. J. Physiol. (Lond.). 2010; 588: 1129-1138.
- Tingting Liu, Dan Xu, He Li, Hailong Pei, Mingyue Zhu, Jufang Wang, et al. Risk assessment of space radiation during manned space flights. Rend. Fis. Acc. Lincei. 2014; 25: 17-21. https://goo.gl/sdZcB2
- Bulinina T, Vorozhtsova S, Abrosimova A, et al. Biomedical effects of protons with different levels of LET[C]//40th COSPAR Scientific Assembly. 2014, 40.
- Li M, Holmes V, Zhou Y, Ni H, Sanzari JK, Kennedy AR, et al. Hindlimb suspension and SPE-like radiation impairs clearance of bacterial infections. PLoS One. 2014; 9: 85665. https://goo.gl/3yYge6
- Mothersill C, Moriarty M J, Seymour C B. Bystander and other delayed effects and multi-organ involvement and failure following high dose exposure to ionising radiation. Br. J. Radiol. 2014; 27: 1-6. https://goo.gl/EX4Br5
- Cucinotta F A. Review of NASA approach to space radiation risk assessments for Mars exploration. Health Phys., 2015. 108: 131-42. https://goo.gl/j1LJz1
- Ren H T, Zhao J, Peng S X, P N Lu, Q. F Zhou, Y Xu, J Chen, et al. Handling radiation generated during an ion source commissioning a. Rev. Sci. Instrum. 2014; 85: 02A930. https://goo.gl/QYdFfE
- Sihver L, Sato T, Puchalska M, Reitz G. Simulations of the MATROSHKA experiment at the international space station using PHITS. Radiat. Environ. Biop. 2010; 49: 351-7. https://goo.gl/zGhcMT
- Reitz G, Berger T. The MATROSHKA facility-dose determination during an EVA. Radiat. Prot. Dosim. 2006; 120: 442-5. https://goo.gl/4DqSeS
- Zhou D, Semones E, O’Sullivan D, E.R. Benton. Radiation measured for MATROSHKA-1 experiment with passive dosimeters. Acta Astronaut. 2010; 66: 301-8. https://goo.gl/pFEhA2
- Dettmann J, Reitz G, Gianfiglio G. MATROSHKA-The first ESA external payload on the International Space Station. Acta Astronaut. 2007; 60: 17-23. https://goo.gl/2jBE2a
- Shurshakov V, Kartashov D, Tolochek R. Space Radiation Dose Calculations for the Space Experiment Matroshka-R Modelling Conditions. In 40th COSPAR Scientific Assembly. 2014; 3: 7-14. https://goo.gl/V2NR2W
- Zhang B, Davidson M M, Hei T K. Mitochondria regulate DNA damage and genomic instability induced by high LET radiation. Life Sci Space Res (Amst). 2014; 1: 80-88. https://goo.gl/iRa2xP
- Trani D, Scott A Nelson, Bo-Hyun Moon, Jan J Swedlow, Elizabeth M Williams, et al. High-energy particle-induced tumorigenesis throughout the gastrointestinal tract. Radiat Res. 2014; 181: 162-71. https://goo.gl/G5bRoa
- Barcellos-Hoff M H, Adams C, Balmain A, Sylvain V. Costes, Sandra Demaria, Irineu Illa-Bochaca, et al. Systems biology perspectives on the carcinogenic potential of radiation. J Radiat Res. 2014; 55: 145-54. https://goo.gl/32i89Y
- Farin A M, Manzo N D, Kirsch D G, Stripp BR. Low-and high-LET radiation drives clonal expansion of lung progenitor cells in vivo. Radiat Res. 2015; 183: 124-32. https://goo.gl/jZbGqf
- Cheema A K, Suman S, Kaur P, Singh R, Fornace AJ Jr, Datta K. Long-term differential changes in mouse intestinal metabolomics after γ and heavy ion radiation exposure. PloS one. 2014; 9: 87079. https://goo.gl/F6YaXo
- Haddy N, Tartier L, Koscielny S, Rubino C, Brugières L, Pacquement H, et al. Repair of ionizing radiation-induced DNA damage and risk of second cancer in childhood cancer survivors. Carcinogenesis. 2014; 35: 1745-1749. https://goo.gl/mvFcXr
- Chishti A A, Hellweg C E, Berger T, , Baumstark-Khan C, Feles S, Kätzel T, et al. Constitutive expression of tdTomato protein as a cytotoxicity and proliferation marker for space radiation biology. Life Sci Space Res (Amst). 2015; 4: 35-45. https://goo.gl/LtpPhs
- Ding N, Pei H, Hu W, Jinpeng He, He Li, Jufang Wang, et al. Cancer risk of high-charge and-energy ions and the biological effects of the induced secondary particles in space. Rend. Lincei. 2014; 25: 59-63. https://goo.gl/vsYKai
- Han W, Wu L, Hu B, Zhang L, Chen S, Bao L, et al. The early and initiation processes of radiation-induced bystander effects involved in the induction of DNA double strand breaks in non-irradiated cultures. Br J Radiol. 2007; 80: 7-12. https://goo.gl/cnpgfH
- Saloua KS, Sonia G, Pierre C, Léon S, Darel HJ. The relative contributions of DNA strand breaks, base damage and clustered lesions to the loss of DNA functionality induced by ionizing radiation. Radiat Res. 2014; 181: 99-110. https://goo.gl/qVK6eK
- Sahbani S K, Rezaee M, Cloutier P, Sanche L, Hunting DJ. Non-DSB clustered DNA lesions induced by ionizing radiation are largely responsible for the loss of plasmid DNA functionality in the presence of cisplatin. Chem Biol Interact. 2014; 217: 9-18. https://goo.gl/itTLtM
- Kraft G, Arndt U, Becher W, et al. Heavy ion therapy at GSI[J]. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers. Detectors and Associated Equipment. 1995; 367: 66-70.
- Du Y, Zhang J, Zheng Q, Li M, Liu Y, Zhang B, et al. Heavy ion and X-ray irradiation alter the cytoskeleton and cytomechanics of cortical neurons. Neural Regen Res. 2014; 9: 1129-1137. https://goo.gl/yVSJqH
- Grigor’ev A I, Krasavin E A, Ostrovskii M A. Assessment of the risk of the biological actions of galactic heavy ions to interplanetary flight. Neuroscience and Behavioral Physiology. 2015; 45: 91-95. https://goo.gl/6XpiRB
- Cucinotta F A, Wang H, Huff J L. Risk of acute or late central nervous system effects from radiation exposure. Human health and performance risks of space exploration missions: evidence reviewed by the NASA Human Research Program. 2009: 191-212. https://goo.gl/FVxVgz
- Nelson G, Fike J, Limoli C, André Obenaus, Jacob Raber, Ivan Soltesz, et al. Responses of the central nervous system to high linear energy transfer radiation: NSCOR project highlights. J Radiat Res. 2014; 55: 22-23. https://goo.gl/gE9yYe
- Popov D, Jones J, Maliev S. Acute Cerebrovascular Radiation Syndrome: Radiation Neurotoxicity, mechanisms of CNS radiation injury, advanced countermeasures for Radiation Protection of Central Nervous System[C]//40th COSPAR Scientific Assembly. 2014, 40.
- Rubin P, Fajardo L. Biophysiopathology of the Microvasculature and Microcirculation[M]//ALERT-Adverse Late Effects of Cancer Treatment. Springer Berlin Heidelberg, 2014: 27-39.
- Warrington J P, Csiszar A, Johnson D A, Herman TS, Ahmad S, Lee YW, et al. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am. J. Physiol-Heart C. 2011; 300: 736-744. https://goo.gl/oC7a9d
- Tseng B P, Giedzinski E, Izadi A, Suarez T, Lan ML, Tran KK, et al. Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid Redox Signal. 2014; 20: 1410-1422. https://goo.gl/XPRNRB
- Gaeta M C, Herrmann K A. PET/MRI in Evaluating Lymphomas: Preliminary Experience and Potential Future Applications[M]//PET/MRI. Springer Berlin Heidelberg. 2014: 71-78. https://goo.gl/h1PWyt
- Parihar V K, Allen B D, Tran K K, Chmielewski NN, Craver BM, Martirosian V, et al. Targeted overexpression of mitochondrial catalase prevents radiation-induced cognitive dysfunction. Antioxid Redox Signal. 2015; 22: 78-91. https://goo.gl/e8xbn4
- Huff J L. NASA Space Radiation Program Element: Research Overview. Radiation Health Working Group Meeting. 2014; Hiroshima; Japan. https://goo.gl/5vkRST
- Moncaster J, Wojnarowicz M, Tagge C, Andrew Fisher, Katisha Gopaul, Omid Syed, et al. Effects of space radiation on hippocampal-dependent learning and cognition aged mice. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2014; 10: 482. https://goo.gl/adG8uX
- Parihar V K, Pasha J, Tran K K, Craver BM, Acharya MM, Limoli CL. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct. Funct. 2015; 220: 1161-1171. https://goo.gl/AhzFCn
- Pauli, N. Treating radiation-induced trismus in head and neck cancer; Exercise intervention and risk structures. Institute of Clinical Sciences, Section for Anesthesiology, Biomaterials and Orthopaedics. Department of Otorhinolaryngology. 2015. https://goo.gl/7AnWhT
- Gourmelon P, Marquette C, Agay D, et al. Involvement of the central nervous system in radiation-induced multi-organ dysfunction and/or failure. Brit J Radiol. 2005: 62-68.
- Meineke V, Fliedner T M. Radiation-induced multi-organ involvement and failure: challenges for radiation accident medical management and future research. Brit J Radiol. 2005: 196-200. https://goo.gl/bUXVjZ
- Rödel F, Frey B, Multhoff G, Gabriele Multhoff, Udo Gaipl. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. 2015; 356: 105-113. https://goo.gl/hrgJHL
- Lawrence Y R, Dicker AP. Radiation therapy and the immune system: learning to live together. Future Oncol. 2014; 10: 777-780. https://goo.gl/3iFf9L
- Lim J Y H, Gerber S A, Murphy S P, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8+ T cells. Cancer Immunol. Immunother. 2014; 63: 259-271. https://goo.gl/qYtsRo
- Demaria S, Pilones K A, Vanpouille-Box C, Golden EB, Formenti SC. The optimal partnership of radiation and immunotherapy: from preclinical studies to clinical translation. Radiat Res. 2014; 182: 170-181. https://goo.gl/YCx2ek
- Zhou Y, Ni H, Balint K, Dentchev T, Diffenderfer ES, Wilson JM, et al. Ionizing radiation selectively reduces skin regulatory T cells and alters immune function. PloS one. 2014; 9: 100800. https://goo.gl/tU22tR
- Keisari Y, Hochman I, Confino H, Korenstein R, Kelson I. Activation of local and systemic anti-tumor immune responses by ablation of solid tumors with intratumoral electrochemical or alpha radiation treatments. Cancer Immunol Immunother. 2014; 63: 1-9. https://goo.gl/2cnzg6
- Anthony D C, Couch Y. The systemic response to CNS injury. Exp Neurol. 2014; 258: 105-111. https://goo.gl/AwT3rr
- Ran Y, Wang R, Hasan M, Jia Q, Tang B, Shan S, et al. Radioprotective effects of dragon's blood and its extracts on radiation-induced myelosuppressive mice. J. J Ethnopharmacol. 2014; 154: 624-634. https://goo.gl/V2z3aJ
- Ran Y, Wang R, Gao Q, Jia Q, Hasan M, Awan MU, et al. Dragon’s blood and its extracts attenuate radiation-induced oxidative stress in mice. J Radiat Res. 2014; 55: 699-706. https://goo.gl/XP2EXz
- Xin N, Li Y J, Li X, Wang X, Li Y, Zhang X, et al. Dragon's blood may have radioprotective effects in radiation-induced rat brain injury. Radiat Res. 2012; 178: 75-85. https://goo.gl/afrgUK
- Ma H, Rao L, Wang H L, Mao ZW, Lei RH, Yang ZY, et al. Transcriptome analysis of glioma cells for the dynamic response to γ-irradiation and dual regulation of apoptosis genes: a new insight into radiotherapy for glioblastomas. Cell Death Dis. 2013; 4: 895. https://goo.gl/6XwcPK
- Ullah K, Xie B, Iqbal J, Rasool A, Qing H, Deng Y. Arterial vascular cell line expressing SSAO: a new tool to study the pathophysiology of vascular amine oxidases. J Neural Transm (Vienna). 2013; 120: 1005-1013. https://goo.gl/BWSWJz
- Jervis H R, Donati R M, Stromberg L R, et al. Histochemical investigation of the mucosa of the exteriorized small intestine of the rat exposed to x-radiation. Strahlentherapie. 1969; 137: 326.
- S. H. Fatemi, M. Antosh, G. M. Cullan, J. G. Sharp. Late ultrastructural effects of heavy ions and gamma irradiation in the gastrointestinal tract of the mouse. J Submicrosc Cytol Pathol. 1985; 48: 325-340. https://goo.gl/HtEw6X
- Carr K E, Hayes T L, Abbas B, Ainsworth EJ. Collared crypts in irradiated small intestine. J Submicrosc Cytol Pathol. 1990; 22: 265-271. https://goo.gl/vz6Apo
- Finegold S M, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002; 35: 6-16. https://goo.gl/qRs7rF
- Song Y, Liu C, Finegold S M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004; 70: 6459-6465. https://goo.gl/vsN8Sx
- Williams B L, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PloS one. 2011; 6: 24585. https://goo.gl/wYKKYi
- Adams J B, Johansen L J, Powell L D, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC gastroenterol. 2011; 11: 22. https://goo.gl/hoJFFR
- Joly F, Mayeur C, Bruneau A, Noordine ML, Meylheuc T, Langella P, et al. Drastic changes in fecal and mucosa-associated microbiota in adult patients with short bowel syndrome. Biochimie. 2010; 92: 753-761. https://goo.gl/wk5zko
Sign up for Article Alerts