Recovery Assays: Determination of Methylparaben in Sediment Samples?
Julia Martin1*, Diego Trujillo2, Jose M. Jurado3 and Agustin G. Asuero2
1Department of Analytical Chemistry, Escuela Politecnica Superior, University of Seville
2Department of Analytical Chemistry, Faculty of Pharmacy, University of Seville
3Department of Analytical Chemistry, Faculty of Chemistry, University of Seville
*Address for Correspondence: Julia Martin, Department of Analytical Chemistry, Escuela Politecnica Superior, University of Seville, Spain, Tel: +349-545-562-50; ORCID: orcid.org/0000-0003-3621-1291; E-mail: jbueno@us.es
Submitted: 03 January 2018; Approved: 24 February 2018; Published: 01 March 2018
Citation this article: Martin J, Trujillo GD, Marcos JJ, Asuero AG. Recovery Assays: Determination of Methylparaben in Sediment Samples. Int J Pharma Anal Acta. 2018;2(1): 001-006.
Copyright: © 2018 Martin J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Validation; Acuracy; Recovery assays; Confidence Ellipse; Emerging Pollutants; Sediments
Download Fulltext PDF
Since were discovered by their antimicrobial activity, parabens have been widely used in many cosmetics, pharmaceuticals, personal care products and food, among others consumer products. These compounds, after human consumption, reach wastewater treatment plants, where are not efficiently removed, ending up in the environment, as well as by the direct discharge of detergents, soaps or products that may contain these compounds in their formulation. This concern has significantly boosted the number of publications in recent literature, although the number of papers on aqueous samples is still much higher compared to solid matrices, probably due to the complexity of these last. In this work, the accuracy of a new developed analytical method for the determination of emerging pollutants (Methylparaben (MeP)) in sediment samples has been carried out using recovery assays and evaluated by mean of the cofindence ellipse. The sample treatment involve steps of lyophilization, solvent extraction and clean-up of the extracts with dispersive sorbents prior to liquid chromatography – tandem mass spectrometry analysis. Fortified blank samples for MeP at seven different analyte concentration levels (2.5, 12.5, 25, 50, 125, 250y 500 ng/g) were analyzed. Recovery calculations from fortified samples were performed from linear regression of the data found for each amount (level) of fortified analyte versus data added. Finally, the methodology to build the confidence ellipse, necessary operations required and the graphical representation, is applied in order to evaluate the acuracy of the method. Successful recoveries between 84 and 108% were achieved at all tested levels, although significantly different from 100% from a statistical point of view according with the confidence ellipse. However, these values are acceptable from the % practical point of view as contemplated in the recovery table of the AOAC.
Introduction
Marine ecosystem tends to end up accumulating a huge variety of organic pollutants, primarily resulting from anthropogenic activities, through rivers, direct discharges, or atmospheric deposition. Over the past few decades a large number of previously unrecognized pollutants [1,2], called emerging contaminants, have been identified in this scenario. Many of these substances are especially relevant, in particular those with the capacity to disrupt the hormonal (endocrine) system of living organisms. Since were discovered by their antimicrobial activity, parabens have been widely used as bactericides, fungicides and preservatives agents in many cosmetics, pharmaceuticals, personal care products and food, among others consumer products. Although the toxicity of these compounds is very low, they present a weak estrogenic activity and are considered as endocrine disruptors, that is why they have been classified as emerging contaminants arousing a great interest in the scientific world [3,4]. These compounds, after human consumption, reach wastewater treatment plants, where are not efficiently removed, ending up in the environment, as well as by the direct discharge of detergents, soaps or products that may contain these compounds in their formulation. This concern has significantly boosted the number of publications in recent literature, although the number of papers on aqueous samples is still much higher compared to solid matrices, probably due to the complexity of these last. The development of new methodologies in solid waste matrices is key to estimate the persistence and risk of these compounds in the environment. The choice of analytical instrumentation and sample treatment should be based on the intended purpose and scope of the analytical method. The important parameters that may be evaluated during method development are accuracy, linearity, precision, sensibility and robustness [5-7]. This paper deals on the accuracy [8-12]. According to ISO 5725-1 [7], the general term "Accuracy" is used to describe the closeness of a measurement to the true value. When the term is applied to sets of measurements of the same measurand, it involves a component of random error and a component of systematic error. In this case trueness is the closeness of the mean of a set of measurement results to the actual (true) value and precision is the closeness of agreement among a set of results. Several methods of determining accuracy are available:
i) Use of certified reference materials
ii) Comparison of a proposed method with a reference method
iii) The use of recovery assays on samples and matrices
iv) Interlaboratory studies [13], which will not be considered here because in this paper we are dealing with internal quality control of accuracy.
It can also be inferred once precision, linearity and specificity have been established. In the field of environmental and pharmaceutical analysis, reference standards or alternative methods are usually not available. The recovery assays are then the tool of choice to check the accuracy [14-22]. Recovery calculations from matrices or fortified samples can be performed either from individual recoveries for each amount (level) of fortified analyte or from linear regression [23,24] of the data found versus data added:
y = xfound = a0 + a1xadded (1)
Where xfound and xadded refer to analyte concentrations found and fortified, respectively. The theory predicts a value of unity for the slope a1 and a zero value for the intercept a0 although the presence of systematic and random errors in the analytical process can produce deviations (bias) of this ideal situation.Equations (2, a, b) provide the confidence intervals of the intercept, a0 and of the slope, a1, of a straight line obtained by the least squares method in which the values of α0 and α1 represent the true values of the intercept and of the slope, which could be obtained by making an infinite number of measurements, and t is the tabulated Student’s t for a significance level of 1-α/2 [24-28].
(2, a, b)
It is possible to build a confidence rectangle drawing the limits of these intervals for the 95% confidence level in a diagram whose abscissa is the slope, and the ordinate, the intercept (or vice versa).
However, statistical tests on slope and intercept of a straight line obtained by linear regression (ta = a/sa; tb = ABS (b - 1)/sb), although frequently used by the workers, are not entirely reliable when the strong correlation existent between the slope and intercept are not taken into account [29-31]. The covariance between two variables is as important as the variances and both contribute to the total analytical error significantly. That is to say, the confidence rectangle has to be substituted by the confidence ellipse (joint effect) that describes the behaviour of the system in a more rigorously way [23,24,32-38].
(3)
Instead of these individual tests, the confidence ellipse derived by Working and Hotelling [37] and adopted by Mandel and Linnig [38] is recommended. It is therefore preferable to calculate a set interval for these coefficients. The formula that describes this interval is an ellipse [23,24,37,38] centered on the point (a1, a0).
(4)
Here, F (2, ν, α) is the tabulated F-distribution with a significance level of and 2 and (n - 2) are the degrees of freedom (n is the total number of samples), and sy/x2 is the regression variance.
(5)
SYY and SXX are the corresponding sums of squares with respect to the means of y and x, respectively.
This ellipse is difficult to represent [38-42]. In order to simplify the calculations, a change of axes can be made, taking the values (A0, A1), as the center of the ellipse, defined as
A0 = a0- α0 A1 = a1- α1 (6)
Eq. (4) becomes:
(7)
Taking into account that:
(8)
We get the following equation which describes the intersections of the ellipse with a line parallel to the x - axis.
(9)
The two possible solutions after solving the eq. (9) are:
(10)
To have real solutions, the root discriminant must be greater than zero. Note that
(11)
(12)
and
(13)
To build the ellipse, A1 is varied in small increments in positive and negative directions, calculating the discriminant value. When this is positive, two real roots are obtained, getting the two branches of the curve. In order to make this diagram easier to use, the coordinates in the initial space (α1, α0) are now calculated by applying the change of variables in the reverse direction. In this regard, the study of the confidence ellipse is a very powerful and precise tool order to evaluate the acuracy of the method. In spite of the difficulties involved in representing the ellipse, it is preferable to use this joint confidence interval because it is much more efficient from the statistical point of view. To do this, one may use a spreadsheet like Excel and through a graphic visualization we can easily detect how reliable are the slope and the intercept of the regression line. Additionally, the procedure described in this work may be applied in a variety of areas of analytical importance such as calibration, recovery assays and comparison of methods. In this work, the evaluation of accuracy of a new developed analytical method [1] for the determination of emerging pollutants (Methylparaben (MeP)) in sediment samples has been carried out by mean of recovery assays. Recovery calculations from fortified samples are performed from linear regression of the data found for each amount (level) of fortified analyte versus data added and the methodology described above to build the confidence ellipse is applied in order to evaluate the acuracy of the method.
Materials and Methods
Reagents
The compound studied MeP, was supplied by Dr. Ehrenstorfer GmbH (between 97 - 99.5% purity). The Internal Standard (IS) Ethylparaben - d5 (EtP - d5), was supplied by Cambridge isotope laboratories (MA, USA). The stock solution (1000 mg/L) was prepared in methanol and stored in a refrigerator at 4°C working solutions were prepared by diluting the stock standard solution in methanol. Acetonitrile, water and methanol all of HPLC quality purity, were supplied by Romil Ltd. (Barcelona, Spain). Ammonium acetate (reagent grade analysis), was supplied by Panreac (Barcelona, Spain). Water, methanol and Acetonitrile (AcN) (all of chromatographic analysis grade) were purchased from Romil Ltd. (Barcelona, Spain). Octadecyl functionalized silica (C18) was provided by Sigma - Aldrich (Steinheim, Germany). Ammonium acetate obtained from Panreac (Barcelona, Spain).
Sample collection
Marine sediments were hand collected by scuba divers at random locations in March 2016 along a transect (50m long and 7-13m deep) in the infralittoral zone of Marina del Este beach (Almuñecar, Southern Spain). Sediment samples were freeze-dried, homogenized, grounded into powder and frozen at - 20ºC until analyses [1].
Preparation of matrix-matched calibration curves
Matrix-matched calibration standards were prepared at seven different analyte concentration levels (2.5, 12.5, 25, 50, 125, 250 and 500 ng/g de MeP). Working with matrix-matched standard in environmental samples could lead to representativeness problems, therefore a pool of different sediment samples were used. The mixtures were vortexed for 2 min and then left to stand for 24 h at 4ºC in the dark before analysis. This allows the analyte to come into full contact with the sample.
Sample treatment
Aliquots of the samples (0.5 g) were weighed into 12 mL glass vials, containing 100 µL of a methanol solution (250 ng/mL) of the IS (EtP - d5). The samples were vortexed for homogenization twice in 7 mL of acetonitrile (2 min) and centrifuged for 10 min at 4050 × g. In order to decrease the matrix co-extractives in the extract that could cause the matrix effect, a clean-up of the extract based on disperse solid phase extraction (d-SPE) was was carried out. The supernatants obtained from each extraction step were combined and transferred to a 50 mL polypropylene conical tube containing 800 mg of C18 sorbent. The mixture was hand-shaken for 2 min and centrifuged for 5 min at 4050 × g. Next, the solvent was evaporated to dryness at room temperature under a nitrogen stream and the extract was reconstituted in 0.25 mL of a mixture methanol: water (50:50 v/v), filtered through a 0.22 µm nylon filter and a 20 - µL aliquot was injected into the HPLC instrument.
High performance liquid chromatography – tandem mass spectrometry operating conditions
Chromatographic separation of MeP was performed using a HALO C-18 Rapid Resolution (50 × 4.6 mm i.d., 2.7 µm particle size) column. The compound was separated using a gradient mobile phase consisting of an aqueous buffer solution of acetic acid/ammonium acetate (pH 4.4) (solvent A) and methanol buffered with the same composition (solvent B). The gradient program was as follows: 0 - 14 min, linear gradient from 28 to 70% of solvent B, from 70% to 80% of solvent B in 5 min, and then increased to 100% in 6 min and held for 2 min. Flow rate was fixed at 0.6 mL min-1. The injection volume was 20 µL. The column temperature was maintained at 30ºC. The HPLC system is coupled to a triple quadrupole mass spectrometer with ESI working in negative mode. The parameters selected for the spectrometer are: capillary voltage, 3000V; nebulizer pressure, 40psig; drying-gas flow rate, 9.0 L/min and drying-gas temperature, 355°C. The mode of operation of the spectrometer is MRM (Multiple Reaction Monitoring). Instrument control and data acquisition were carried out with MassHunter software (Agilent, USA). A previous optimization of the conditions of fragmentation was made using the Optimizer software. Negative mode was selected because it showed higher sensitivity for all compounds of interest. The two transitions, one for quantification and the other for confirmation, corresponding to the most abundant ion products were selected after the rupture of the precursor ion. The most abundant transition ion was selected to obtain maximum sensitivity for quantification. The parameters optimized for product ions were fragmentation voltage and collision energy. The parameters selected to obtain optimum responses are presented in table 1.
Results and Discussion
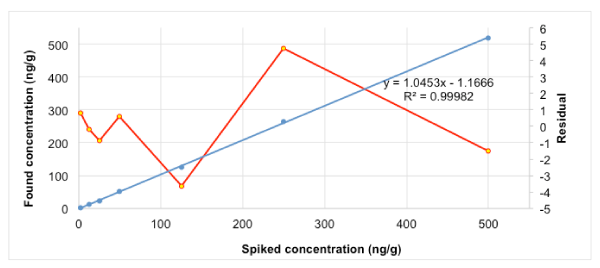
Quantification was carried out using matrix-matched calibration curves. Calibration curves were constructed by linear regression of the peak area ratio of the analyte and its corresponding internal standard against their respective concentrations. Figure 1 shows the found concentration versus the added concentration. The Linest; function in Excel provides us with the value of the parameters of the straight line. The values obtained for a0, a1 and their standard deviations in the case of simple linear regression are shown in table 2.
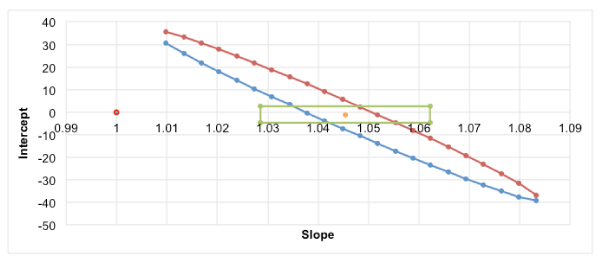
Once the values of the intercept and slope, a0 and a1, respectively are estimated, and before evaluating the recovery, a residual analysis should be undertaken [29-31] to check the validity of the model. Certain assumptions fundamental to regression, such as independence of errors, homocedasticity (uniform or regular variance) and Gaussian distribution must be fulfilled. If the model represents the data properly, the residuals must be randomly distributed around the normal (Gaussian) predicted values or about the x values. In the secondary axis of figure1 is plotted the residual analysis, which shows absence of spurious data as well as an increase in the error with the concentration level. The methodology described in the introduction to build the confidence ellipse is treated in short in the following. Accuracy was calculated as the percentage of analyte extracted, quantified using matrix-matched calibration curves, in relation to the spiking level. Successful recoveries between 84 and 108% were achieved (Table 3). The theory predicts a value of unity for the slope a1 and a zero value for the intercept a0. After application of the statistical test for the intercept and the slope, it can be concluded that the intercept is not significantly different from zero, since the value of zero falls in the range or confidence interval of this parameter (texp = a0/s (a0) = 0.813 < ttab), while the slope is significantly different from one (texp = (a0 - 1) / s (a1) = 6.924 > ttab), and hence the recovery is far from 100%. Nonetheless, statistical tests on the intercept and the slope of a straight line obtained by the least squares method are not completely reliable when performed independently. We must therefore proceed to obtain the confidence interval together, which requires the drawing of the confidence ellipse, better than the confidence rectangle corresponding to the individual tests. Point (1, 0) falls far enough away from both the rectangle and the confidence ellipse (Figure 2). We can see how the study of the confidence ellipse is a very powerful and precise tool for graphic visualization of how reliable, are the slope and the intercept in the regression line. Nevertheless, once the recovery is computed, it should be checked for fulfilling the accuracy criteria according to the AOAC guidelines [43]. The AOAC manual for the peer verified methods program includes a table with estimated recovery data as a function of analyte concentration. The concentration should cover the range of concern and should particularly include one concentration close to the quantitation limit. The expected recovery depends on the sample matrix, the sample processing procedure and on the analyte concentration. Notes that for trace analysis, e.g. MeP in sediment samples investigated (part per billion levels), recoveries about 50% are often the best that can be achieved. So, the recovery obtained from a practical point of view is acceptable, since it is within the range of values allowed by the table of AOAC. The use of non-statistical criteria for judging accuracy based on recovery percentages as a function of analyte concentration has been previously reported [43,44] for many authors and frequently use in these circumstances.
Conclusion
The calculation of recoveries may be performed from linear regression analysis of estimated against spiked analyte concentration. The theory predicts a value of 1 for the slope and a value of 0 for the intercept. A very powerful and precise tool in these situations is the study of the confidence ellipse. Along this contribution the confidence ellipse has proven to be a powerful and precise tool for the evaluation of accuracy of a new developed analytical method for the determination of MeP in sediment samples. In spite of the difficulties to build it, through a graphic visualization we can easily detect how reliable are the slope and the intercept of the regression line. The recoveries obtained for MeP in sediment samples, of interest at the ppb level, ranged between 84 - 108%, significantly different from 100% from a statistical point of view although acceptable from the practical point of view contemplated by the AOAC table. The procedure described in this work may be applied in a variety of areas of analytical importance such as calibration, recovery assays and comparison of methods.
- Martin J, Zafra-Gomez A, Hidalgo F, Ibanez-Yuste AJ, Alonso E, Vilchez JL. Multi-residue analysis of 36 priority and emerging pollutants in marine echinoderms (holothuria tubulosa) and marine sediments by solid-liquid extraction followed by dispersive solid phase extraction and liquid chromatography-tandem mass spectrometry analysis. Talanta. 2017; 166: 336-348. https://goo.gl/qtL192
- Ruhi A, Acuna V, Barcelo D, Huerta B, Mor JR, Rodriguez-Mozaz S, et al. Bioaccumulation and trophic magnification of pharmaceuticals and endocrine disruptors in a mediterranean river food web. Sci Total Environ. 2016; 540: 250-259. https://goo.gl/UoM2ZT
- Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol. 2005; 43: 985-1015. https://goo.gl/Y6YWf3
- Boberg J, Taxvig C, Christiansen S, Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol. 2010; 30: 301-312. https://goo.gl/tjbL4j
- Eurachem G. The fitness for purpose of analytical methods—a laboratory guide to method validation and related topics. Teddington. UK: 1998; 54. https://goo.gl/1rghAH
- Thompson M, Stephen LRE, Roger W. Harmonized IUPAC guidelines for single-laboratory validation of methods of analysis. Pure Appl. Chem. 2002; 74: 835-855. https://goo.gl/hrceBP
- BS ISO 5725-1. Accuracy (trueness and precision) of measurement methods and results - Part 1: general principles and definitions. 1994; 1. https://goo.gl/JPZGUH
- Thompson M. Regression methods in the comparison of accuracy. Analyst. 1982; 1279: 1105-1296. https://goo.gl/27axyc
- Ellison SL, Barwick VJ, Norris P, Griffiths M. Complete curve fitting of extraction profiles for estimating uncertainties in recovery estimates. Analyst. 2003; 128: 493-498. https://goo.gl/uKAVSi
- Gonzalez AG, Herrador MA, Asuero AG. Intra-laboratory assessment of method accuracy (trueness and precision) by using validation standards. Talanta. 2010; 82: 1995-1998. https://goo.gl/Eg8Mdw
- S. Kemeny, Deak A, Banfai B. Testing accuracy of analytical methods by regression. J Chemom. 2009; 23: 211-216. https://goo.gl/NfcC6X
- Koscielniak P, Wietecha R. A critical look at the recovery test as a way for evaluation of analytical accuracy. Anal Lett. 2003; 36: 861-870. https://goo.gl/szbJJK
- Grant TW. Use of statistics to develop and evaluate analytical methods. 3rd ed. William S. Arlington: AOAC; 1985. https://goo.gl/iphg42
- Barwick VJ, Ellison SLR. Measurement uncertainty: approaches to the evaluation of uncertainties associated with recovery. Analyst. 1999; 124: 981-990. https://goo.gl/9yXRN3
- Burns DT, Klaus D, Townshend A. Use of the terms “recovery” and “apparent recovery” in analytical procedures. Pure Appl Chem. 2002; 74: 2201-2205. https://goo.gl/cyBpzc
- Dybkaer R. Recovery revisited. Accred Qual Assur. 2005; 10: 302-303. https://goo.gl/TmTWy5
- Koscielniak P. On analytical usefulness of the recovery method. Anal Lett. 2004; 37: 2625-2640. https://goo.gl/JW5ToD
- Thomas PJL. Use of recovery and bias information in analytical chemistry and estimation of its uncertainty contribution. TrAC Anal Chem. 2008; 27: 916-923. https://goo.gl/eoS2dT
- Alicia M, Ricard B, Riu J, Rius FX. Estimation of measurement uncertainty by using regression techniques and spiked samples. Anal Chim Acta. 2001; 446: 131-143. https://goo.gl/DY52Mf
- Alicia M, Ricard B, Riu J, Rius FX. Measurement uncertainty in analytical methods in which trueness is assessed from recovery assays. Anal Chim Acta. 2001; 440: 171-184. https://goo.gl/H55YSH
- Thompson M, Ellison SLR, Fajgelj A, Willetts P, Wood R. Harmonised guidelines for the use of recovery information in analytical measurement. Pure Appl Chem. 1999; 71: 337-348. https://goo.gl/YtcMPs
- Thompson M. The estimation use of recovery factors. Anal Methods Committee. 2008; TB 21A. https://goo.gl/CLb7EC
- Gonzalez AG, Herrador MA, Asuero AG. Intra-laboratory testing of method accuracy from recovery assays. Talanta. 1999; 48: 729-736. https://goo.gl/ibfkeB
- Martin J, Cabana HA, Asuero AG. The confidence ellipse: calibration, recovery assays and comparison of methods. Curr Topics Anal Chem. 2016; 19: 79-100. https://goo.gl/dbHhYm
- Feinberg M. La validation des methodes d analyse: une approache chimiometrique de i assurance qualite au laboratoire. Paris: Masson; 1996. https://goo.gl/8apwtx
- Feinberg M. Labo-stat. guide de validation des methodes d analyse. Paris: Editions Tec & Doc; 2009. https://goo.gl/oeQVEC
- Feinberg M. L assurance qualite dans les laboratoires agroalimentaires et pharmaceutiques. 2nd ed. Paris: Editions Tec & Doc; 2001. https://goo.gl/eN85sP
- Feinberg M, Boulanger B, Dewe W, Hubert P. New advances in method validation and measurement uncertainty aimed at improving the quality of chemical data. Anal Bioanal Chem. 2004; 380: 502-514. https://goo.gl/14QLNn
- Agustin GA, Julia M. Fitting straight lines with replicated observations by linear regression IV. transforming data. Crit Rev Anal Chem. 2011; 41: 36-69. https://goo.gl/azuoxt
- Sayago A, Boccio M, Asuero AG. Fitting straight lines with replicated observations by linear regression: the least squares postulates. Crit Rev Anal Chem. 2004; 34: 39-50. https://goo.gl/ULAi7A
- Asuero AG, Sayago A, Gonzalez AG. The correlation coefficient: an overview. Crit Rev Anal Chem. 2006; 36: 41-59. https://goo.gl/rhK2Pi
- Charles KB, Ira BR. Practical experimental design and optimization methods for chemists. New York: VCH Publishers; 1986; 64: 194.
- Bruce RK. Chemometrics. Anal Chem. 1980; 52: 112-122. https://goo.gl/yPF7ZZ
- Asuero AG, Gonzalez AG. Some observations on fitting a straight line to data. Microchem J. 1989; 40: 216-225. https://goo.gl/rCyzJw
- Sayago A, Asuero AG. Fitting straight lines with replicated observations by linear regression: part II. testing for homogeneity of variances. Crit Rev Anal Chem. 2004; 34: 133-146. https://goo.gl/ipfg4x
- John. M, Linning FJ. Study of accuracy in chemical analysis using linear calibration curves. Anal Chem. 1957; 29: 743-749. https://goo.gl/EBvL3k
- Holbrook W, Harold H. Applications of the theory of error to the interpretation of trends. J Am Stat Assoc. 1929; 24: 73-85. https://goo.gl/avWPfv
- Lark PD, Craven BR, Bosworth RCL. The handling of chemical data, pergamon press, oxford. 1968. https://goo.gl/i1aMXL
- Nalimov VV. The application of mathematical statistics to chemical analysis. Reading MA: Addison- Wesley; 1963. https://goo.gl/Vvxc4z
- Rocchi MBL, Sisti D, Ditroilo M, Calavalle A, Panebianco R. The misuse of the confidence ellipse in evaluating statokinesigram. Ital J Sport Sci. 2005; 12: 169-172. https://goo.gl/XK2yKd
- Sadler WA. Joint parameter confidence regions improve the power of parametric regression in method comparison studies. Accred Qual Assur. 2010; 15: 547-554. https://goo.gl/dPgcqU
- Lowell MS. Multiparameter models and statistical uncertainties. Anal Chim Acta. 1980; 122: 291-301. https://goo.gl/iKx5C4
- AOAC. Peer verified methods program, manual on politics and procedures. Arlington: 1993. https://goo.gl/X4UqW7
- Hubert L. Validation of analytical methods: review and strategy. LC-GC Int. 1998; 2: 96-105.

Sign up for Article Alerts