Formulation, Development and Optimization of Itraconazole Cubosomal Gel for the Treatment of Candidiasis?
Nitika, Nikita, Iram Khan and Yasmin Sultana*
Department of Pharmaceutics, School of Pharmaceutical Education & Research, Jamia Hamdard, New Delhi, India
*Address for Correspondence: Yasmin Sultana, Head of Department, Department of Pharmaceutics, School of Pharmaceutical Education & Research, Jamia Hamdard, New Delhi, India, Email: yas2312003@yahoo.com
Submitted: 22 May 2020; Approved: 26 May 2020; Published: 09 June 2020
Citation this article: Nitika, Nikita, Khan I, Sultana Y. Formulation, Development and Optimization of Itraconazole Cubosomal Gel for the Treatment of Candidiasis. Int J Pharma Anal Acta. 2020;3(1): 015-019.
Copyright: © 2020 Khan NI, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Candidiasis; Itraconazole; Cubosomes; Cubosomal gel
Download Fulltext PDF
Dermatological conditions are known to be one of the most common medical conditions and 67-90% of fungal infections are known to be caused by Candida non-albicans. Since topicaldrug administration is the most preferred route for dermatological treatment. Unlike classic liposomal vesicles that become trapped within the top layer of stratum corneum cells, cubosomes penetrate the skin and allow improved delivery of various compounds to the deep strata of the skin or to the systemic circulation and can release therapeutic dose. Itraconazole is known to interact with 14-α demethylase interfering with the formation of ergosterol, the main component of the fungal cell membrane.Therefore, the present study is aimed to prepare a cubosomal gel of itraconazole.Cubosomal gel was prepared to increase the penetration of itraconazole into the deeper layers of the skin and hence enhancing the bioavailability by avoiding first-pass metabolism. The prepared cubosomes were optimized on the basis of vesicle size, drug entrapment efficiency,shape, appearance and in-vitro drug release study. The vesicle size of the prepared cubosomes was found to be 86.69nm and PDI was found to be and 0.258 with 37%, entrapment efficiency, in-vitro study follows Korsemeyer –Peppas order release pattern. The appearance of the cubosomes was confirmed by SEM and the cubosomal gel shows good pH, homogeneity, spreadability, and hardness properties. Hence, with the above results it could be interpreted that the cubosomal gel formulation of itraconazole can be a promising treatment approach for candidiasis with improved skin penetration of the drug.
Introduction
Candida species are the most common cause of opportunistic fungal infection worldwide causing diseases ranging from superficial mucosal infections to disseminated, systemic infections that are often life-threatening. Cutaneous Candidiasis or Skin Candidiasis is a fungal infection of the skin caused by the fungus Candida. Epidemiological survey data from India showed that 67 to 90% of nosocomial candidemia cases were due to Candida non-albicansof which C. tropicaliswas the most dominant [1]. In the last few decades, there have been numerous reports of Candida infections in India. C. albicansis the major cause of serious fungal infections in the United States, and Candida species are the fourth most commonly cultured microbe from the blood. The attributable mortality from bloodstream C. albicans infections in adults is at least 15% [2]. Skin disease (dermatological conditions') affects the population and has been cited as one of the top 15 medical conditions for which prevalence and healthcare spending increased in the last decade. The outcome of topical dermatological drug treatment is significantly influenced by the choice of vehicle or delivery system. Non-invasive drug delivery systems provide alternative routes of administration and improved delivery of drugs to localized target sites in the body [3]. Itraconazole is an azole derivative antifungal drug belongs to BCS class II that interacts with 14-α demethylase, a cytochrome P-450 enzyme necessary to convert lanosterol to ergosterol; as ergosterol is an essential component of the fungal cell membrane [4,5]. The aim of the present study is to increase penetration of Itraconazole into deeper layers of skin by formulating cubosomes of Itraconazole into a topical gel that helps in enhancing the bioavailability by avoiding first-pass metabolism. Cubosomes are a novel lipid particulate delivery system that is discrete, sub-micron, nanostructured particles of the bicontinuous cubic liquid crystalline phase. Cubosomes are nanoparticles that are self-assembled and are thermodynamically stable; they have a structure like “honeycombed” with bicontinuous domains of water and lipid in which surfactant assembles into bilayers and twisted into a three-dimension, periodic, and minimal surface, forming a tightly packed structure [6]. Cubosomes acts as an ideal drug delivery system for the treatment of topical fungal infections reportedly for drugs having poor loading and permeability [7]. Unlike classic liposomal vesicles that become trapped within the top layer of stratum corneum cells, cubosomes penetrate the skin and allow enhanced delivery of various compounds to the deep strata of the skin or to the systemic circulation and can release therapeutic dose. In the present study the cubosomal formulation is developed with Glyceryl Monooleate (GMO) with stabilizing polymer poloxamer forming a bicontinuous cubic phase in excesswater these concentration [8] are optimized on the basis of vesicle size and size distribution, drug entrapment efficiency, and shape- appearance, in-vitro, as well as characterization of cubosomal gel loaded with itraconazole pH, homogeneity, spreadability, and hardness properties are studiedis . Objective of the study that the cubosomal drug administered in a semisolid form (gel) produce high patient compliance avoiding first pass metabo.
Materials and Methods
Materials
Itraconazole was obtained as a gift sample by Kusum Healthcare, Delhi, India. Glyceryl Monooleate (GMO) and Poloxamer 407 were purchased from Sigma Aldrich and Carbopol 934 was purchased from S.D.Fine Chemicals Ltd., Mumbai, India. All the reagents used in this study were of analytical grade.
Preparation of Itraconazole loaded cubosomes vesicles
Cubosomes were prepared using the top-down method by mixing lipid with stabilizer, then the resultant mixture is dispersed into aqueous solution by high energy input of such as High-Pressure Homogenization, to form vesicle-like structures [9]. The varying concentrations of cubosomal vesicles (F1-F9) were prepared as described in table 1. Varying concentrations of Glyceryl monooleate (3.2 to 4.8%) and poloxamer 407 polymers (0.2 -1.8%) were mixed and melted in a water bath at 60°C. To this mixture, 100mg of itraconazole was added and stirred until completely dissolved. Then preheated (up to 70°C) distilled water of suitable quantity (95%) was added drop by drop to this solution by continuous stirring, after complete addition of water it was kept aside for one day to attain equilibration and resulted in the formation two-phase system which was disturbed by stirring. This whole system was then subjected to homogenization at 8000-10000 rpm for 2hr at room temperature. The liquid dispersion of cubosomes thus formed was kept at room temperature in dark to avoid direct sunlight.
Preparation of Itraconazole cubosomal topical gel
On the basis of particle size and entrapment efficiency study F2 was selected to be converted into the gel system using Carbopol 934. The topical gels were prepared by allowing cubosome dispersions to swell using Carbopol 934 (1%, 2%, and 3%) in distilled water at 800 rpm for a few hours. To this, the drug-loaded cubosomal suspension was added drop by drop with continuous stirring to form a homogeneous mixture. Triethanolamine (0.5%) was then added to it until a transparent gel was obtained. Finally, 0.01% benzalkonium chloride was added as a preservative. The formulations with different concentrations (G1-G3) as described in table 2 were then evaluated for pH, homogeneity, spreadability, and hardness.
Optimization and characterization of cubosomes vesicles
The effect of Poloxamer 407 concentration, GMO concentration, and sonication time on the formation of cubosomes was characterized by using optical electron microscopy.
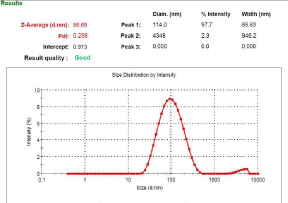
Particle Size Determination: The mean size of the cubosomal vesicle was analyzed by a dynamic light scattering technique also known as photon correlation spectroscopy using Malvern Zeta-sizer. The sample was placed in quartz cuvette after diluting (50 times) with an appropriate medium and size measurements were carried out at a scattering angle of 90º and at a temperature of 25 ± 1°C [10].
Entrapment Efficiency: Entrapment Efficiency (E.E.) of Itraconazole loaded cubosomal vesicleswas determined by the centrifugation method [11]. Vesicle preparations were kept overnight at 4˚C and ultra-centrifuged (Remi cooling centrifuge C-24, India) for 2 hrs. At 17000 rpm. The free (un entrapped) Itraconazole concentration was determined in the supernatant spectrophotometrically (Shimadzu UV–1601 PC Double Beam, Kyoto, Japan) at λmax 262 nm. The Itraconazole entrapment percentage was calculated from the following formula:
Where,
Cp: concentration of Itraconazole analyzed after lysing with triton X-100 as described in the above methods
Ci: The initial concentration of Itraconazole added into the formulation
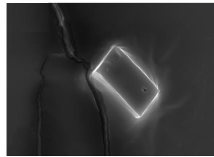
Vesicular Shape and Morphology: Scanning Electron Microscopy (SEM) was conducted to characterize the surface morphology of the optimized cubosomal vesicles. One drop of cubosomal system was mounted on clear glass stub, air-dried, and coated with Polaron E 5100 Sputter and visualized under SEM at an accelerating voltage of 20 kV and at 15mm working distance [12].
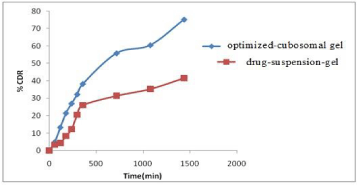
in-vitro drug release study: The in vitro release profile of optimized-cubosomal gel and drug-suspension-gel was determined by dialysis membrane-diffusion technique. Both the formulations were filled in a preactivated dialysis bag (MW~12000–14000 Da) and then fix them to the shafts positioned vertically in a beaker containing release medium i.e. pH 6.8phosphate buffer saline with continous stirring at 100 rpm at the maintained temperature i.e. 37 ± 2°C. The samples were at equal time intervals and also add fresh release medium to maintain the sink condition. The samples collected are then analyzed by UV and graph was plotted between % cumulative drug release and time (hours). To find the release pattern the data obtained from in-vitro release study were fitted to various release kinetic models such as zero order, first order, Higuchi's matrix and Korsmeyer-Peppas [13-16].
Characterization of cubosomal gel
Physical parameters of gels: Cubosomal gel formulations G1 to G3 were characterized for pH using pH meter, spreadability, consistency and homogeneity, and hardness.
a) pH: The pH of the various gel formulations was determined by using digital pH meter.
b) Spreadability: It was determined by a wooden block and glass slide apparatus. Weights about 10g were added to the pan and the time was noted for the upper slide (movable) to separate completely from the fixed slides. Spreadability was then calculated by using the formula:
S = M.L / T Eq.2
Where,
S = Spreadability M = Weight tide to upper slide L = Length of glass slide T = Time taken to separate the slide completely from each other.
c) Consistency: The measurement of the consistency of the prepared gels was done by dropping a cone attached to a holding rod from a fixed distance of 10cm in such a way that it should fall on the center of the glass cup filled with the gel. The penetration by the cone was measured from the surface of the gel to the tip of the cone inside the gel. The distance traveled by cone was noted down after 10sec.
d) Homogeneity: All developed gels were tested for homogeneity by visual inspection after the gels have been set in the container. They were tested for their appearance and presence of any aggregates.
e) Firmness: Peak or the maximum force by the probe to break away from the gel when starting to ascend.
Results and Discussion
Characterization of cubosomal vesicles
Particle size, PDI and entrapment efficiency: Particle size analysis and PDI (Figure 1) which was found to be 86.69 nm and 0.258 respectively which is primarily suitable for cubosomal delivery. Entrapment efficiency is a direct commentary on the ability of the drug to integrate with lipoidal content to form vesicles of suitable integrity. The Entrapment Efficiency (EE) of was found to be 37% while the effect of GMO can be seen and it was concluded that on increasing GMO contents, Formulations Entrapment efficiency increases, and the maximum entrapment efficiency was found in formulation F2.
Vesicular Shape and Morphology: The optimized formulation F2 was subjected to Scanning Electron Microscopy and micrographs obtained revealed the presence of cubical shape and smooth and even surface of the cubosomes as shown in figure 2.
in-vitro drug release study: The % cumulative drug release optimized-cubosomal gel and drug-suspension-gel were found to be 79.11 ± 2.03 and 41.85 ± 3.19 respectively after 24 h (Figure 3). By studying the kinetic models it was found that the cubosomal vesicles follow Korsmeyer‐Peppas release pattern as it showed maximum R2 value (Table 5) and according to this model the cubosomal formulation released the drug through non‐Fickian super case‐II transport (n > 0.1).
Characterization of cubosomal gel
a) pH: The pH value of cubosomal gel G1, G2, and G3 was found to be 6.8, 6.7, and 6.4 respectively.
b) Spreadability: The value of the spreadability of G1, G2, and G3 was 13.8, 14.01, and 14.3 (g.cm/sec) respectively. The values of spreadability indicate that the gel is easily spreadable by a small amount of shear.
c) Consistency: The consistency reflects the capacity of the gel, to get ejected in uniform and desired quantity when the tube is squeezed. Consistency in terms of distance travel by cone was 10 mm for all developed batches.
d) Homogeneity: The developed gel containing 1% carbopol showed good homogeneity with the absence of lumps. The gel containing 2% and 3% carbopol 934 showed lumps and it was found that the last two gels were more viscous.
e) Firmness: G1 has the least hardness 6.010 g, then G2 (6.859 g), and maximum hardness was found in G3 (6.929 g).
The parameters which were evaluated for the cubosomal gels including the pH, homogeneity, drug content, spreadability, and firmness are given in table 4. The Carbopol-934 gel concentration was optimized on the basis of mechanical (Firmness) property. The Firmness of the gel represents the consistency and firmness of the sample which showed maximum for G3 than G2 and least in G1. The pH value of cubosomal gel G1, G2, and G3 were found to be 6.8, 6.7, and 6.4 respectively.The drug content was also 99.45 ± 0.25%.
Conclusion
This study included the development and optimization of Itraconazole cubosomal gel for the treatment of candidiasis. They are prepared by using top- down method and was optimized. The developed cubosomes formulation showed cubical shape with smooth surfaces confirmed by electron microscopy. The developed cubosomal gel formulation of itraconazole showed better in-vitro release study and also has pafromising potential approach for a treatment for candidiasis with enhanced drug penetration through skin layers.lism.
- A Kothari, V Sagar. Epidemiology of Candida bloodstream infections in a tertiary care institute in India. Indian journal of medical microbiology. 2009; 27: 171-172. PubMed: https://pubmed.ncbi.nlm.nih.gov/19384050/
- Suzanne M Noble, Sarah French, Lisa A Kohn, Victoria Chen, Alexander D Johnson. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nature genetics. 2010; 42: 590-598. PubMed: https://pubmed.ncbi.nlm.nih.gov/20543849/
- Bhowmik D, Gopinath H, Kumar BP, Duraivel S, Kumar KS. Recent advances in novel topical drug delivery system. The Pharma Innovation. 2012; 1: 12. https://tinyurl.com/y85zeq8h
- Elena Shekhova, Olaf Kniemeyer, Axel A Brakhage. Induction of mitochondrial reactive oxygen species production by Itraconazole, Terbinafine, and Amphotericin B as a mode of action against. Antimicrobial Agents and Chemotherapy. 2017; 61: e00978-17. PubMed: https://pubmed.ncbi.nlm.nih.gov/28848005/
- B Venkatesh, S Indira, Prathima Srinivas. Formulation and evaluation of miconazole nitrate as a cubosomal topical gel. 2014, 5: 2037-2047. https://tinyurl.com/y9g5ebtc
- Sherif A, Gaballa, Omar HE. Garhy, Hamdy Abdelkader. Cubosomes: Composition, preparation, and drug delivery applications. Journal of advanced Biomedical and Pharmaceutical Sciences. 2020; 3: 1-9. https://tinyurl.com/ybzfk9h6
- Samia Omar email, Aliaa Ismail, Kariman Hassanin, Sara Hamdy. Formulation and evaluation of cubosomes as skin retentive system for topical delivery of Clotrimazole. Journal of Advance Pharmacy Research. 2019; 547: 68-82. https://tinyurl.com/yag5vxq8
- Rizwan SB, Dong YD, Boyd BJ, Rades T, Hook S. Characterisation of bicontinuous cubic liquid crystalline systems of phytantriol and water using cryo field emission scanning electron microscopy (Cryo FESEM). 2007; 38: 478-485. PubMed: https://pubmed.ncbi.nlm.nih.gov/17011783/
- Basavaraj K Nanjwade, Yallappamaharaj R Hundekar, Meghana S Kamble, Teerapol Srichana. Development of cuboidal nanomedicine by nanotechnology. Austin Journal of Nanomedicine & Nanotechnology. 2014; 2: 1-8. https://tinyurl.com/yaaaffme
- S M Kawish, ShakeebAhmed, AzkaGullb, MohammedAslama, JayamantiPandita, Mohd.Aqila, et al. development of nabumetone loaded lipid nano-scaffold for the effective oral delivery; optimization, characterization, drug release and pharmacodynamic study. J Mol Liq. 2017; 231: 514-522. https://tinyurl.com/y85rhzba
- Yosra S R Elnaggar, Samar M Etman, Doaa A Abdelmonsif, Ossama Y Abdallah. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer's disease: Pharmaceutical, biological, and toxicological studies. Int J Nanomedicine. 2015; 10: 5459-5473. PubMed: https://pubmed.ncbi.nlm.nih.gov/26346130/
- M Alam, S Ahmed, Nikita, G Moon, M Aqil, Y Sultana. Chemical engineering of a lipid nano-scaffold for the solubility enhancement of an antihyperlipidaemic drug, simvastatin; preparation, optimization, physicochemical characterization and pharmacodynamic study. Artif Cells Nanomed.Biotechnol. 2018; 46: 1908–1919. https://tinyurl.com/yctjzuum
- Danish M, Ahmed S, Sarim S, Khan I, Singhal S. Journal of Drug Delivery Science and Technology CCD Based Development and Characterization of Nano-Transethosome to Augment the Antidepressant effect of Agomelatine on Swiss Albino Mice. J Drug Deliv Sci Technol. 2019; 54: 101234.
- T Higuchi. Mechanism of sustained‐action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963; 52: 1145-1149. PubMed: https://pubmed.ncbi.nlm.nih.gov/14088963/
- Richard W Korsmeyer, Robert Gurnya, Eric Doelker, Pierre Buri, Nikolaos A Peppas. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983; 15: 25-35. https://tinyurl.com/y9lxxb3k
- N A Peppas. Analysis of fissckian and non-fickian drug release from polymers. Pharm Acta Helv. 1985; 60: 110-111. PubMed: https://pubmed.ncbi.nlm.nih.gov/4011621/

Sign up for Article Alerts