Ceftaroline Fosamil in the Treatment of Experimental Meningitis Caused by Methicillin-Resistant Staphylococcus aureus?
Brenda K Hansen1,2, Dena L Toffaletti1, Lawrence P Park1, Bobby Warren1, Charles Giamberardino1, John R Perfect1, Vance G Fowler Jr1, Richard H Drew1# and Batu K Sharma-Kuinkel1#*
1Department of Medicine, Duke University Medical Center, Durham, North Carolina, United States of America
2Currently at Pediatric Gastroenterology, University of North Carolina, Chapel Hill, North Carolina, United States of America
#These authors contributed equally
*Address for Correspondence: Batu K. Sharma-Kuinkel, Division of Infectious Diseases Department of Medicine, Duke University Medical Center Box 102359 Medical Center, Durham, NC 27710, ORCID ID: orcid.org/0000-0002-7217-3213; Tel: (919) 668-5366; Fax: (919) 613-5175; E-mail: batu.sharma@duke.edu
Submitted: 15 July 2020; Approved: 19 July 2020; Published: 21 July 2020
Citation this article: Hansen BK, Toffaletti DL, Park LP, Drew RH, Sharma-Kuinkel BK, et al. Ceftaroline Fosamil in the Treatment of Experimental Meningitis Caused by Methicillin-Resistant Staphylococcus aureus. Int J Pharma Anal Acta. 2020;3(1): 020-024.
Copyright: © 2020 Hansen BK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Staphylococcus aureus; MRSA; Meningitis; Ceftaroline; Vancomycin
Download Fulltext PDF
Introduction: Meningitis caused by methicillin resistant Staphylococcus aureus (MRSA) is rare and often fatal. The recommended treatment for MRSA meningitis, vancomycin, is limited by poor cerebrospinal fluid (CSF) penetration. Ceftaroline fosamil is a novel fifth-generation cephalosporin with potent antibacterial activity in vitro against MRSA. Several case reports in humans suggest that ceftaroline might be a potential treatment option for MRSA meningitis. Our primary objective was to compare the efficacy of ceftaroline and vancomycin for the treatment of MRSA meningitis using a rabbit meningitis model. We then characterized CSF drug concentrations over time.
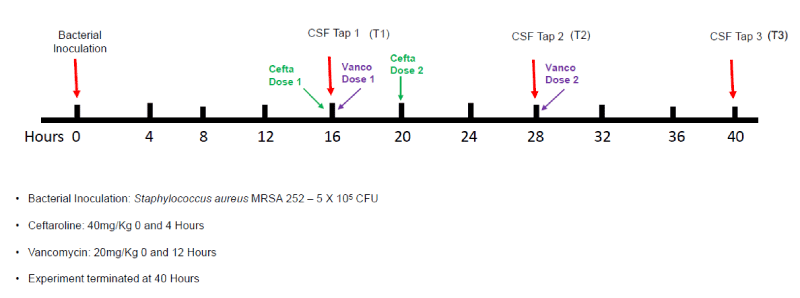
Methods: Ninety rabbits received a direct intracisternal injection of 5 x 105 CFU S. aureus (MRSA 252). Following a 16-hour incubation period, approximately 0.5ml of CSF was withdrawn via intracisternal aspiration immediately prior to treatment for quantitative bacterial counts. Rabbits then received (by 1:1:1 random assignment) either no treatment (control group), vancomycin 20 mg/kg at 0 and 12hrs, or ceftaroline 40mg/kg at 0 and 4 hrs via marginal ear vein. CSF collection was repeated every 12 hours for up to 40 hours. All animals were humanely euthanized at 40 hours post inoculation. The primary endpoint (difference in bacterial load [expressed as CFU/mL] for each animal between the initial (T1) and terminal (T3) taps were characterized and compared between groups using the Kruskal-Wallis test. CSF drug concentrations were determined using liquid chromatography with tandem mass spectrometry (LC/MS/MS) assay.
Results: Among the forty-three evaluable animals with established MRSA meningitis, there was no statistical difference in median CFU observed between the groups in pretreatment CFU counts (p = 0.16). Reductions in bacterial load from baseline were 88%, 79% and 75% in the control, ceftaroline and vancomycin-treated groups, respectively. There was no statistical difference observed between vancomycin and either control or ceftaroline at any time point. While animals treated with ceftaroline exhibited a significant increase in clearance rate (log ∆) between T1 and T2 when compared to control (p = 0.019), these differences were no longer statistically significant by T3 (p = 0.43) as CSF ceftaroline concentrations were undetectable by that time point.
Conclusions: While ceftaroline may be a reasonable alternative to vancomycin for MRSA meningitis, additional animal and clinical studies are required to determine optimal dosing to establish its effectiveness.
Abbreviations
MRSA: methicillin resistant Staphylococcus aureus; CSF: cerebrospinal fluid; CFU: colony forming units; LC/MS/MS: liquid chromatography–mass spectrometry; T1: time point one (16 hours post inoculation); T2: time point 2 (28 hours post inoculation); T3: time point 3 (40 hours post inoculation)
Introduction
Methicillin resistant Staphylococcus aureus (MRSA) is a common and often devastating cause of nosocomial bacterial meningitis [1]. Currently, the standard treatment for MRSA meningitis is vancomycin [2], but its penetration into the cerebral spinal fluid (CSF) is inconsistent and (at times) poor [3]. Adjunctive treatments, such as intrathecal administration of vancomycin, are of unproven inefficacy and can confer significant risks to the patient. As a result, alternative treatments for MRSA meningitis are greatly needed.
Cephalosporins have a rich history in the optimal management of bacterial meningitis in humans [4]. Ceftaroline fosamil (Teflaro®-Allergan, Madison, NJ, USA) is a fifth-generation cephalosporin approved for treatment of community-acquired bacterial pneumonia and acute skin and soft tissue infections [5]. Recent animal studies suggest that ceftaroline can penetrate the blood-brain barrier in rabbits [6,7], and therefore might play a role in the treatment of MRSA meningitis in humans. While a few cases have reported some promise with ceftaroline for the treatment of S. aureus and Streptococcus pneumoniae meningitis [8-10], the efficacy of ceftaroline against MRSA meningitis and its efficacy relative to vancomycin has yet to be comparatively tested experimentally. Our purpose was to evaluate the efficacy of ceftaroline in the treatment of MRSA meningitis using a rabbit meningitis model [11]. Our primary objective was to compare ceftaroline- and vancomycin-treated animals for the difference in bacterial load (expressed as CFU/mL) between the initial and terminal taps. Our secondary objective was to characterize CSF drug concentrations over time.
Materials and Methods
Ethics
All animal procedures were reviewed and approved by the Duke Institutional Animal Care and Use Committee prior to institution of any study-related procedures (Duke IACUC #A006-15-01).
Test organism
An isolated colony of S. aureus MRSA 252 [12], a well characterized and widely used MRSA isolate, was incubated overnight in tryptic soy broth at 37oC/220 RPM. The following day, a subculture was permitted to grow until the bacteria reached the log-phase of growth (approximately 2 hrs.). Cells were then washed twice in phosphate buffered saline (PBS) and re-suspended in PBS containing 20% glycerol at a concentration of ~106 cfu/µL. The suspension was divided into 1 mL glycerol stock aliquots, and stored at -80°C. Bacterial inoculations were derived from frozen glycerol stock, and re-suspended at 5 X 105 cfu in 300 µL sterile PBS.
Animal model
The overall experimental design is illustrated in figure 1. Ninety 6-8-week-old male New Zealand white rabbits (~2.5 kg) were acquired from Robinson Services Inc. (Mocksville, NC, USA). Following a week of habituation, animals were anesthetized with an intramuscular injection of ketamine (38 mg/kg of body weight) and xylazine (5 mg/kg of body weight) prior to each intracisternal procedure. Animals were assigned to treatment groups 1:1:1 using a random number generator in Microsoft Excel (Microsoft Corp., Redmond, WA) at the beginning of the experiment. Each animal received an intracisternal inoculation of MRSA 252 at 0 hours, following the method of Perfect and Durack [11]. At 16 Hours Post-Inoculation (HPI), we collected the first CSF sample followed immediately by antibiotic administration (timepoint 1[Tl]). We chose an inoculation dose of 5 X 105 cfu in order to attain sufficient bacterial load without resulting in unacceptable mortality, which meant that most animals (regardless of treatment) cleared infection by the terminal time point. Dosing regimens for ceftaroline and vancomycin were based on the published literature [6], and our experience in clinical practice in human beings, respectively. Commercial vials of ceftaroline fosamil for injection for human use (Teflaro®-Allergan, Madison, NJ, USA) 600 mg/vial was reconstituted with sterile water for injection, USP to a final volume of 100 mg/ml. Vancomycin hydrochloride USP (Sigma-Aldrich, St. Louis, MO, USA) was reconstituted in sterile water for injection, at a final concentration of 50 mg/ml. Ceftaroline (40 mg/kg dose repeated at 0 and 4 hours) or vancomycin (20 mg/kg dose repeated at 0 and 12 hours) were administered via the marginal ear vein. Control animals received no treatment. Additional CSF samples were similarly collected at 28 HPI (timepoint 2 [T2]), and at the terminal time point of 40 hours HPI (timepoint 3 [T3]). Blood samples were taken at Tl and T3 to identify potential secondary blood infection. At T3, animals were humanely euthanized using an intravenous injection of 1 mL Euthasol.
Quantification of bacterial burden
All CSF samples were plated following a serial dilution (50 µL). Bacterial load was determined by manual counting after 24 hours, and expressed as colony forming units (CFU) /ml. Blood samples were serially plated for culture.
CSF antibiotic concentrations
CSF samples (approximately 50 µl) were analyzed by the Duke Cancer Institute’s Pharmacokinetic/Pharmacodyamic Core Laboratory at various timepoints (Tl as negative controls as well as T2 and T3) to determine ceftaroline and vancomycin concentrations using LC/MS/MS Assay. Briefly, ceftaroline T2 samples were diluted 1/50 and vancomycin T2 and T3 samples 1/10 with artificial CSF. A 20 mL aliquot of the sample was transferred to a polypropylene autosampler vial containing a 20 mL aliquot of aminopterin (internal standard), vortexed, centrifuged at 6000 g for 5 minutes, and placed in the refrigerated autosampler for analysis.
Agilent 1200 series LC system interfaced with Applied Biosystems/SCIEX API 5500 QTrap hybrid triple quadrupole-linear trap MS/MS spectrometer equipped with electrospray ionization (ESI) source was used for analysis. Analyst (version 1.6.2) software was used for mass tuning, data acquisition, and quantification. The assay compounds were individually infused as 100 nM solutions in 50%A/50%B at 10 µL/min flow rate and internal ion path parameters optimized to provide maximal ion count for “parent” and collision-produced (“daughter”) MS/MS ions. Samples were prepared by adding pure standard of the measured compound to artificial CSF. Lower limit of quantification (LLOQ) at 80% accuracy limit for both vancomycin and ceftaroline was 0.81 ng/mL from 20 μL CSF sample. The response of the peak area standard/internal standard to nominal concentration was linear with r2 = 0.998 or better.
Data analyses
Only animals with CSF samples meeting the minimum criteria of 103 CFU/mL at Tl who successfully underwent CSF collection at T3 were included in the final analyses. For the primary endpoint, the difference in bacterial load for each animal between Tl and T3 were characterized and compared between groups. Absolute bacterial loads as well as proportional changes between Tl and T2 and between T2 and T3 were examined. Descriptive statistics were performed for both bacterial loads, changes in these loads and drug concentrations. Differences in these measures by treatment group and specific time points were assessed for statistical differences using the Kruskal-Wallis test. These tests were performed for all three treatment groups together and for pairwise comparisons of the treatment groups. Drug concentrations were characterized at two time points (T2 and T3) using descriptive statistics.
Results
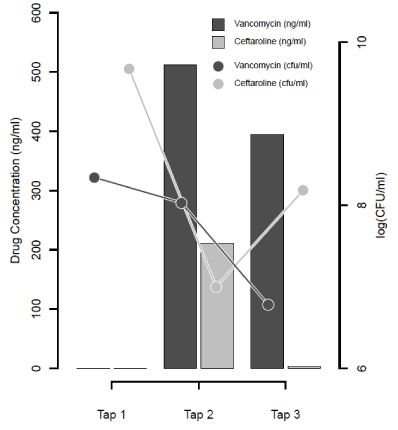
Of the ninety animals used in this experiment, forty-three met our minimum criteria for inclusion, with fourteen animals analyzed in each of the control and ceftaroline groups and fifteen in the vancomycin group. CSF bacterial loads at Tl, T2 and T3 were comparable among all three treatment groups (Tl: p = 0.16, T2: p = 0.17 and T3: p = 0.47; Table 1). Reductions in bacterial load from baseline were 88%, 79%, and 75% in the control, ceftaroline and vancomycin-treated groups, respectively (Table 1). No statistically significant differences occurred at any time point between controls or those receiving either vancomycin or ceftaroline (Tl: p = 0.16, T2: p = 0.17 and T3: p = 0.47; Table 1). The rate of bacterial clearance among the three groups approached significance between Tl and T2 (p = 0.063), with ceftaroline clearing at a much faster rate than control (p = 0.019), finally reaching statistically not significant difference by T3 (p = 0.43). The bacterial clearance by ceftaroline was reversed by end of T2-T3, period with CSF bacterial counts increasing in the ceftaroline group, while the control group continued to clear bacteria (p = 0.0039). This result was partly explained by a notable drop in ceftaroline concentrations from T2 to T3, while the vancomycin group maintained relatively high drug concentrations (Figure 2).
Discussion
Nosocomial bacterial meningitis caused by MRSA is a devastating disease [1], and the standard treatment is vancomycin [2]. Vancomycin is associated with inconsistent and poor penetration into the cerebral spinal fluid (CSF), is inconsistent and (at times) poor [3]. In this study, we attempted to explore alternative treatments for MRSA meningitis using ceftaroline and rabbit meningitis model. We compared CSF bacterial clearance over different time points among the rabbits treated with ceftaroline, vancomycin and control groups. In consistence with the previous studies, we demonstrated a very rapid bacterial clearance from the CSF in rabbits [13], suggesting that shorter time points would potentially help to properly assess bactericidal potential of newer antibacterial agents. We noticed a much faster bacterial clearance in ceftaroline treated rabbits compared to control between Tl and T2 (p = 0.019) however, it reached statistically not significant difference by T3 (p = 0.43). This result was partly explained by a notable drop in ceftaroline concentrations from T2 to T3, while the vancomycin group maintained relatively high drug concentrations (Figure 2). Others have demonstrated a similarly short half-life of ceftaroline in rabbit CSF compared to humans [14], suggesting that additional or higher doses of ceftaroline are necessary for persistent necessary drug exposure in the rabbit model.
This study has several limitations. First, as about fifty percent of the rabbits included didn’t meet the inclusion criteria, we didn’t have substantially enough rabbits for a robust statistical comparison among the treatment groups. Second, the lack of prior information about the natural clearance of Staphylococcus aureus by rabbits complicated the study. Despite these limitations, using a very challenging rabbit meningitis model, this study is able to provide some useful information about the potential use of ceftaroline in selected MRSA meningitis patients unable to take vancomycin, or for patients in whom vancomycin has failed [8,9]. Future work could consider developing a swine meningitis model, as pigs are physiologically similar to humans. Additionally, implantation of a CSF reservoir would be easier in the swine model, allowing for increased sampling.
We observed comparable early central nervous system action against MRSA meningitis between ceftaroline and vancomycin in our animal model. Further work is needed to define optimal ceftaroline dosing in both the animal model of CSF infection as well as for use in selected MRSA meningitis patients unable to take vancomycin, or for patients in whom vancomycin has failed [8,9].
Conflict of Interests
V.G.F. served as Chair of the V710 Scientific Advisory Committee (Merck); has received grant support from Cerexa/Actavis/Allergan, Pfizer, Advanced Liquid Logics, National Institutes of Health (NIH), MedImmune, Basilea Pharmaceutica, Karius, ContraFect, Regeneron Pharmaceuticals and Genentech; has been a consultant for Achaogen, AmpliPhi Biosciences, Astellas Pharma, Arsanis, Affinergy, Basilea Pharmaceutica, Bayer, Cerexa, ContraFect, Cubist, Debiopharm, Destiny Pharmaceuticals, Durata Therapeutics, Grifols, Genentech, MedImmune, Merck, The Medicines Company, Pfizer, Novartis, NovaDigm Therapeutics, Theravance Biopharma, XBiotech, Integrated BioTherapeutics, and C3J; and has received honoraria from Theravance Biopharma and Green Cross; and has a patent pending in sepsis diagnostics.
Acknowledgements
We thank Ivan Spasojevic and Duke Cancer Institute’s PK/PD core laboratory for developing the assay and measuring the vancomycin and ceftaroline concentration in CSF samples, and to the Duke Animal Facility personnel for their excellent care of our animals.
This work was supported by an investigator-initiated grant (contract ID: 105711) to BKSK from Allergan Inc. Dr. Fowler was supported by K24-AI093969 from National Institutes of Health. The funding agency has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
BKH, BW and BKSK carried out the experiments. BKSK, VGF and RHD conceptualized and designed the experiments. DLT, CG and JRP provided trainings to develop the rabbit meningitis model. LPP carried out the statistical analysis. BKH, BKSK, RHD and VGF wrote the manuscript.
- Peter Gerber, Armin Stucki, Fernando Acosta, Marianne Cottagnoud, Philippe Cottagnoud. Daptomycin is more efficacious than vancomycin against a methicillin-susceptible Staphylococcus aureus in experimental meningitis. J Antimicrob Chemothe. 2006; 57: 720-723. DOI: 10.1093/jac/dkl007
- Allan R Tunkel, Rodrigo Hasbun, Adarsh Bhimraj, Karin Byers, Sheldon L Kaplan, W Michael Scheld, et al. 2017 Infectious diseases society of America's clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017; 64: e34-e65. DOI: 10.1093/cid/ciw861
- Bettina Pfausler, Heinrich Spiss, Ronny Beer, Andreas Kampl, Klaus Engelhardt, Maria Schober, et al. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: A prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J Neurosurg. 2003; 98: 1040-1044. DOI: 10.3171/jns.2003.98.5.1040
- Reese A Cosimi, Nahal Beik, David W Kubiak, Jennifer A Johnson. Ceftaroline for severe methicillin-resistant Staphylococcus aureus infections: A Systematic Review. Open Forum Infect Dis. 2017; 4: ofx084. DOI: 10.1093/ofid/ofx084
- Allergan-Inc. Ceftaroline Product Insert: https://tinyurl.com/y39b9kws
- Cottagnoud P, Cottagnoud M, Acosta F. Efficacy of ceftaroline fosamil against penicillin-sensitive and -resistant Streptococcus pneumoniae in an experimental rabbit meningitis model. Antimicrob Agents Chemother. 2013; 57: 4653-4655.
- A Stucki, Acosta F, Cottagnoud M, Cottagnoud P. Efficacy of ceftaroline fosamil against Escherichia coli and Klebsiella pneumoniae strains in a rabbit meningitis model. Antimicrob Agents Chemother. 2013; 57: 5808-5810. DOI: 10.1128/AAC.00285-13
- Muhammad Adnan Balouch, Rizma Jalees Bajwa, Ali Hassoun. Successful use of ceftaroline for the treatment of MRSA meningitis secondary to an infectious complication of lumbar spine surgery. J Antimicrob Chemothe. 2015; 70: 624-625. DOI: 10.1093/jac/dku392
- Safia S Kuriakose, Mohamed Rabbat, Jason C Gallagher. Ceftaroline CSF concentrations in a patient with ventriculoperitoneal shunt-related meningitis. J Antimicrob Chemothe. 2015; 70: 953-954. DOI: 10.1093/jac/dku464
- George Sakoulas, Poochit Nonejuie, Ravina Kullar, Joseph Pogliano, Michael J Rybak, Victor Nizet. Examining the use of ceftaroline in the treatment of Streptococcus pneumoniae meningitis with reference to human cathelicidin LL-37. Antimicrob Agents Chemother. 2015; 59: 2428-2431. DOI: 10.1128/AAC.04965-14
- Perfect JR, Durack DT. Pharmacokinetics of cefoperazone, moxalactam, cefotaxime, trimethoprim and sulphamethoxazole in experimental meningitis. J Antimicrob Chemothe. 1981; 8: 49-58. DOI: 10.1093/jac/8.1.49
- Matthew T G Holden, Edward J Feil, Jodi A Lindsay, Sharon J Peacock, Nicholas P J Day, Mark C Enright, et al. Complete genomes of two clinical Staphylococcus aureus strains: Evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A. 2004; 101: 9786-9791.
- Sato K, Lin TY, Weintrub L. Bacteriological efficacy of nafcillin and vancomycin alone or combined with rifampicin or amikacin in experimental meningitis due to methicillin-susceptible or -resistant Staphylococcus aureus. Jpn J Antibiot 1985; 38: 2155-2162.
- Norrby SR. Role of cephalosporins in the treatment of bacterial meningitis in adults. Overview with special emphasis on ceftazidime. Am J Med. 1985; 79: 56-61. DOI: 10.1016/0002-9343(85)90262-1

Sign up for Article Alerts