Prevalence of Malaria among Febrile Patients attending Government Hospitals in Ondo State, South-West Nigeria
Omoya F.O and Ajayi KO*
Department of Microbiology, Federal University of Technology, Akure PMB 704, Akure, Nigeria
*Address for Correspondence: Ajayi K. Oluyemi, Department of Microbiology, Federal University of Technology, Akure PMB 704, Akure, Nigeria, Tel: +234-070-389-905-88; E-mail: ooluyhemikehinde@yahoo.com
Submitted: 10 November 2020; Approved: 19 November 2020; Published: 03 December 2020
Citation this article: JOmoya FO,Ajayi KO. Prevalence of Malaria among Febrile Patients attending Government Hospitals in Ondo State, South-West Nigeria. American J Epidemiol Public Health. 2020 Dec 03;4(4): 017-024. doi: 10.37871/ajeph.id40
Copyright: © 2020 Omoya FO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Febrile patients; Malaria prevalence; Parasitaemia; Plasmodium falciparum
Download Fulltext PDF
Background: The use of malaria infection prevalence among febrile patients is a valuable epidemiological surveillance tool. In this study, a cross sectional study was conducted among febrile patients in selected government Hospitals in Ondo State for malaria prevalence.
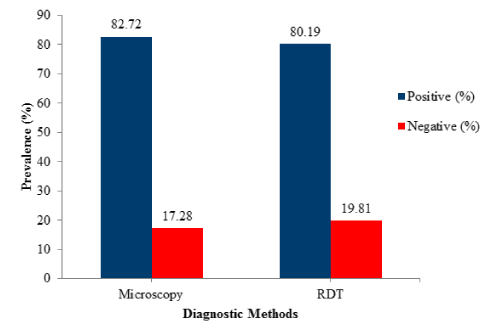
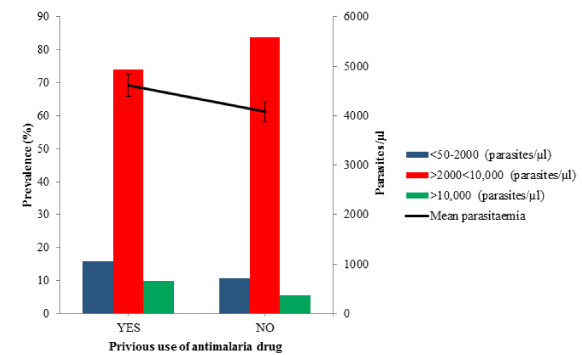
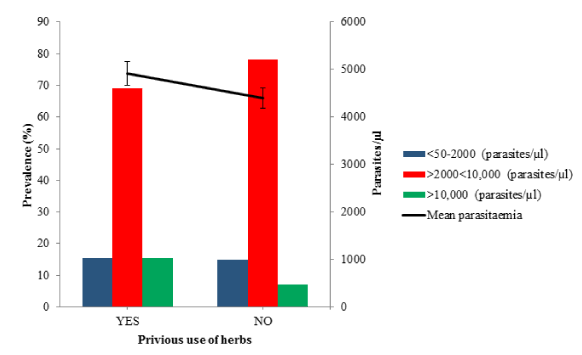
Results: Plasmodium falciparum is the only encountered malaria parasite with prevalence values of 82.72% (426/515) and 80.19% (413/515) were obtained for microscopy and RDTs respectively. The prevalence of malaria among the males (86.59%) was higher than the females (80.65%), all age groups in this study were vulnerable with highest infection rate of 89.66% among age group 11-20 years. The parasites densities ranged between 209 and 22310 parasites/μl with a mean parasitaemia of 5522.17 ± 183.30 parasites/μl. The prevalence of malaria among the febrile participants that have taken antimalarial drug before visiting the hospital is 82.94% (389/469) with the mean parasitaemia of 4615.21 ± 188.14 parasites/μl while among the participants that have taken herbs before visiting the hospital the prevalence is 85.03% (142/167) with the mean parasitaemia of 4913.81 ± 330.20 parasites/μl.
Conclusion: There was high prevalence of malaria among febrile patients and this finding will help improve the diagnosis and treatment of other febrile (non-malaria) infections, limit antimalarial usage to only malaria parasite-based test true positives and serve as a guide to combat malaria drug resistance in the study area.
Background
Febrile illness (fever) may be caused by a wide range of bacterial, fungal, parasitic, and viral infections [1,2], as well as by noninfectious conditions such as drug-related reactions, toxidromes, inflammatory, malignancy and so on [3]. Fever is one of the most common symptoms reported by patients seeking health care in the tropics, it may occur solely or in combinations with other symptoms, like cough or diarrhea [2,4].
Among all the febrile illness, malaria is the most deadly worldwide, especially in the tropical nations of the world and accounted for the highest causes of fever in Nigeria [5]. According to World Malaria Report by WHO [6], Nigeria accounted for the highest global malaria burden (25%), malarial death (24%) and 99.7% of the malaria cases are caused by Plasmodium falciparum [6].
During the erythrocytic stage of malaria infection, antibody mediates cellular killing, blocks adhesion of infected red blood cells to endothelium, and neutralizes parasite toxins to prevent the induction of excessive inflammation [7,8]. This stage is also known by proinflammatory cytokine response that activates macrophages. The activated macrophages then release cytokines, such as tumor necrosis factor (TNF-alpha), and other substances like as interleukins IL1, IL6 and IL8 into the circulation which cause fever and other malarial symptoms [7,9].
In Ondo state, different febrile illness caused by infectious agent such as Typhoid fever, Lassa fever, yellow fever [10-12] and others have been reported. There is need to constantly differentiate non-malaria febrile illness from malaria febrile illness to monitor the progression of non-malaria febrile illnesses, avert wrong malaria treatment and reduce drug resistance to malaria [2]. This study therefore gives an insight on febrile patient blood tested for falciparum malaria with focus on different approach of surveillance ranging socio-demographic characteristics, parasitaemia, knowledge and awareness of malaria and individuals therapeutic means of treating malaria as home management.
Methods
Study area, Selection and enrollment of participants for the study
During this study, blood samples were collected from 515 febrile patients between October, 2018 and August, 2019 from randomly selected 10 government hospitals in Ondo State, Nigeria. Multiple choice structured questionnaires were used to obtain the socio-demographic characteristics of all the participants.
Inclusion criteria for participants were: residency within 60 km of the study clinic, informed consent and present febrile symptom.
The sample size was determined using standard epidemiological formula (Fisher’s formula for cross-sectional descriptive study) as follows; in equation 1 [13].
where; Z = 1.96
p = prevalence of malaria in Nigeria (45% Rapid Diagnostic Test (RDT), 27% Microscopy) [14]
e = error rate = 0.05
The sample size is thus calculated (using the highest prevalence of RDT 45% = 0.45) as;
Therefore, the sample size for this study will be ≥ 380 samples
Ethical consideration and informed consent
The ethical approval was issued by Ondo State Health Research Ethics Committee (OSHREC). A written informed consent was obtained from the participants, parents/guardians of the children in line with the Helsinki Declaration [15].
Collection of blood samples from febrile patients
Two (2.0) milliliter of whole blood was collected from the consented patients into sample bottle containing EDTA (Ethylene Diaminetetraacetic acid) as anticoagulant by venipuncture method while 15 μL of capillary blood was collected from some of the children into capillary tube containing anticoagulant as described by Cheesbrough [16]. All blood samples were collected by professionals (Medical Laboratory Scientist and/or physician) working in each of the hospitals visited and processed for parasitological study using microscopic examination and RDT within 30 minutes of collection.
Smear preparation, microscopy and RDT
Blood smear microscopy method was used for examination of malaria parasite, thick smear was used for parasite estimation and thin film was used to determine the type of malaria parasite. Thin blood films were fixed with absolute methanol and later stained together with thick blood films using 10% Giemsa solution and subsequently washed (after 10 min) using buffered distilled water (pH 7.2).
A drop of immersion oil was applied on the dried stained slide and examined microscopically for malaria parasites using 100x objective lens. The films were examined following standard procedure for the detection and identification of malaria parasites [16]. As a quality control measure, slides were read by two independent microscopists in microbiology laboratory, and in the case of any disparity, the third microscopist was employed. Slides were considered positive when the ring / trophozoite form of Plasmodium species was observed in the blood film.
Parasitaemia was determined using the100x objective lens and calculated as stated in equation 2,
Number of parasites/μL of blood=
Blood was tested for the presence of malaria using Plasmodium falciparum specific Rapid Diagnostic Test (RDT) kit (SD bioline® malaria Ag P.f) following the manufacturer specification.
Statistical analysis of the data
Data was analysed using IBM-Statistical Package (IBM-SPSS) version 20. Relationships between parameters were evaluated using Pearson’s Chi Square (χ2) and p-value <0.05 was considered significant. Relationship between risk factors of malaria and malaria prevalence was compared by odd ratio and p < 0.05 was considered significant.
Results
Prevalence of malaria among the febrile patients attending government hospitals in Ondo State, Nigeria
In this study, a total of 515 patients with suspected malaria (febrile patients) based on clinical presentation were enrolled and screened for Plasmodium parasite. Among the febrile patients, 82.72% (426/515) of the participants were malaria positive by microscopy method, all observed parasite were P. falciparum infection while 80.19% (413/515) of the participants were malaria positive using Rapid Diagnostic Test (RDT) specific for P. falciparum antigen (Figure 1) and there was significant (p < 0.001, df = 1, χ2 = 217.002) difference in the prevalence of malaria in the two diagnostic methods used. The study showed that the parasites densities of malaria positive patients ranged between 209 and 22310 parasites/μL with a mean parasite density of 5522.17 ± 183.30 parasites/μl of blood using microscopy. P. falciparum is the only malaria parasite species encountered.
Prevalence of malaria among the febrile patients attending Government hospitals in Ondo State, Nigeria in relation to socio-demographic factors
The result in table 1 showed the prevalence of malaria among the febrile patients attending government hospitals in Ondo State, Nigeria in relation to demographic and socio-economic factors and microscopy result was used as gold standard. The prevalence of malaria in relation to gender was higher in male 86.59% (155/179) than female 80.65% (271/336. Based on age groups, malaria prevalence ranged from 78.50% (84/107) to 89.66% (52/58) in age group 31-40 and 11-20 years respectively.
The prevalence of malaria based on the level of education were 84.38% (27/32), 83.55% (254/304), 82.98% (39/47) and 80.30% (106/132) among primary, tertiary, those that has no formal education and secondary respectively. Also, based on the types of occupation, the highest prevalence was among students 86.42% (70/81) and pensioner 86.49% (32/37) while the least was noted among farmers 79.66% (47/59).
The prevalence of malaria based on the three major tribes in the study area showed higher prevalence among Yoruba 83.37% (401/481) followed by Igbo 75.00% (18/24) and Hausa 70.00% (7/10). Based on the religion of the participants, the prevalence of malaria were 82.19% (360/438), 85.14% (63/74) and 100% (3/3) among Christians, Muslims and other religions respectively. Furthermore, based on the marital status the highest prevalence was noted among the divorced 92.59% (25/27) closely followed by widower 88.89% (8/9) and the least was noted among the widowed 80.95% (17/21).
Prevalence of malaria based on travel history, previous infection, awareness and knowledge of malaria
Prevalence of malaria based on travel history, previous infection, awareness and knowledge of malaria among the febrile patients attending Government hospitals in Ondo State is shown in table 2. Based on the travel history, the prevalence of malaria among those that travelled out of the study area two weeks before the onset of malaria symptoms is 80.77% (21/26) while the prevalence rate among those that did not is 82.82% (405/489) (OR = 0.87, p = 0.477). The result revealed high malaria prevalence 80.65% (50/61) among those that had malaria previously within last six month before the present infection and there was no significant (OR = 0.85, p = 0.379) difference among them and those that does not. It was noted that there was no significant (OR =0.94, p = 0.494) difference in the malaria prevalence among those that sleep under 81.97% (50/61) ITN and those that does not 82.82% (376/454) in the study area. The prevalence of malaria among the participants that agreed that only mosquito can transmit malaria was higher 83.43% (345) compared with those that did not 81.81% (81/99) and there was no significant (OR = 1.08, p = 0.446) different. Also, environmental factors like closeness of the house to bush and drainage/stagnant water were considered and there was higher 84.43% (179/212) malaria prevalence among those the live close to bushes (OR = 1.23, p = 0.229). Furthermore, the knowledge of mosquito as only vector of malaria (OR = 1.08) and closeness of house to bushes (OR = 1.23) showed increased risk factor of malaria in the study statistically.
Prevalence of malaria in relation to symptoms presented by the febrile patients
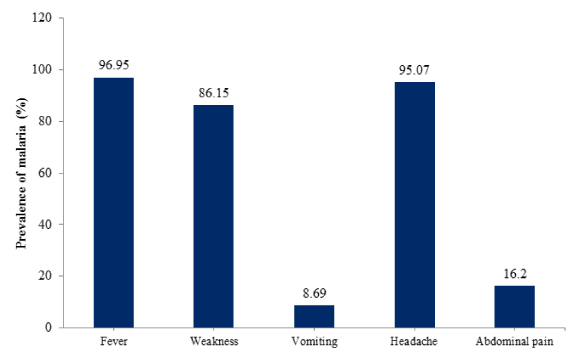
Prevalence of malaria in relation to the symptoms presented by the febrile patients is shown in figure 2. Fever, weakness, vomiting, headache and abdominal pain had prevalence of 96.95%, 86.15%, 8.69%, 95.07% and 16.20% respectively and, there was significant association between malaria and these symptoms; weakness (p = 0.045, df= 1, χ2 = 4.031) and abdominal pain (p = 0.020, df= 1, χ2 = 5.436) while fever (p = 0.456, df= 1, χ2 = 0.555), vomiting (p = 0.864, df= 1, χ2 = 0.029) and headache (p = 0.508, df= 1, χ2 = 0.438) showed no significant association.
Prevalence of malaria parasite in Ondo State among febrile patients, based on the use of commercially available antimalarial drug (Microscopy)
Prevalence rate of malaria parasite in Ondo State among febrile patients, based on the use of commercially available antimalarial drug (Microscopy) during the active infection was studied and showed in figure 3 and table 3. The overall prevalence of malaria among the febrile patients that have taken antimalarial drug (YES) before visiting the hospital is 82.94% (389/469) with the mean parasitaemia of 4615.21 ± 188.14 (ranges from 0.00 to 22310 parasites/µl) compared with those that did not (NO) take prior antimalarial drug 80.43% (37/46) with the mean parasitaemia of 4085.00±513.98 (ranges from 0.00 to 13280.00 parasites/µl. Also, 74.04% (288/389) of febrile patients that have used antimalarial drug before visiting the hospital had the parasitaemia within the range 2000 to 10000 parasites/µl. However, the differences in the parasitaemia is not significant (p = 0.884, df= 334, χ2 = 303.37) statistically.
Prevalence rate of malaria parasite in Ondo State among febrile patients, based on the use of herbal therapy (Microscopy)
Prevalence rate of malaria among febrile patients based on the use of herbal therapy as antimalarial drug (Microscopy) before visiting the hospital is shown in figure 4 and table 3. The overall prevalence of malaria among the participants that have taken herbs (YES) before visiting the hospital is 85.03% (142/167) with the mean parasitaemia of 4913.81 ± 330.20 (range from 0.00 to 19030 parasites/µl) compared with those that did not take herbs (NO) 81.61% (284/348) with the mean parasitaemia of 4397.38 ± 208.62 (range from 0.00 to 22310 parasites/µl. Also, 78.17% (222/284) of febrile patients that have not used herbs before visiting the hospital had the parasitaemia within the range 2000 to 10000 parasites/µl. However, the differences in the parasitaemia is not statistically significant (p = 0.318, df= 334, χ2 = 345.67).
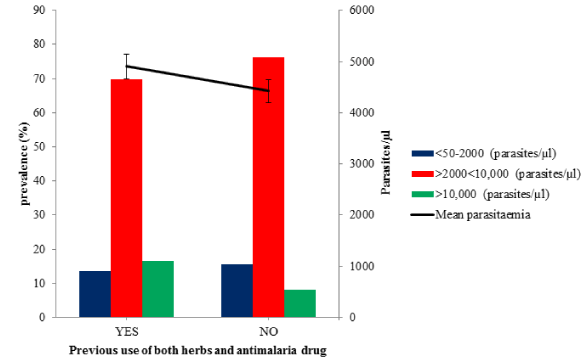
Prevalence of malaria parasite in Ondo State among febrile patients, based on the use of both herbal therapy and commercially available antimalarial drug (Microscopy)
Prevalence of malaria parasite in Ondo State among febrile patients, based on the use of both herbal therapy and commercial antimalarial drug (Microscopy) during the active infection was studied and showed in figure 5 and table 3. The overall prevalence of malaria among the participants that have taken both (YES) herbal therapy and commercial antimalarial drug before visiting the hospital is 84.62% (132/156) with the mean parasitaemia of 4902.60 ± 346.34 (ranges from 0.00 to 19030 parasites/µl) compared with those that did not take both (NO) 75.21% (270/359) with the mean parasitaemia of 4423.72 ± 205.17 (ranges from 0.00 to 22310 parasites/µl). Also, higher number of patients (76.30% (206/270) that have not used both herbs and commercial antimalaria drug before visiting the hospital had parasitaemia that falls within the range of 2000 to 10000 parasites/µl. However, the differences in the parasitaemia is not significant (p = 0.240, df= 334, χ2 = 351.92).
Discussion
The prevalence of malaria in this study is 82.72% and 80.19% using microscopy and RDT respectively, this prevalence is higher than the estimated risk map of 20% to 70% prevalence in some areas in Nigeria [17-19] and what was observed in other parts of Ondo state [20,21], they all reported prevalence of less than 70%. However, the prevalence of malaria in febrile patients reported by Nas, et al. [22] in Kano, Northern Nigeria was 84% which is higher than the findings of this study. This variation in prevalence of malaria in different places in Nigeria could be due to inadequate protection against mosquito bites or insufficient knowledge about malaria transmission, climatic differences, period of study and socio-cultural factors.
The prevalence of malaria using microscopy was significantly (p < 0.001, df = 1, χ2 = 217.002) higher than RDT method in this study. This difference was previously reported by Pembele, et al. [23] and Wogu and Nduka [24], that microscopy has higher malaria positive than RDT. The sensitivity of the RDT could be affected by storage temperature which may have been responsible for low sensitivity in this study. The better microscopic diagnosis is a gold standard for malaria examination and control [23]. Also, while microscopy permit parasite differentiation and quantification, RDT is very fast and it require little expert [26]. However, RDTs should be used alone when expert microscopy is unavailable else it should complement microscopy [24]. The findings from this study confirmed the World Health Organization’s policy that all febrile illness must be differentiated into malaria and non-malaria febrile by a laboratory parasite-based diagnosis before the administration of antimalarial to prevent malaria misdiagnosis and drug resistance [25].
This study showed that some febrile patients have a parasites density that is less than 2000 parasites/μL which is classified as low parasitaemia [25] Contrary to the study that high parasite density during active infection could result to complicated febrile illness [27]. Low parasitaemia observed in some febrile patients in this study could be that those patients have weak immune system or they may have taken an antimalarial drug prior their visit to the hospital. The occurrence of only P. falciparum during this study is not surprising, as stated by WHO [6] in world malaria report, P. falciparum accounted for over 98% of malaria cases in sub-Sahara Africa.
This study revealed that the prevalence of malaria is higher in males than females, which corroborates the findings of Oladele, et al. [18] and Nwaorgu and Orajaka [28]; who stated that males may be more prone to the disease than the females and the findings of this study contradict the report of Jemimah, et al. [19] and Houmsou, et al. [29]. There was no significant (p < 0.05) association between malaria and gender in this study. Till date, there has not been any scientific evidence documented to prove the higher prevalence of malaria was associated to sex susceptibility because Anopheles mosquito which is vector of malaria is not gender discriminatory during biting [19].
Age is an important determinant of malaria infection [17], the highest prevalence of malaria based on age groups is 11-20 years (89.66%) in this study, and there was no significant (p < 0.05) association between age and prevalence therefore age may not be the risk factor for the acquisition of malaria in this study area, this negates the study of [19] that age is a risk factor in the study area. This finding also corroborates other findings that reported higher prevalence among age groups less than 20 years [30] while other findings disagree with this report [18,31]. Improper use of malaria control strategies like Insecticide Treated Nets (ITNs) usage, poor knowledge and awareness of malaria might be the reason for a high prevalence among those aged 11-20 years.
The findings of this study also showed that there was no significant (p < 0.05) relationship between prevalence of malaria and socio-cultural factors such as level of education, occupation, tribes, religion and marital status in the study area. However, there was high prevalence of malaria of 70% and above among all the socio-cultural factors examined during this study and this could be that the patients were febrile. Ofori, et al. [32] stated that religious belief and practice could influence high prevalence of malaria infection. Adepeju [20] and Kepha, et al. [26], stated that occupation and level of education respectively may contribute to high malaria infection.
Travel history, history of malaria infection, use of Insecticide Treated Nets (ITNs) and closeness of the house to drainage/stagnant water does not contribute significantly as a risk factor of malaria among the febrile patients in the study area. Lack of difference in prevalence of malaria between patients that use ITNs and those that does not in this study is of concern, notwithstanding, inquiry was not made as to whether the nets were within their life span or used properly was not considered during the study. Public enlightenment on the proper use of ITNs should be further encouraged in the study area, Dada-Adegbola, et al. [33] stated that ITNs have been shown to be effective in malaria control and Jemimah, et al. [19] observed low malaria prevalence among those that use ITNs. Also, the patients might have been exposed to the vector (mosquito) from the environment probably, before sleeping under the nets as well as noncompliance to the use of ITNs by majority of the people living in the study area because more than half of the people in a community must use ITNs for it to be effective [34].
However, knowledge of mosquito as a vector of malaria and very closeness of houses to the bush (OR > 1) could predisposes people to malaria infection in the study area and this correspond to other findings [18,35]. Base on this finding, it is suggested that there should be continual awareness through different media about mosquito as vector of malaria and effective environmental sanitation channeled towards the clearing of bushes and gutters could reduce the spread and breading of mosquitos.
Parasite density and clinical symptoms may define the severity of malaria infection [35], in this study symptoms presented by the patients were evaluated in relations with malaria prevalence. There was higher prevalence of malaria among the febrile patients that have fever, headache and weakness and statistically, there is relationship between malaria weakness and abdominal pain in the study area. Although, malaria infection is associated with a broad spectrum of clinical manifestations [36], symptoms presented by febrile patients in this study could be used as diagnostic measure and prescription for prophylactic treatment of malaria in the study area.
There was higher prevalence of malaria with higher mean parasite load among the febrile patients that have taken non-recommended antimalarial drug before visiting the hospital compared with those that have not. The higher prevalence and parasite load could be that the parasite have developed resistance to the antimalarial used hence the high parasitaemia while the higher parasite load could increase the severity of malaria among them. This finding supports the claimed that reported that the use of non-recommended malaria chemo prophylactic medication such as Chloroquine did not significantly reduce malaria infection among patients [37]. Though the type of antimalarial drug used by the patients were not considered in this study, however there should be more awareness to discourage the use of non-recommended drug for the treatment of malaria in the study area to reduce the spread of antimalarial drug resistance which has been previously reported in the study area by Simon-Oke, et al. [21].
The higher prevalence malaria and mean parasitaemia among febrile patients that have taken herbs as a preventive measure before visiting the hospital seen in this study could be that the herbs taken had could not totally suppress the parasite. Higher percentage (78.17%) of those that took herbs had parasitaemia within the range 2000 to 10000 parasites/µl which could be the indication of severe malaria because high parasite count is a sign of severe infection [34]. This finding did not support the report of Adefioye, et al. [38] and Omololu-Aso, et al. [37], who stated that the use of herbs significantly reduce malaria in their study. This discordance may be due to differences in the herbs used, and it has been reported that some herbs only suppress parasite, lack scientific validation for the treatment of malaria and there have not been a well-established recommended dosage for herbal therapy [39,40]. Also, Oladunmoye and Kehinde [41] stated that time of harvest and mode of processing also affect the efficacy of herbs which might have contributed to the high prevalence among the herb user in this study. Therefore, Government can educate those preferred the use of herbs regarding the normal dose taken, so as not to make it excessive; it is also advisable that the food and drug agencies of Nigeria should do more research in local herbs in order to develop new and more effective drug for prevention and control of malaria.
This study also showed that the use of both herbal therapy and commercial antimalarial drug by febrile patients did not reduce the parasite load which could be as a result of factors stated earlier in the use of only herbs and antimalarial drug for the management of malaria.
Conclusion
This study reported high prevalence of malaria among febrile patients and P. falciparum is responsible for the cause. The use of ITNs did not have effect on the malaria prevalence however, knowledge of mosquito as a vector of malaria and very closeness of houses to the bush could play a significant role in the transmission of malaria in the study area. Malaria infection could also result into case fertility in the study area as headache and weakness had significant association with malaria in this study. This finding will help improve the diagnosis and treatment of other febrile (non-malaria) infections, limit antimalarial usage to only malaria parasite-based test true positives. The limitation of this study could be that only febrile patients were considered for the study.
- Mayxay M, Castonguay-Vanier J, Chansamouth V, Dubot-Pérès A, Paris DH, Phetsouvanh R, Tangkhabuanbutra J, Douangdala P, Inthalath S, Souvannasing P, Slesak G, Tongyoo N, Chanthongthip A, Panyanouvong P, Sibounheuang B, Phommasone K, Dohnt M, Phonekeo D, Hongvanthong B, Xayadeth S, Ketmayoon P, Blacksell SD, Moore CE, Craig SB, Burns MA, von Sonnenburg F, Corwin A, de Lamballerie X, González IJ, Christophel EM, Cawthorne A, Bell D, Newton PN. Causes of non-malarial fever in Laos: a prospective study. Lancet Glob Health. 2013 Jul;1(1):e46-54. doi: 10.1016/S2214-109X(13)70008-1. PMID: 24748368; PMCID: PMC3986032.
- Prasad N, Murdoch DR, Reyburn H, Crump JA. Etiology of Severe Febrile Illness in Low- and Middle-Income Countries: A Systematic Review. PLoS One. 2015 Jun 30;10(6):e0127962. doi: 10.1371/journal.pone.0127962. PMID: 26126200; PMCID: PMC4488327.
- Niven DJ, Laupland KB. Pyrexia: aetiology in the ICU. Crit Care. 2016 Sep 1;20(1):247. doi: 10.1186/s13054-016-1406-2. PMID: 27581757; PMCID: PMC5007859.
- Feikin DR, Olack B, Bigogo GM, Audi A, Cosmas L, Aura B, Burke H, Njenga MK, Williamson J, Breiman RF. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS One. 2011 Jan 18;6(1):e16085. doi: 10.1371/journal.pone.0016085. PMID: 21267459; PMCID: PMC3022725.
- Akinlua JT, Meakin R, Umar AM, Freemantle N. Current Prevalence Pattern of Hypertension in Nigeria: A Systematic Review. PLoS One. 2015 Oct 13;10(10):e0140021. doi: 10.1371/journal.pone.0140021. PMID: 26461923; PMCID: PMC4603956.
- World Health Organization. Malaria Report Sheet for 2018. WHO‘s World Malaria factsheet. Geneva, Switzerland. 2019; 7-9.
- Gomes PS, Bhardwaj J, Rivera-Correa J, Freire-De-Lima CG, Morrot A. Immune Escape Strategies of Malaria Parasites. Front Microbiol. 2016 Oct 17;7:1617. doi: 10.3389/fmicb.2016.01617. PMID: 27799922; PMCID: PMC5066453.
- Belachew EB. Immune Response and Evasion Mechanisms of Plasmodium falciparum Parasites. J Immunol Res. 2018 Mar 25;2018:6529681. doi: 10.1155/2018/6529681. PMID: 29765991; PMCID: PMC5889876.
- White NJ, Breman JG, Malária In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL and Loscalzo J. Harrison medicina interna. 17th ed. Rio de Janeiro: McGraw-Hill do Brasil. 2008. p. 1280-1294. https://bit.ly/3mFnXvR
- Olajubu FA. Evaluation of diagnostic methods for Typhoid fever diseases in Ondo state, Nigeria. British Journal of Medicine and Medicinal Research. 2014;4(36):5812-5817. https://bit.ly/3quNgTk
- Fatiregun AA, Isere E, Dosumu M, Agunbiade O, Onyibe R. Lassa fever awareness and knowledge among community residents in Ondo state, Nigeria. Journal of community medicine and primary health care. 2019;31(2):26-35. https://bit.ly/3lJok78
- Nigeria Centre for Disease Control (NCDC). Yellow fever outbreak in Nigeria: NCDC situation report 5. February 11-17, 2019. https://bit.ly/3gcBhoP
- Kwenti TE, Kwenti TDB, Njunda LA, Latz A, Tufon KA, Nkuo-Akenji T. Identification of the Plasmodium species in clinical samples from children residing in five epidemiological strata of malaria in Cameroon. Trop Med Health. 2017 Jun 15;45:14. doi: 10.1186/s41182-017-0058-5. PMID: 28630585; PMCID: PMC5471890.
- Nigeria Malaria Indicator Survey (NMIS). Atlas of Key Indicators, Rockville, Maryland, USA: National Malaria Elimination Program (Nigeria), and ICF International. 2015;1-16.
- Associazione Medica Mondiale (AMM) dichiarazione di Helsinki. Principi etici per la ricerca medica che coinvolge soggetti umani [World Medical Association (AMM). Helsinki Declaration. Ethical principles for medical research involving human subjects]. Assist Inferm Ric. 2001 Apr-Jun;20(2):104-7. Italian. PMID: 11942195.
- Cheesbrough M.. District Laboratory Practice in Tropical Countries. 2nd Edition. Cambridge University Press; Cambridge, UK: ISBN-13:9781139449298. 2010;50:165-176. https://bit.ly/2VCKgq4
- Abdulazeez AM, Ya'u M, Kurfi B. Association of hypertension and activity of angiotensin converting enzyme in malaria patients attending Sheik Muhammad Jidda General Hospital, Kano State, Nigeria. Nigeria Journal of Basic Clinical Science. 2017;14:121-126. doi: 10.4103/njbcs.njbcs_6_17
- Oladele OV, Onuoha SC, Hamafyelto HS, Omisope O, Fauziyya A, Akindigh M, Abdullahi T, Ilu ML, Ikeh, E. Prevalence of malaria infection among patients attending Murtala Muhammed specialist hospital Kano, Nigeria. African Journal of Clinical and Experimental Microbiology. 2018;19(3):214-220. doi: 10.4314/ajcem.v19i3.9
- Jemimah Y, Victor O, Elizabeth A, Akpu P, and Lynda A. Plasmodium falciparum infection among febrile patients attending a tertiary healthcare facility in central Nigeria: Prevalence, hematologic and sociodemographic factors. International Journal Tropical Diseases. 2019;2(019):1-6. doi: 10.23937/IJTD-2017/1710019
- Adepeju IS. Prevalence of malaria parasite among asymptomatic and symptomatic students of Federal University of Technology, Akure, Ondo State. British Journal of Research. 2017;4: 5. doi:10.21767/2394-3718.100005
- Simon-Oke IA, Obimakinde ET, Afolabi OJ. Prevalence and distribution of malaria, Pfcrt and Pfmdr 1 genes in patients attending FUT Health Centre, Akure, Nigeria. Beni-Suef University Journal of Basic and Applied Sciences.2018;7: 98-103. doi: 10.1016/j.bjbas.2017.07.009
- Nas FS, Yahaya A, Ali M. Prevalence of malaria with respect to age, gender and socio-economic status of fever related patients in Kano city, Nigeria. Greener Journal of Epidemiology and Public Health. 2017;5(5):44-49.
- Pembele GN, Rivero LR, Fraga J. Detection and species identification of malaria parasites by nested-PCR: Comparison with light microscopy and with SD BIOLINE malaria Ag test in Luanda, Angola. International Journal of TROPICAL DISEASE and Health.2015;10(1):1-13. doi: 10.9734/IJTDH/2015/18744
- Wogu MN, Nduka FO. Evaluating malaria prevalence using clinical diagnosis compared with microscopy and rapid diagnostic tests in a tertiary healthcare facility in Rivers State, Nigeria. Journal of Tropical Medicine. 2018;2018(3954717):1-4.
- World Health Organisation. World Malaria Report. Geneva: World Health Organisation. 2015. https://bit.ly/3ol96XI
- Ojurongbe O, Adegbosin O, Taiwo S. Assessing of clinical diagnosis, microscopy, rapid diagnostictests and polymerase chain reaction in the diagnosis of Plasmodium falciparum in Nigeria. Malaria Research and Treatment. 2013;5: 308069. https://bit.ly/3otdPa1
- Kepha S, Nikolay B, Nuwaha F, Mwandawiro CS, Nankabirwa J, Ndibazza J. Plasmodium falciparum parasitaemia and clinical malaria among school children living in a high transmission setting in western Kenya. Malaria Journal. 2016;15: 157. https://bit.ly/3quyiwE
- Nwaorgu OC, Orajaka BN. Prevalence of malaria among children 1 – 10 years old in communities in Awka North Local Government Area, Anambra State South East Nigeria International Multidisciplinary Journal, Ethiopia. 2011;5(5): 264-281. doi: 10.4314/afrrev.v5i5.21
- Houmsou RS, Amuta EU, Sar TT, Adagba AH. Malarial infection among patients attending a Nigerian semi-urban based hospital and performance of HRP-2 pf Rapid Diagnostic Test (RDT) in screening clinical cases of Plasmodium falciparum malaria. Translational Biomedicine.2011;2:1-5. doi: 10:3823/422
- Obimakinde ET, Simon-Oke IA. The Prevalence of malaria infection among patients attending the health centre of the Federal University of Technology, Akure, Nigeria. International Journal of Tropical Disease and Health. 2017;27(4):1-7. doi: 10.9734/IJTDH/2017/35340
- Okokon II, Ubong AU, Kenneth OI, Anthony AI. Climate and Plasmodium falciparum infection on the Jos Plateau, Nigeria. International Journal of Microbiology and Biotechnology. 2017);2:161-165. https://bit.ly/3qrGM7X
- Ofori M, Ansah E, Agyepong I, Ofori-Adjei D, Hviid L, Akanmori B. Pregnancy-associated malaria in a rural community of ghana. Ghana Med J. 2009 Mar;43(1):13-8. PMID: 19652749; PMCID: PMC2709171.
- Dada-Adegbola HO, Brown BJ, Labaeka AA. Prevalence of malaria and performance of a rapid diagnostic test for malaria in febrile children with sickle cell disease. Pediatric Hematology Oncology Journal. 2018;3(2018):42-45. doi: 10.1016/j.phoj.2018.06.003
- Olawumi HO, Fadeyi A, Babatunde SK, Akanbi AA 2nd, Babatunde AS, Sani MA, Aderibigbe SA. Malaria parasitaemia among blood donors in Ilorin, Nigeria. Afr J Infect Dis. 2015;9(1):10-3. doi: 10.4314/ajid.v9i1.3. PMID: 25722845; PMCID: PMC4325353.
- Belete EM, Roro AB. Malaria prevalence and its associated risk factors among patients attending Chichu and Wonago Health Centres, South Ethiopia. J Res Health Sci. 2016 fall;16(4):185-189. PMID: 28087849; PMCID: PMC7189928.
- Deku DJ, Lokpo YK, Kye-Amoah KK, Orish VN, Ussher FA, Esson J, Aduko RA, Dakorah MP, Osei-Yeboah J. Malaria burden and trend among clients seeking healthcare in the western region: A 4-year retrospective study at the Sefwi-Wiawso Municipal Hospital, Ghana. The Open Microbiology Journal. 2018;12:404-411. doi: 10.2174/1874285801812010404
- Omololu-Aso J, Omololu-Aso OO, Oluduro AO, Ajayi OO, Adejuwon A, Otusanya OO. Assessment of Plasmodium falciparum case-based surveillance at the two major University Teaching Hospital South Western Nigeria. A comparative study. British Biomedical Bulletin. 2017;5(2):300. https://bit.ly/3qsrYWM
- Adefioye OA, Adeyeba OA, Hassan WO, and Oyeniran OA. Prevalence of malaria parasite infection among pregnant women in osogbo, southwest, Nigeria. American-Eurasian Journal of Scientific Research. 2007;2:43-45.
- Ene AC, Atawodi SE, Ameh DA, Kwanashie HO, Agomo PU. Locally used plants for malaria therapy amongst the Housa, Yoruba and Ibo communities in Maiduguri, Northeastern Nigeria. Indian Journal of traditional Knowledge. 2010;9(3): 486-490. https://bit.ly/3lCN5SA
- Lagnika L, Djehoue R, Yedomonhan H, Sanni A. Ethnobotanical survey of medicinal plants used in malaria management in South Benin. Journal of Medicinal Plants Research. 2016;10(4):748-756. doi: 10.5897/JMPR2016.6219
- Oladunmoye MK, Kehinde FY. Ethnobotanical survey of medical plants used in treating viral infections among Yoruba tribe of South Western Nigeria. African Journal of Microbiology Research. 2011; (19):2991-3004. doi: 10.5897/AJMR10.004

Sign up for Article Alerts