Life-Saving Drugs in Sudan: A Matter of Definitions and Concepts
Esraa Mahadi Ali Mohamed1, Khalid Sayid Abdelgadir Habbani1 and Mohamed Awad Mousnad2*
1Alsudani Center for Training in Health Economics (ACTHE), Khartoum, Sudan
2Health Economic Centre, University of Khartoum, Khartoum, Sudan
*Address for Correspondence: Mohamed Awad Mousnad, Adjunct Assistant Professor, Health Economic Centre, University of Khartoum, Khartoum, Sudan, Tel: +249-912-325-864; ORCID: orcid.org/0000-0003-2680-2450; E- mail: m_abdalaziz@yahoo.com
Submitted: 10 December 2020; Approved: 04 January 2021; Published: 08 January 2021
Citation this article: Ali Mohamed EM, Abdelgadir Habbani KS, Mousnad MA. Life-Saving Drugs in Sudan: A Matter of Definitions and Concepts. American J Epidemiol Public Health. 2021 Jan 08;5(1): 001-000. doi: 10.37871/ajeph.id41
Copyright: © 2021 Ali Mohamed EM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Life-saving drugs; Essential medicines; Sudan; Pharmaceutical sector.
Download Fulltext PDF
Background: Drugs are one of the most crucial pillars in the provision of healthcare services and achieving Universal Health Coverage (UHC). Life-Saving Drugs (LSDs) are the drugs that save someone’s life, require immediate administration in most of the cases, as they sustain life, and prevent complications. They are top priority healthcare needs, which required to be available and affordable at all time in adequate quantities and low cost for the whole population and thus achieving equity. Since pharmaceutical sector in Sudan is highly fragmented, this study is conducted to determine the degree of stakeholders’ knowledge about the definition of LSD, whether there is a policy governing them, if they have a standard list and their availability status in Sudan.
Methods: Grounded theory qualitative (exploratory) study design. The theory is grounded from the actual data after being collected and analysed.
Results: Only essential medicines is the well-known term for the stakeholders -mainly at the governance level- with its policy and list. The other LSDs terminologies were found to be ambiguous, with no policies or standard lists. The status of the availability of the different LSDs terminologies (as it perceived by each stakeholder) was found to be poor and there is a significant shortage mainly due to the economic issues.
Conclusions: Unification of pharmaceutical organization in Sudan is a must and a priority issue due to its ultimate importance in the provision of healthcare services. Consensus and participation of all the relevant stakeholders in designing policies is an important input for a well-designed health system governance. Further researches in different areas must be conducted, and awareness of policymakers and service providers must be raised.
Key MessagesImplications for policymakers:
• All of the drugs specified to be priority LSDs should be unified in one list, a standard definition, under a policy that is disseminated for the whole country.
• Policy formulation should be in the bottom-up form to raise the awareness of all of the stakeholders.
• Manage between National Essential Medicines List (NEML) and Free of Charge (FOC) list in one policy that is disseminated and financed.
• Re-adoption of the pooled procurement method using one tender for the whole country to achieve equity through improved availability and affordability of quality medicines, reduce the expenditure on medicines, improve efficiency in utilization of country’s resources, and reduce fragmentation.
• The gradual adoption of the decentralization policy after successfully passing the transitional period and engagement of the community in the decision-making process, which will result in improving access to the local and community needs in a more focused pattern, more accountability and transparency for both government and community, and improve the community’s sense of ownership.
• A one-day workshop to discuss this issue can be conducted.
Implications for the public:
• Health system research to strengthen the governance system in Sudan.
• Policy brief through a systematic review for a better understanding of the issue.
• Operational research in the field for the rapid assessment of the lists and their need to update.
• Investigation of explanatory research to determine the reason behind the improved availability only at National Medical Supplies Fund (NMSF), but there is still significant shortage at Revolving Drug Fund (RDF) and thus the whole country, and determine whether it is an information system issues, financial hardships, or other factors are contributing.
• Further researches on the impact of LSDs shortage in the community must be investigated.
Abbreviations
DTC: Drug and Therapeutic Committee; EDM: Essential Drugs and Medicines Policy; EML: Essential Medicines List; FBPP: Federal Board of Pharmacy and Poisons; FMOH: Federal Ministry of Health; GDP: General Directorate of Pharmacy; LSDs: Life-Saving Drugs; NDP: National Drug Policy; NEDL: National Essential Drugs List; NMPB: National Medicines and Poisons Board; NMSF: National Medical Supplies Fund; RDF: Revolving Drug Fund; SMOH: State Ministry of Health; UHC: Universal Health Coverage; VEN: Vital, Essential, and Necessary or Non-essential; WHO: World Health Organization.
Background
Drugs play a prominent and crucial role in the provision of healthcare and thus achieving UHC. Many policies had been adopted to regulate and provide medicines through various scenarios [1]. LSDs generally means: the drugs that save someone’s life [2]. They were defined by the Drugs Bank in 2017 as: “Life Saving Drugs are those drugs which save lives in case of emergency. Also, these drugs have the capability to hold life or prevent further damage and complications. These drugs are used in emergency situation, intensive care unit. These drugs help patient close to life”. They are the drugs used for the treatment of specified emergency situations including: severe bleeding, hypertensive emergency, Myocardial Infraction (MI), respiratory failure, anaphylactic shock, status epilepticus, peripheral respiratory collapse, hypoglycemia, sever angina attack, pain relief in emergency rescue situations, acute asthmatic attack, anti-snake venom inj., rabies vaccine, and tetanus toxoid injection [3].
Many terms have been used interchangeably worldwide with a similar but paradoxical meaning including: Essential Medicines, Vital Drugs, Critical Drugs and Emergency Drugs. So as a situation under consideration that has been dealt with or discussed, matter has been mentioned to indicate this meaning [4]. A definition is: “the act of defining, or of making something definite, distinct, or clear [5]. There are three types of definition: formal definition which is used when the terms used are not familiar for the reader, informal definition is about using known words or synonymous to explain unknown terms, and extended definition that is consisting of both components of formal and informal definitions [6]. Any definition should imply certain criteria, including: clarity, integrity, usefulness, and withstand scrutiny [7]. Concept has been defined as an idea, thought, notion, mental representation, or theory about particular subject [8].
“Sudan, once the largest and one of the most geographically diverse states in Africa, split into two countries in July 2011 after the people of the south voted for independence.” It is a lower-middle income country with a high percentage of population living below poverty line [9].
In Sudanese health system, pharmaceutical sector has organizational, legal, and regulatory framework which consists of several key players including: The General Directorate of Pharmacy (GDP) of the Federal Ministry of Health (FMOH) who is responsible of the formulation and monitoring of policies such as the National Drug Policy (NDP), National Essential Drugs List (NEDL), and the Rational Drug Use (RDU). The second key player is the National Medicines and Poisons Board (NMPB) where the regulatory authority, quality control, marketing authorization, licensing, registration, the regulation and control of importation, exportation, manufacturing, distribution, sale, and use of the medicines, cosmetics and medical devices is its responsibility through Medicines and Poison Act. The third key player is the Central Medical Stores (CMS) which is now the National Medical Supplies Fund (NMSF), it is a semi-autonomous public organization, and it strives to function independently, delegated by the government to provide medical supplies for the entire population in Sudan. It is responsible of the selection, procurement, storage, and distribution of medicines. There is a project within the CMS called the Revolving Drug Fund (RDF) which is responsible of facilitation of supply and distribution of medical supplies to different parts of Sudan through its state branches. The last body is the Federal Board of Pharmacy and Poisons (FBPP), it has an informal role and considered as a coordination body in which all of the three bodies mentioned above are gathered. It was established in 2001 as a representative to the governmental, private and any other pharmaceutical sector, the Pharmacy and Poison Law, 2001 which covers all the regulatory related areas including marketing authorization, good manufacturing practice, licensing and inspection had been put by FBPP which is considered as a national medicines regulatory authority in which the plans are implemented through the directorate of pharmacy in each state who is considered as a regulatory body [10].
The term LSDs is ambiguous in many departments of FMOH/GDP, so there is important need to investigate and explore the basic definitions and concepts of the different LSDs terminologies.
This study is conducted to determine the degree of stakeholders’ knowledge about the definition of LSD, whether there is a policy governing them whether it is implemented or not, and if there is a standard list for LSD list that is used by the whole pharmaceutical sector. This study is also carried to determine whether there are shortages of LSDs (or the other differently used terminologies) at the public sector, to make the necessary reforms of the system, improving their availability and affordability, and achieving equity. Enabling evaluation of potential solutions for this issue is of a great importance and concern.
Materials & Methods
Study design
Grounded theory qualitative (exploratory) study design. The theory is grounded from the actual data after being collected and analysed. It is an interpretive (inductive) approach of generating a new theory of an unknown idea from the data that has been collected systematically.
Study area
All of the areas concerned with the provision of the required information were covered, including: (FMOH/GDP, SMOH/GDP, NMPB, RDF, NMSF, WHO).
In addition, five hospitals containing emergency departments including: Ibrahim Malik Hospital (IMH), Gaafar Ibn Ouf Hospital (GIOH), Khartoum Bahri Teaching Hospital (KBTH), Police Hospital (PH), and Sharg Elnile Hospital (SNH).
Study population
The study population were one or two representatives of each organization/health entity who found to be informative and helpful in the provision of the required data.
Operational definition of variables
Essential medicines: In 1976, Non-aligned Summit Conference was held in Sri Lanka which traced the concept of essential drugs that have been defined by the WHO as: “Essential medicines are those that satisfy the priority health care needs of the population and are intended to be available at all times in adequate amounts in the appropriate dosage forms”. However, essential drugs and medicines policy (EDM) is mainly aiming to save lives and procure LSDs [11].
Life-saving drugs: WHO has defined life-saving medicines as: priority medicines that improving health, saving lives, and having the biggest impact on reducing morbidity and mortality. All of the life-saving medicines are included within the Essential Medicines List (EML) and WHO treatment guidelines, with an exception for some drugs to prioritize those medicines can be used throughout healthcare systems [12].
Vital drugs: In November 2015, Jamaican Ministry of Health released the sixth edition of Jamaica’s Essential Drug List which reflects its policy as an element of the NDP, taking on the viewpoint of the WHO, whereas the drugs were classified according to their health impact and categorized into: Vital (V), Essential (E), and Necessary or Non-essential (N), which is called “VEN” analysis. The term “Vital Drugs” means the drugs that are potentially lifesaving, crucial to provide basic health services and of major public health importance, and considered as the ‘first line’ drug or the ‘drug of choice’. The “Essential Drugs” are those drugs which act as a back-up for the vital drugs, used for less severe but significant forms of illness, and they are the ‘second line’ drugs. The third category is the “Necessary or Non-essential Drugs” and these drugs considered to be of questionable therapeutic efficacy and higher cost, and they are used for minor or self-limiting illnesses [13].
Critical drugs: According to the National Health Services (NHS), UK, Critical Drugs have been defined as: the drugs administered to patients with life-threatening conditions, in which if its administration is omitted or delayed will result in a risk of patient harm. These drugs include: anticoagulants, antiepileptic, antimicrobials, antiplatelet & thrombolytic, antiviral, chemotherapy, clozapine, corticosteroids, desmopressin, immunosuppressant, insulin and other hypoglycemic agents, neuromuscular agents, opioid analgesics, Parkinson’s disease medications, and nebulized bronchodilator therapy [14].
Emergency medicines: Emergency medicines have been defined by the WHO as: “medicines used for the prevention and treatment of life-threatening emergency medical cases of the community [15].”
Concepts
Many studies revealed that the concept of LSD is adopted. A cross sectional study conducted in Tigray region, Ethiopia to assess the availability and affordability of LSD and it was found that the drugs are of lower availability and affordability, and higher prices which indicates a failure to implement health policies [16]. Also, In Jinja district of Uganda, a cross sectional survey was conducted in 32 lower level public facilities to investigate the availability and utilization for the LSD which have been recommended by the WHO to be provided for the children under 5 at Uganda’s public health facilities and the priority LSD for diarrhea and sepsis were available and highly prescribed, where the pneumonia and malaria medications availability and utilization was very low at Uganda’s public health facilities [17]. In Tanzania a study conducted to describe the experience of the managers of the rural health facilities, ensuring the availability of drugs and medical supplies for emergency obstetric care. It was found that the obtained emergency obstetric drugs and supplies were unreliable, the funds provided by the government were insufficient, and the lack of accountability within the drug supply system, all were contributing to this shortage. Many approaches must be used to tackle the problem of accessibility to essential drugs for maternal health, such as: improving governance of the drug delivery system, enhancing accountability and transparency of information and drug funds, and involvement of stakeholders in decision making [18].
Policy issues
In Sudan, NDP has been formulated by the Ministry of Health in 1981, in which its first component to be implemented was the selection of National List of Essential Drugs (NLED), which was published in 1982, and then updated in 1985 in the workshop conducted in Khartoum. It was revised in 1987 by the Technical Committee for Drug Products Registration where it is firstly adopted by Ministerial Order. In 2004, the NLED name was changed to National Essential Medicines List (NEML) by the WHO. In 2013, consensus on the updated NLED was convened in the Khartoum’s NEML Consensus Workshop [19].
South Africa’s NDP has been established in 1993, and the responsibility of its implementation was delegated to the South African Drug Action Program (SADAP), which coordinates the activities and strategies of the different stakeholders, and also responsible of the policy monitoring and evaluation. This policy was found to address many issues, including: drugs pricing, inspection, development of essential drugs list and treatment guidelines, ensure effective procurement and distribution of medicines, licensing, promote availability with the lowest possible cost, rational prescribing and dispensing, good storage practice and management of expiry, and development of human resources for effective implementation of the policy [20].
Systems for Improved Access to Pharmaceuticals and Services (SIAPS) Program in Ethiopia, is mainly responsible of achieving desired health outcomes through ensuring availability of quality pharmaceutical products and effective pharmaceutical services. SIAPS program had conducted many interventions and achievements including: strengthen pharmaceutical sector governance (laws, regulations, legislations, policies, guidelines, and standard operating procedures) so as to improve transparency, accountability, and efficiency, development of the drugs formulary has a key step in prioritizing LSD for maternal and child health at all levels of health system, emphasize the importance of Pharmacy Directorate as a separate body at FMOH which help creating leadership for the pharmaceutical sector at both national and regional levels which will result in sustainability, structuring of in-service training to raise the awareness of policy makers, and managers at FMOH and hospitals [21].
A qualitative research was conducted in Kenya to investigate the impact of engagement of external actors in the improvement of the NDP (pharmaco-governance), and addressing the pharmaceutical sector fragmentation issue. It was found that the complete dependency on external actors is inappropriate, and they can be only engaged in consultative, collaborative and cooperative procedures, while depending on internal actors [22].
List
WHO stated that the selection of drugs on EML is the responsibility of Drug and Therapeutic Committee (DTC) based on the standard treatment guideline in the hospital or the specified health facility [23].
Also, the quantification of drug requirements at national or regional level is a responsibility of multidisciplinary team of pharmacists, epidemiologists, physicians, and administrators that are aware of drug management processes, and informed about the use of epidemiological information [24].
Availability status and issues
Many initiatives have been done in many countries to improve the availability of LSD. Free of charge project was adopted in Sudan in 1996 and implemented in 2005. It was mainly aiming to provide the most urgently required medicines without any fees for the public sector health facilities. It was found to be useful for urban hospitals, but interrupted in rural ones. As a result of this free project with its separate list, the NEML in Sudan became neglected [25].
In Somalia, there were many activities proposed by clusters in response to IDPs in Kimaiyo and Baidoa included but not limited to: introduction of a funding modality called “Reverse Plus” for better prioritization, providing medical supplies and LSDs to strengthen primary health facilities, and regular provision of them [26].
In Australia there is an initiative to subsidize the patients with rare diseases that require expensive medications to improve their access to LSDs, which called Life Saving Drugs Program (LSDP). It has been found through this technical assessment that the drugs funded by LSDP are both safe and effective, but in some cases, they are facing uncertainty, and sustainability problems. To tackle these problems many approaches must be adopted such as registry for drug surveillance to address the uncertainty and ensure whether the drugs are performing as expected, especially when governance, and resourcing are going in the right direction [27].
In Ethiopia interviews were conducted with health facility staff and revealed that facilities use their budget to procure essential drugs, with a priority given to LSD such as antibiotics and antimalarial. Rational Pharmaceutical Management (RPM) Plus and the United Nations Children’s Fund (UNICEF) carry out drug procurement for U.S. government-supported PMTCT program sites. This program is aiming to strengthen existing structures and improve the availability of supplies at the facility level [28].
In Philippines, from 2011 to 2016, strategic directions to improve the access for medicines have been developed. In Philippines, at both national and local levels the drug procurement system is fragmented, the drug prices are widely variable due to the disunited financing and distributing chain, lack of transparency and clear objectives which regulate the prices, and uneven availability of drugs at the public sector [29].
Lack of access to essential medicines in developing countries lead to creation of a medicine patent pool through non-governmental organizations, private sector, and stakeholders’ partnerships that was responsible of the licensing process, facilitate the production and distribution of affordable medicines and ensure access to life-saving treatments [30].
Across national boundaries, pooled procurement was found to be a very effective methodology that consolidate purchasing having a greater benefit represented in decrease of the purchasing prices, increase in the quality of medicines, elimination of corruption, more informed selection, less operational costs, improved equity, and finally improved access to essential medicines [31]. On the basis of this study, NMSF has conducted a retrospective study tender procedures starting from the bids and up to awarding the contract. All of the public health organizations in Sudan (Armed forces medical services (police and military), National Health Insurance Fund (NHIF), and RDF of Khartoum and the other states were included in this event. It was characterized mainly by the high transparency, security, low cost, increased purchasing power with negotiable prices in which the saving that had been made from this initiative reach Euros 10 million in 2015, for the first time in Sudan’s history [32].
Sampling technique
Non-probability purposive sampling. Data was collected until the principle of saturation was reached.
Inclusion criteria: All of the stakeholders who seem to be informative about the issue, including: stakeholders at governance level, service providers, and WHO.
Exclusion criteria: Any other component of pharmaceutical sector outside the research border, such as: community pharmacies, drug companies, and pharmaceutical industries.
Data collection methods and tools
In-Depth Interviews (IDIs) using an interview guide were used as a data collection technique and tool respectively. Document review was the second data collection method that has been used to fulfill the process of triangulation and reduce systematic bias.
Data analysis method
Data were analyzed using grounded theory framework approach. An inductive approach has been used to create an understanding between the research objectives and the summary of the findings from the interviews [33]. A coding frame applied for conceptualization of the data.
Ethical consideration
The approval of this study was obtained from the Research and Ethical Committee from University of Medical Sciences and Technology and Khartoum state Ministry of health research department and other health institutions. The participants have right to voluntary informed consent, ensuring privacy and confidentiality.
Results
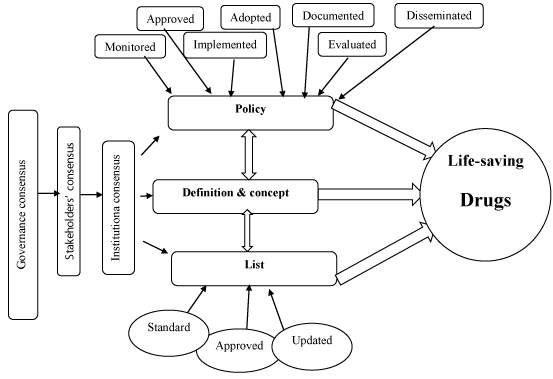
Definitions and concepts
All of the respondents at governance level were mainly familiar with the term “essential medicines”, whereas service providers were not.
Discrepancies were clear between the respondents in defining all of the other terms “life-saving drugs, vital drugs, emergency medicines, and critical drugs”; since any one or two of the study participants has/have different response than the others.
Although there was a consensus in defining the term “vital drugs as a life-saving drugs” but still the concept is not clear, because the respondents were unfamiliar with what is LSDs its self.
Policy
Stakeholders at governance level were familiar with the availability of essential medicines policy, whereas service providers were not.
Two participants from the same governance body (FMOH) were not agreed about the process of policy operation (FMOH/K1, and FMOH/K2). Also, another two participants from the same governance level (NMPB, and NMSF), their response regarding the evaluation of the policy was differing. The process of policy operation was not clear for the other study participants -service providers- (IMH, KBTH, GIOH, PH, and SNH).
Study participants at governance level were all agreed that the responsibility of policy implementation is delegated to GDP/SMOH. Service providers were not familiar with the process.
List
There is a consensus by all study participants that essential medicines have a standard list. FMOH/K1 and FMOH/K2 agreed that LSDs are included within the FOC list. NMSF and NMBP each using their own lists in which policy stated that they have to apply EML and they are not allowed to tailor another list, and they both have a common drug with NEML but adding or excluding some medicines according to the needs and the institutional objectives. Service providers (IMH, KBTH, GIOH, and SNH) all obtaining their lists from the FOC list and not the NEML. PH obtaining their list from the NEML. All of the lists mentioned above are documented and reviewed.
All of the study participants agreed that the establishment of the NEML is the responsibility of GDP/FMOH, whereas FOC list is the responsibility of Curative Medicine Department/FMOH. NMPB and NMSF established their own lists.
NEML is last updated in 2014, which has to be updated every 2-3 years as has been stated by the policy.
The drugs of the NEML are selected through a selection committee. NMPB drugs are selected based on the most common chronic diseases. NMSF list selected based on VEN classification through a task force. Hospitals select their lists according to their needs. Policy stated that: “Commitment and regulation of the national list of essential medicines in all public sector institutions. The drugs should be selected through selection committee”.
Availability of policy governing availability, procurement, licensing, distribution, financing, prescribing, dispensing, storage, and expiry of the drugs on EML were not clear for all respondents at governance level (each has varying response). Since NMSF has their own list “they have policy governing the availability, procurement, distribution, financing, storage, and expiry of the drugs at the VEN classes”, and NMPB “started to make focus programs for LSDs, their availability and affordability, and aiming to establish a policy of their LSDs list”. Others wasn’t clear about the process.
Various responses obtained from the different stakeholders regarding whether the list is responding to the needs of the community or not. The policy stated the list established to address the real health needs of the citizens.
At governance level different bodies were found to be responsible of the quantification of the community’s needs, whereas the policy stated that the responsibility is delegated to GDP and medical consultants. In hospital -service providers- the responsibility of the quantification is differing from one to another hospital.
Availability
Regarding the status of the availability of medicines, there were a consensus that there is a significant shortage of LSDs due to the economic instability. Many factors contributing to this shortage were mentioned by the respondents including: there is no specified body responsible of organizing and governing the medical services, there is no priority settings, the conflicting interests of the stakeholders, the importation problems, limited number of drugs at the NMSF’s list, the large number of patients (not less than 30.000 patient/month), the limited funding, sorting out problems between cold and emergency cases, documentation problem, no restrictions for the distribution of the drugs from RDF to the hospitals, no transportation system from the RDF to the hospitals but each hospital has to prepare its transportation by its self, the managerial/administrative problems; no rewarding system which affects the satisfaction of health workers, political instability, irrational use, and the poor prescribing and dispensing behaviors.
Unlike all of the above-mentioned responses, NMSF stated that the availability of drugs is improved, mainly vital drugs, from 46% in 2009, up to 91% in 2019, and the factors contributing for this improvement: investment in training for ordering system and quantification, and improvements in the monitoring system. The drugs other than vital might have shortage issues. This might be an indication that the drugs are available at NMSF but not affordable because they are not included in the free of charge or EML, so they cannot be obtained by RDF and thus hospitals.
Discussion
Production of scientific knowledge has a greater impact on policy decision-making, formulation, and implementation through creating a concrete evidence supporting or challenging the existing inventions and call for change [34].
The definition and concept of essential medicines were obvious for all the stakeholders at governance level. There was a consensus, as they have defined the term as it was defined by the WHO using the same keywords of the standard definition [11]. They were also found fulfilling the criteria of the definition starting from clarity, integrity, and up to usefulness [7]. However, service providers were not informed about the definition of essential medicines; since the concept of essential medicines is old and there is a lack of reminding update that might have a negative impact regarding the awareness of the new generations about it. Another factor contributing to the poor knowledge of the service providers about the definition of essential medicines is a policy dissemination issue.
The term LSDs has been defined differently –no consensus- by the different study participants. It has been defined informally and each of the respondents was using synonymous trying to explain the term, for example, (SMOH, IMH, KBTH, and RDF) have concurred that: “LSDs are emergency drugs” while (FMOH/K2, SNH, and GIOH) have the same definition of LSDs which is: “they are the drugs that used in critical situations”, (SMOH) added that “they are part of the essential medicines”, and PH thinks that “LSDs are those in class V&E of the VEN classification, however, all of them neither fulfill the criteria of the definition ( no consensus which means the definition is not clear) of the term ‘life-saving drugs’ [7], nor mimic the standard definition of LSDs (12). However, here in Sudan the definition and concept of LSDs is completely vague unlike many other neighboring countries, for example, Ethiopia, Uganda, and Tanzania that moved from the general understanding of the concept to the affordability issues of LSDs [16-18].
All of the other terminologies ‘emergency medicines, vital drugs, and critical medicines’ have also been defined informally, overlapping and confusion in defining them were prominent despite the fact that many international health references such as WHO, and NHS have clearly defined them [12-15]. Although there was a consensus regarding the definition of the term ‘vital drugs’ that they are LSDs, but the concept is still unclear since the meaning of the term ‘life-saving drugs’ itself is unknown. So, the synonymous doesn’t reflect the original [13].
The essential medicines’ policy is poorly functioning due to the fragmentation of the pharmaceutical sector. The fragmented pharmaceutical sector in Ethiopia passively affect pharmaceutical sector governance [21]. Also, in case of Kenya, the fragmented pharmaceutical sector was found to have a negative impact on implementation of NDP [22]. In reverse to this, the consensus between the stakeholders in the case of pooled procurement that had been made in Sudan resulted in a very affirmative outcome for the whole country represented in more quality drugs (the main aim), with a reduced cost, and savings up to Euros 10 million [31,32]. Also, another study confirmed that medicines patent pool through stakeholders’ partnership will result in the increased access of LSDs [30].
There is a consensus that essential medicines have a standard list (NEML) that have been established through GDP and lastly updated in 2014 (must be updated at least every 2-3 years) [19]. Free of charge (FOC) project has also a standard drugs list that has been established by Curative Medicine Department/FMOH, which has no definition or policy but it is adopted and disseminated as a list. In FMOH, two key informants agreed that LSDs are included within the free of charge list. However, practically the NEML is neglected and the more concern is for the FOC list (hospitals derive their lists from it); the drugs selected on NEML are more concerned about the cost-effectiveness and affordability criteria rather than the importance and life-saving action. The discrepancy is clear that the NEML has a policy but not operating, while FOC list has no policy but operating; and that is mainly due to the priority issues of service providers who are more concerned with dispensing of medicines and those included within the FOC list are fully financed from the government.
FMOH, NMPB, and NMSF are all at the same governance level under FBPP which is responsible of the national medicine regulatory authority of the country [10]. NMPB and NMSF each has their own list other than NEML and FOC lists that has been chosen according to institutional objectives and requirements. This indicates that FBPP is not functioning, overriding policy statement “commitment and regulation of the national list of essential medicines in all public sector institutions” [35], and also an indication of the fragmentation of pharmaceutical sector.
In hospitals the responsibility of drugs selection is different from one hospital to another where it might be delegated for medical supplier, head of pharmacy, or quality department. WHO stated that “there must be a specified DTC in each hospital taking the responsibility of the selection of the drugs in the list”, without surpassing anybody of concern, because the poor drug selection will result in waste of resources [36].
Quantification of the populations’ needs is crucial to determine the number of selected drugs requires to cover the population, and provide the required financial means. Stakeholders’ responses on whether the list is responding to the needs of community or not was varied. However, one of the main pillars of the policy is to address the populations’ most common needs. The quantification of the community’s needs conducted by different bodies in health institutions at governance level and service providers, despite of quantifying the needs of the same community. This responsibility must be delegated to a predetermined staff of pharmacists, physicians, epidemiologists, and managers who are familiar with the use of epidemiological data, and the drug management procedures [24].
Upon list review, NMSF’s VEN list as has been stated by the respondent, was found to be the most comprehensive list sharing many drugs with the NEML in addition to other medicines that are not available at NEML, which found to be basic drug requirements for many sectors within the pharmaceutical organization, including: armed forces, health insurance schemes, and population at the states. Vital (life-saving) drugs in VEN represent 17% of the total number of medicines. Most of the drugs on the FOC list is included within NEML with exception to IV fluids and many other items. Hospitals lists has been chosen based on FOC list (and not NEML) according to their needs, with exception to police hospital that use NEML because it is directly supplied from NMSF. WHO critical or LSDs list including all of the drugs in NEML in addition to another drugs [37]. Many countries such as Egypt and India have a standard LSDs list that is used by the whole country, unlike the situation of Sudan [38,39].
The existence of policy governing availability, procurement, licensing, distribution, financing, prescribing, dispensing, storage, and expiry of the drugs on EML was ambiguous for all the stakeholders at all levels. NDP, for example, in case of Sudan and South Africa both emphasize that the policy should cover those key issues have been mentioned above and the policy strategies and activities to achieve them [19,20,35].
Regarding the availability status of LSDs in Sudan, whatever the term used or the definition is, there was a consensus –by the majority of respondents- that there is a significant shortage that is mainly due to the economic instability and deficiency of the hard currency. Free of charge project was introduced to address this issue of availability, but still the subsidy provided by the government was found to be insufficient to cover the populations needs, and all of the hospitals revealed that the insufficient subsidy from the government lead them sometimes to use their own budget for procurement of LSDs which exactly resemble the situation of Ethiopia which is then supported through U.S. government-supported PMTCT program by the aid of UNICEF (25,28). Also, in Somalia drug shortages were mainly due to economic issues in which new funding modality was introduced to tackle this problem [26]. In Australia the affordability of LSDs for patients with rare diseases were improved through a subsidy program called Life-saving Drugs Program (LSDP) [27].
All of these factors are resulted from the fragmentation of the pharmaceutical sector which affect the access of medicines, resembling the issue of Philippines in which the drug procurement system is fragmented at both national and local levels [29].
NMSF stated that the availability of drugs is improved, mainly vital drugs, from 46% in 2009, up to 91% in 2019 and drugs other than vital might have shortage issues. This might be an indication that the drugs are available at NMSF but not affordable because they are not included in the free of charge or EML, so they cannot be obtained by RDF and thus hospitals.
Therefore, only essential medicines is the well-known term for the stakeholders -mainly at governance level- with its policy and list. The other LSDs terminologies were found to be ambiguous, with no policies or standard lists. The status of the availability of the different LSDs terminologies (as it perceived by each stakeholder) was found to be poor and there is a significant shortage mainly due to the economic issues.
Finally, LSDs is an open term that has no any boundaries, and any drug can be LSD under a certain condition. That doesn’t prevent to have a consensus at each level (emergency, ICU, and CCU) for priority LSDs lists that must be affordable, free of charge, for the whole population regardless of their socioeconomic status, even if they are not included in the free of charge list, and only available, for example, in private sector.
Conclusion
In Sudan, the only term that was found to be clear for the stakeholders - at governance level mainly- was essential medicines, its definition, policy, and the standard list. However, all of the other synonymous of the term LSD were defined informally, with no consensus between the stakeholders in one definition, policy, or list. There is a consensus that LSD with their different terms recognized by the stakeholders are in a significant shortage in Sudan, which resulted from the economic and political constraints. Governance is central to achieve UHC, and consensus and unification of pharmaceutical organization in Sudan is a must and a priority issue due to its ultimate importance in provision of healthcare services.
Authors’ Contributions
E.M and K.H conceived of the presented idea. E.M developed the theory and performed the computations. M.M. and K.H verified the analytical methods. K.H encouraged M.M to investigate and supervised the findings of this work. E.M and M.M drafted the manuscript. All authors discussed the results and contributed to the final manuscript. K.H and M.M. are guarantor of the paper.
- Bigdeli M, Laing R, Tomson G, Babar ZU. Medicines and universal health coverage: challenges and opportunities. J Pharm Policy Pract. 2015 Feb 16;8(1):8. doi: 10.1186/s40545-015-0028-4. PMID: 25825675; PMCID: PMC4350289.
- Collins Dictionary. Definition, Thesaurus and Translations [Internet]. Collinsdictionary.com. 2019. https://bit.ly/2Lk3ZsB
- Life Saving Drugs - DrugsBank [Internet]. DrugsBank. 2019. https://bit.ly/2LugWAk
- MATTER. Meaning in the Cambridge English Dictionary [Internet]. Dictionary.cambridge.org. 2019. https://bit.ly/2L5cY13
- The definition of definition [Internet]. www.dictionary.com. 2019. https://bit.ly/2KWNxPj
- NROC Developmental English Foundations [Internet]. Content.nroc.org. 2018. https://bit.ly/3bdWFtm
- Smithy Smithy [Internet]. Stoyko.net. 2019. https://bit.ly/3bapGGs
- CONCEPT. Meaning in the Cambridge English Dictionary [Internet]. Dictionary.cambridge.org. 2019. https://bit.ly/3hJpY8w
- Sudan country profile. 2019. https://bbc.in/3okZoVM
- Sudan Pharmaceutical Country Profile. 2010. https://bit.ly/3pWEs82
- Quick JD, Hogerzeil HV, Velasquez G, Rago L. Twenty-five years of essential medicines. Bull World Health Organ. 2002;80(11):913-4. PMID: 12481216; PMCID: PMC2567676.
- Priority life-saving medicines for women and children 2012. World Health Organization. 2012. https://bit.ly/35eG1WC
- World Health Organization. List of vital essential and necessary drugs and medical sundries for public health institutions. 2019. https://bit.ly/2LphaIK
- National Health Services. Ipswich and east Suffolk. 2017. https://bit.ly/3rXciLR
- Alamne Y. Emergency Medicines List (EML). WHO. 2014. https://bit.ly/3nhRv1V
- Abrha S, Tadesse E, Atey TM, Molla F, Melkam W, Masresha B, Gashaw S, Wondimu A. Availability and affordability of priority life-saving medicines for under-five children in health facilities of Tigray region, northern Ethiopia. BMC Pregnancy Childbirth. 2018 Nov 29;18(1):464. doi: 10.1186/s12884-018-2109-2. PMID: 30497441; PMCID: PMC6267819.
- Nsabagasani X, Ogwal-Okeng J, Mbonye A, Ssengooba F, Muhumuza S, Hansen EH. Availability and utilization of the WHO recommended priority lifesaving medicines for under five-year old children in public health facilities in Uganda: a cross-sectional survey. J Pharm Policy Pract. 2015 May 11;8(1):18. doi: 10.1186/s40545-015-0038-2. PMID: 25995847; PMCID: PMC4438460.
- Mkoka DA, Goicolea I, Kiwara A, Mwangu M, Hurtig AK. Availability of drugs and medical supplies for emergency obstetric care: Experience of health facility managers in a rural District of Tanzania. BMC Pregnancy Childbirth. 2014 Mar 19;14:108. doi: 10.1186/1471-2393-14-108. PMID: 24646098; PMCID: PMC3995093.
- World Health Organization. National Essential Medicines List 2014. https://bit.ly/3pUGcOO
- Gray A, Suleman F, Pharasi B. South Africa’s National Drug Policy: 20 years and still going?. South African Health Review. 2017; 2017: 49-58. https://bit.ly/2Mynazn
- SIAPS Ethiopia End of Project Report. SIAPS program. 2017. https://bit.ly/35dMm4X
- Moscou K, Kohler JC. Matching safety to access: global actors and pharmacogovernance in Kenya- a case study. Global Health. 2017 Mar 23;13(1):20. doi: 10.1186/s12992-017-0232-x. PMID: 28335786; PMCID: PMC5363016.
- Holloway K, Green T. Drug and therapeutics committees: A practical guide. In drug and therapeutics committees: A practical guide 2003. 146-146.
- Management Sciences for Health. MDS-3: Managing access to medicines and health technologies. https://bit.ly/3pV2JeE
- Elgozoli B. Assessment of Emergency Free Medicines Project: Experience of the Blue Nile State – Sudan. Sudan Medical Specialization Board. Pharmacy Specialization Board. 2007. https://bit.ly/3hOdwV9
- Somalia Humanitarian Fund. Revised Strategic Reserve Allocation 2016. https://bit.ly/2MJyCbI
- Department of Health. Other supply arrangements outside the Pharmaceutical Benefits Scheme (PBS) – the Life Saving Drugs Program [Internet]. Health.gov.au. 2019. https://bit.ly/35lmHqW
- World Health Organization. Developing Commodity Procurement Support for the Ethiopia PMTCT Program. Management Science for Health RPM Plus. 2018. https://bit.ly/3hUroNr
- Jean BP, Ala M, David L, Valera M, Ronquillo K, Solon K et al. Strategic Directions to Access to Medicines Filipinos 2011-2016. The Philippine Medicines Policy. [internet] 2011. https://bit.ly/35iAdvC
- Stevens H, Huys I. Innovative Approaches to Increase Access to Medicines in Developing Countries. Front Med (Lausanne). 2017 Dec 7;4:218. doi: 10.3389/fmed.2017.00218. PMID: 29270407; PMCID: PMC5725781.
- Huff-Rousselle M. The logical underpinnings and benefits of pooled pharmaceutical procurement: a pragmatic role for our public institutions? Soc Sci Med. 2012 Nov;75(9):1572-80. doi: 10.1016/j.socscimed.2012.05.044. Epub 2012 Jul 11. PMID: 22835922.
- Ali GK, Fundafunda B. The National Medical Supply Fund and E-Procurement Services- A Success Story. 2015
- Soklaridis S. The process of conducting qualitative grounded theory research for a doctoral thesis: Experiences and reflections. The qualitative report. 2009;14(4):718-33. https://bit.ly/2XnnXWg
- Almeida C, Báscolo E. Use of research results in policy decision-making, formulation, and implementation: a review of the literature. Cad Saude Publica. 2006;22 Suppl:S7-19; discussion S20-33. doi: 10.1590/s0102-311x2006001300002. PMID: 17086340.
- National Drug Policy [Internet]. Apps.who.int. 2005-2009. https://bit.ly/3be7NXa
- Holloway K, Green T. Drug and therapeutics committees: A practical guide. In Drug and therapeutics committees: a practical guide. 2003. 146-146. https://bit.ly/2LnmmNt
- World Health Organization. Critical or Life-saving Drugs [Internet]. Apps.who.int. 2015. https://bit.ly/3okfP4E
- Abdelrahman AA, Saad AA, Sabry NA, Farid SF. Perceptions of Egyptian physicians about drug shortage during political disturbances: Survey in Greater Cairo. Bulletin of Faculty of Pharmacy, Cairo University. 2016 Dec 1;54(2):191-6. doi: 10.1016/j.bfopcu.2016.05.004
- Dharan L. List of Life Saving Drugs [Internet]. data.gov.in. 2012. https://bit.ly/2LliGf0

Sign up for Article Alerts