A Pilot Study: Metal-Induced Immunotoxicity and Deaths of the 100 Vaccinees in the Republic of Korea for 2 Months of 2020 Flu Vaccination
Munhee Jeon<1, Jongsung Oh2, Kyu Yun Jang3 and Ki-Yeob Jeon4*
1Department of Surgery, Presbyterian Hospital, Jeonju, 54987, the Republic of Korea
2Department of Orthopedics, Jeonbuk National University Hospital, JBNU, 54907, the Republic of Korea
3Department of Pathology, Jeonbuk National University Medical School, 567 Baekje-daero, 54836, the Republic of Korea
4Hopkins Jeonil Internal Medicine Clinic, Song-cheon-Joong-ang-Ro 154, 54836, the Republic of Korea
*Address for Correspondence: Ki-Yeob Jeon, Hopkins Jeonil Internal Medicine Clinic, Jeonju, 54836, the Republic of Korea (South Korea), Tel: +82-107-701-5621; E-mail: kjeon@hanmail.net
Submitted: 12 January 2021; Approved: 30 January 2021; Published: 01 February 2021
Citation this article: Jeon M, Oh J, Jang KY, Jeon KY. A Pilot Study: Metal-Induced Immunotoxicity and Deaths of the 100 Vaccinees in the Republic of Korea for 2 Months of 2020 Flu Vaccination. American J Epidemiol Public Health. 2021 Feb 01;5(1): 014-021. doi: 10.37871/ajeph.id43
Copyright: © 2021 Jeon M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Type IV hypersensitivity; Complement Activation-Related Psudoallergy (CARPA); Immunotoxicity; Mercury; Cadmium; Influenza vaccine; COVID-19 vaccine; Vaccine Adverse Event Reporting System (VAERS)
Download Fulltext PDF
Summary: More than 100 persons died within 7 days of influenza vaccination by November 2020 (for two months of 2020 flu vaccination) in the Republic of Korea (South Korea). The current study was conducted to allocate properly any possible causality by examining the presence of heavy metals in a vaccine and metal-induced immunotoxic lesions after flu vaccinations in the experimental mice. It detected cadmium 0.12 ppb (parts per billion = µg/L) and mercury 1.77 ppb in one of the cost-free influenza vaccinees (Lot Number: A14720017) distributed by the Korean government. Lungs of the undiluted-vaccine-injected mice showed significantly more diffuse inflammatory damages than lungs of the 1:4 diluted-vaccine-injected mice which showed no to mild inflammatory changes (p < 0.027 by the method 1, and p < 0.001 by the method 2). Based on this study, it can be presumed that the metals-induced immunotoxicity of type IV hypersensitivity or of psuedoallergy would have caused death in some of persons who coincidentally died within 7 days of vaccinations.
Background: A 17-year-old man died within three days of influenza vaccination (Lot Number: A14720007), a 77-year-old woman died within a day (Lot Number: A14720016), and more than 100 persons died within 7 days of influenza vaccination by November 2020 (for two months of 2020 flu vaccination) in the Republic of Korea (South Korea). Singapore authorities halted two of the seven brands of flu vaccinees even though there was no report of death after flu vaccinations in Singapore. This raises a possibility that there can be a difference between the excipients of the flu vaccinees used in Singapore and in Korea. Our assumptions were that there would have been immunotoxic metals in the flu vaccinees, the metals would have induced type IV hypersensitivity or Complement Activation-Related Psuedoallergy (CARPA), and would have caused some deaths of 100 persons who incidentally died within 7 days after flu vaccinations.
Methods: In this study, we analyzed twice for the presence of any metal components of aluminum, indium, cadmium, gallium, and mercury in the influenza vaccine. Analysis of the metal contents of the 1:29 diluted flu vaccine was assessed by the Inductively Coupled Plasma Mass Spectrometry (ICP-MS) method. Simultaneously, total 10 BALB/c mice were used to analyze any pathological changes after 7 days of flu vaccination. Animals were divided into two groups: one group of 5 mice were injected intraperitoneally with 0.1 ml of 1:4 diluted flu vaccine with injectable distilled water; and the other group of 5 mice were injected intraperitoneally with 0.1 ml of undiluted flu vaccine. They were freely reared for 7 days in a Polycarbonate cage (400 x 255 x 180 mm). The mice were sacrificed after CO2 short-acting gas anesthesia. Brains, hearts, lungs, livers, and kidneys were harvested, prepared with H & E stain, and observed for any histopathological changes.
Findings: In one of the cost-free influenza vaccinees (Lot Number: A14720017), which were distributed by the Korean Government, the current study detected cadmium 0.12 ppb (parts per billion = µg/L), and mercury 1.77 ppb. But neither aluminum, gallium, nor indium was detected.
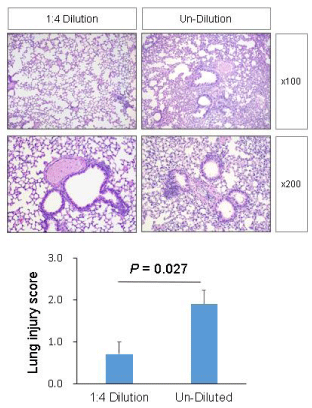
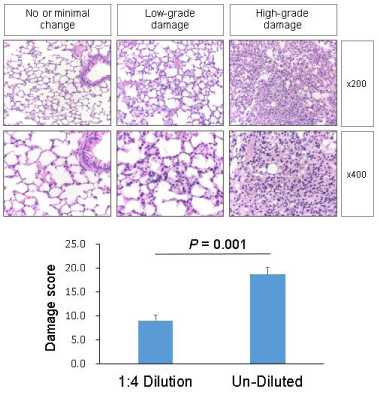
Both experimental groups showed no demonstrable inflammatory changes in the specimens of brains, hearts, livers, and kidneys. However, lungs of the undiluted-vaccine-injected group showed significantly more diffuse damages than lungs of the 1:4 diluted-vaccine-injected group which showed no to mild inflammatory changes. The semiquantitative scores of the diluted-vaccine-injected group and the undiluted-vaccine-injected group were 0.7 ± 0.3 and 1.9 ± 0.3, respectively by method one ([a street-view], mean ± SE, p = 0.027 <0.05); and 9.0 ± 1.1 and 18.6 ± 1.6, respectively by method two ([a sky-view], mean ± SE, p < 0.001).
Interpretation: Mercury (1.77 ppb) and cadmium (0.12 ppb) were found in the freely distributed influenza vaccine by the Korean Government for the season of 2020-2021. Inflammatory damages in the lungs of experimental mice, which occurred within 7 days after influenza vaccination, could be caused by metal-induced type IV hypersensitivity (delayed-type, T-cell-mediated hypersensitivity) or metal nanoparticle-induced CARPA. In application, metal-induced delayed-type hypersensitivity or metal nanoparticle-induced CARPA could explain some deaths of the 100 persons who unintentionally died within 7 days of influenza vaccination by the November 2020 and of the 1,531 persons who coincidentally died within 7 days after Influenza vaccination from the fall of 2019 to the spring of 2020 in South Korea, and of the persons who fortuitously died within one week after influenza vaccinations in the United States-23.2persons/100,000 vaccinees of an age of over 75 and 11.3 persons/100,000 vaccinees of an age between 65 and 75. The results may be helpful for the causal identification of some deaths of COVID-19 vaccinees.
Introduction
Annual vaccination is recommended for every eligible person over 6 months-of-age because flu vaccinations can reduce the prevalence of influenza, the incidence of influenza by 45%, the risk of serious complications and death especially in children and elderly persons [1]. Quadrivalent vaccine, intranasal live-attenuated vaccine, recombinant vaccine, adjuvanted vaccine, and/or high-dose vaccine are options of flu vaccinees recommended for feasible persons. Known adverse effects of flu vaccinees are Guillain-Barre syndrome (about 1-2 additional cases per million vaccinee), influenza infection (about 17 cases per million vaccinee), severe allergic reactions of patients with egg allergy, generalized aching and tenderness at the injection site [1]. CDC estimated that there are 0.27 to 0.71 deaths/4,000 persons (or 6.9 to 17.7 deaths/100,000 persons or 24,000-62,000 flu deaths among 350,000,000 persons in the United States) of flu [2] and that one elderly life was saved for every 4,000 elderly vaccinees [3] in the United States.
There are accidental deaths associated with vaccinees. It was reported that persons who died within one week after influenza vaccination were 23.2/100,000 vaccinees of an age of over 75 and 11.3/100,000 vaccinees of an age between 65 and 75 in the United States [4]. During the 2019-2020 flu season, about 3,000 people died because of flu in the total population of 55,000,000 persons in the Republic of Korea. This means that 5.5 deaths/100,000 persons (0.22 death/4,000 persons) of flu death, where 0.92 deaths/4,000 vaccinees (1,531 persons accidentally died within one week of flu vaccination/ 6,680,000 flu vaccinated persons). It also means that 51% of the total national flu death-number of about 3,000 haphazardly occurred among the flu vaccinees within one week of the influenza vaccination. No direct causal relationships between the flu vaccination and deaths were noticed, but they were regarded as examples of a conditional probability which occurred accidentally [4].
It is worth mentioning that there are confirmed cases of interstitial pneumonia, which occurred associated with the influenza vaccine in one or two months later of the flu vaccinations, and which were aggravated with antibiotics before being cured with supportive care with mechanical ventilation, intravenous methylprednisolone and oral prednisolone [5]. Although its use was recently decreased, thimerosal (organic mercury) has been a common ingredient of vaccinees to serve the purposes of antiseptics and preservatives. It was reported that 10.1% of 1094 children with skin disease showed thimerosal-induced delayed-type hypersensitivity [6], fewer than 10% of 3,162 patients who were suspected for metal allergy showed thimerosal-specific lymphocyte responses by LTT-MELISA test [7]. Heavy metals such as thimerosal or cadmium caused cellular-type hypersensitivity (or Type IV delayed-type hypersensitivity) [7]. Recently, there are some allegations that quantum dots are being used in COVID-19 vaccinees and cadmium is contained in the quantum dot nanoparticles [8,9]. PEGylated liposomes and metal nanoparticles were known to have surface polymer effects to give rise to Complement Activation-Related Psudoallergy (CARPA) [10].
In this context, we conducted this research to see whether one batch of the recent influenza vaccinees which was freely distributed by the Korean Government contained any metals and whether there were any pathologic changes of immunotoxicity (including Type IV delayed-type hypersensitivity or complement activation-related psudoallergy) in the brains, hearts, lungs, livers, or kidneys in the experimental mice within 7 days of flu vaccination (Lot Number: A14720017).
Methods
Study design and participants
Analysis of metal components in the flu vaccine: A new cost-free vaccine (Lot Number: A14720017) was diluted with distilled water. 1: 29 dilution of vaccine contents in distilled water was analyzed twice for the presence of metal components of aluminum, indium, cadmium, gallium, and mercury. Analysis of the metal contents in the 1:29 diluted flu vaccine was assessed by the Inductively Coupled Plasma Mass Spectrometry (ICP-MS) method using 7700 x ICP-MS (Agilent Technologies co.) [11], Quadrupole (a Mass analyzer), and Dual-mode discrete dynode electron multiplier (a detector).
Experimental animals and groups: Total 10 BALB/c mice were used to analyze any pathological changes after 7 days of flu vaccination. Experimental mice were purchased from the Experimental Animal Center of Jeonju, South Korea. All the procedures and mice-using were approved based on the ethical and scientific protocol of the Jeonbuk National University-Institutional Experimental Animal Care and Use Ethical Committee (Approval Number: JBNU 2020-0175). Two vials of cost-free flu vaccine (Lot Number: A14720017) were used in the experiment. Animals were divided into two groups: one group of 5 mice were injected intraperitoneally with 0.1 ml of 1:4 diluted flu vaccine with injectable distilled water; another group of 5 mice were injected intraperitoneally with 0.1 ml of undiluted flu vaccine. They were freely reared in a polycarbonate cage (400 x 255 x 180 mm) for 7 days.
Procedures
Tissue processing: The mice were sacrificed after CO2 short-acting gas anesthesia following the general protocol and procedure. Three medical doctors harvested brains, hearts, lungs, livers, and kidneys of the experimental animals and immerged them in the formalin-filled bottles. All the tissues were embedded in paraffin and sectioned in 6µm thickness for the study.
Hematoxylin and eosin staining: According to a general protocol, hematoxylin and eosin (H & E) staining was done for the histopathological examination.
Histopathological analysis: There were no demonstrable pathological or inflammatory changes in all tissues of brains, hearts, livers, and kidneys of ten experimental animals. Histological analysis for lung lesions was conducted semi-quantitatively using two different methods based on a modified evaluation method of any inflammatory changes of the lung [5,12-14].
In the first method (a street-view method), lung injury was scored by four different researchers as the mean of the scores of 5 items: (1) thickness of the alveolar wall/vascular endothelial wall [12], (2) interstitial lung inflammation predominantly subpleural or peripheral [5], (3) infiltration or aggregation of lymphocytes and eosinophilia/inflammatory cells in airspace, the alveolar wall, or the vessel wall [5,12,13], (4) alveolar congestion/widening of edematous or hemorrhagic interstitium [5,12,13], and (5) lymphocytic alveolitis or regenerative hyperplastic epithelia, scattered granulation tissue plugs within airspaces, or the presence of hyaline membranes [5,14]. Each item was graded on a four-point scale ranged from 0 to 3, where 3 marked the severest damage (Figure 1).
In the second method (a sky-view method), an expert lung pathologist semi-quantitatively scored the lung lesions comprehensively comprising all five aspects above. In brief, damages of the lungs were evaluated based on the degree of histopathological change from point zero (0) to point two (2): point 0—no or minimal histopathological change (minimal changes in the alveolar septa and space); point 1—low-grade damage (mild edema and inflammatory cellular infiltrations in the alveolar septa; scarce red blood cells and mononuclear inflammatory cells in the alveolar spaces); point 2—high-grade damage (moderate to severe distortion of interalveolar septa mostly lined with swollen and hyperplastic type II pneumocytes; moderate to severe edema and infiltration of inflammatory cells mixed with lymphocytes, macrophages and neutrophils in the alveolar septa and perivascular spaces [5,12-14]; and alveolar spaces that are filled with eosinophilic proteinaceous materials, red blood cells, and occasional mononuclear inflammatory cells)
Based on the degree of damage, the number of damaged areas was counted at fields through 10x objective lens (Plan Fluor 10X/o.30NA, Nikon, Japan). Thereafter, the damage scores were calculated as “the damage score = 1x (number of area of low-grade damage at x100 magnification) + 2x (number of area of high-grade damage at x100 magnification)” (Figure 2).
Statistical analysis
The difference of the semi-quantitative scores for histopathological changes between the two groups was analyzed by using SPSS version 20.0 (IBM, CA, USA). All data were presented as mean ± standard error of mean, and a significant difference was considered when P-value was less than 0.05.
Results
Analysis of metal components in the flu vaccine
Korean Government distributed cost-free vaccinees for people of over 62-year-old or people under 18-year-old for the 2020-2021 flu-season. One of the cost-free vaccinees (Lot Number: A14720017) was found to contain cadmium of 0.12 ppb (parts per billion = µg/L), and mercury of 1.77 ppb. Neither aluminum nor gallium, nor indium was detected.
Analysis of histopathological changes induced by flu vaccine
Both experimental groups showed no demonstrable inflammatory changes in the specimens of brains, hearts, livers, and kidneys. However, in both groups, almost all the lungs showed inflammatory changes, with the lungs of the undiluted-vaccine-injected group showing significantly more diffuse damages compared to the lungs of the 1:4 diluted-vaccine-injected group. The semi-quantitative score of the inflammatory damage of each experimental lung was evaluated using two different methods (Figures 1 & 2), and the differences of scores between two groups using two different methods were significant as p < 0.05 by method 1 and as p < 0.001 by method 2 (Table 1).
Discussion
Based on its weekly influenza surveillance data, CDC estimated that there were 39,000,000-56,000,000 flu illnesses among 350,000,000 Americans (11,143-16,000 persons/100,000 persons), 117-211/100,000 flu hospitalization, and 6.9-17.7/100,000 persons (or, 0.27-0.71/4,000 persons) flu deaths [2]. CDC also estimated that the flu vaccination reduced the flu illness by between 40% to 60% and flu death by one elderly person per 4,000 flu-vaccinated elderly persons [3].
Vaccine Adverse Event Reporting System (VAERS) receives any vaccine-associated reports of death, life-threatening illnesses, anaphylactic events, hospitalizations, or prolonged disability [15]. In almost every country, millions of vaccinations are provided to children and adults every year. It should be noted that because of the high volume of vaccinations, there can be reports of coincidental deaths, permanent disability, and prolonged hospitalizations [16]. But an AHRQ (the Agency for Healthcare Research and Quality) study disclosed that fewer than 1% of vaccine adverse events were reported [17].
There are rare cases of immune-mediated side effects associated with vaccinations: IgE mediated, Immune complex (IgG) mediated, T-cell mediated, and autoimmune /inflammatory allergy [18]. Immediate or anaphylactic reactions in children and adolescents can occur at a rate of 0.22 per 100,000 vaccinations, with 31% of them occurring with the first vaccination [18]. There are very rare cases of anaphylactic deaths which have some evidences in favor of causality between vaccination and death. Between 1990 and 2015, VAERS received 2,317 reports of vaccine-related anaphylaxis reactions, of which 828 cases had verifiable medical records, and there were 8 reports of anaphylactic deaths [15]. Those 828 anaphylactic reactions were caused mainly by Influenza vaccine of 330 cases (40%), MMR of 206 of cases (25%), and DTaP/Tdap of 194 cases (23%). Among the 828 reports of anaphylaxis, 341 cases (41% of 828 reports) did not have any previous history of hypersensitivity or allergy. In these newly-occurred anaphylaxis of 341 cases, 163 case (48% of 341 cases) occurred within 30 minutes, while 21 cases (6% of 348 cases) occurred between 8 to 24 hours of vaccination. The VAERS record showed that the reports of anaphylaxis after vaccination had a rate of 1.3 cases per 1 million doses of vaccinations and the main treatments for the 828 anaphylaxis cases were antihistamines, epinephrine, and steroids [15]. Anaphylactic reactions to Polyethylene Glycols (PEG) and polysorbates are more common than we thought, and because COVID-19 mRNA (messenger ribonucleic acid) vaccinees such as Moderna and Pfizer-BioNTech vaccinees contain PEG, they can cause life-threatening anaphylactic reactions of CARPA even in persons who have never experienced severe allergic reactions in the past [19].
The “delayed” type of allergy, which occurs in 1-2 days after initial exposure to the allergen, is cellular-type hypersensitivity and this type of hypersensitivity or allergy can be induced by metals in humans [7]. Heavy metals such as mercury, aluminum, or cadmium were known to cause hypersensitivity and autoimmunity in humans, because heavy metals modulate some parts of immune system and enhance immune responses to unrelated antigens and activate neoantigen-specific T cells [20]. Heavy metals such as mercury, cadmium, and aluminum can have immunotoxic effects through T-cell mediated, cellular-type hypersensitivity (Type IV delayed-type hypersensitivity) [7]. This delayed-type hypersensitivity occurs 24 to 48 hours after exposure and can be diagnosed by patch test, Lymphocyte Transformation Test (LTT), identification of sensitized T cells by MELISA (Memory Lymphocyte Immuno-Stimulation Assay) [21] or morphological confirmation of the presence of activated lymphocytes (lymphoblasts) [7]. This delayed-type hypersensitivity to mercury occurred in 14.8% of healthy persons [21]. As seen in figures 1 & 2 of the current study, this type of metal-induced “delayed” hypersensitivity or immunotoxicity makes alveolar hypoxia, an acute form of Diffuse Alveolar Damage (DAD), and stimulates inflammatory changes, cytokines productions, free radical productions, alveolar congestion, and pulmonary edema [14]. If this kind of “delayed” hypersensitivity and ensuing alveolar hypoxia had occurred after influenza vaccination in a metal hypersensitive vaccinee, it could have caused a lethal effect who had received an influenza vaccine(of Lot Number: A14720017).
Typically, approved vaccinees use aluminum hydroxide, aluminum phosphate, or potassium aluminum sulphate, MF59 (squalene oil), Thimerosal (a neurotoxin, a mercury derivative), gelatin (a stabilizer), Monosodium L-Glutamate (a neurotoxin), Neomycin Sulfate (an immunotoxin), Polymyxin B (a neurotoxin), latex, formaldehyde (a gaseous form of formalin), human cell strains (WI-38, or MRC-5), and animal cell lines as adjuvants [8]. And there are allegations that there are many other excipients of vaccinees including: human embryo cell lines (a human DAN mutant), Sodium Taurodeoxycholate (a carcinogen, an immunotoxin), Xenotropic Murine Leukemia Virus-Related Virus (XMRV, a human DNA mutant), Beta-Propiolactone (a carcinogen), Chick Embryo (a human DNA mutant), Potassium Chloride (a neurotoxin), Polyethylene Glycol (PEG), polysorbates, glyphosate—an organophosphorus herbicide, Genetically Modified Organisms (GMOs), and others [8,22]. However, cadmium is not on the published lists. It is worth noting the nature and effects of cadmium, because small amount of cadmium was detected in the current study of flu vaccine (Batch Number: A14720017). Cadmium is present in sewage sludge-contaminated food and cigarette smoke. Smokers have 4-5 times higher cadmium concentrations in the blood than those of the non-smokers [23]. It is known that cadmium interacts for the production of Reaction Oxygen Species (ROS) and in the DNA repair mechanisms, and affects cell production, differentiation, and apoptosis. Chronic exposure to cadmium can cause Itai-itai disease, decrease the number of sperms and the function of ovary, increase cardiovascular mortality, and lead to Parkinson, Alzheimer, and Huntington’s diseases, and is a lung carcinogen [23].
Cadmium can be found in the core portion of quantum dots and can induce CARPA [9]. When the outer coating is degraded, the “naked” Cadmium Telluride (CdTe) Quantum Dots (QDs) induce the formation of Reactive Oxygen Species (ROS), damage the plasma membrane, mitochondrion, nucleus, impair cellular respiration and mitochondrial oxidative phosphorylation, neuronal dysfunction, and lead to cell death (apoptosis) [22-24]. Cadmium by itself may not cause hypersensitivity and autoimmunity as mercury does, but it may work as a co-stimulation (signal 2) instead of antigen recognition (signal 1) in the two-signal model of lymphocyte activation [20]. It is a weak sensitizer of the immune system and it skews immune responses towards to IgG1 Antibody Secreting Cell (ASC) production, and a T lymphocyte helper type 2 (Th2) isotype [21].
Complement activation-related pseudoallergy is a non-IgE-mediated immune reaction and this kind of CARPA can be occurred when a person is exposed to PEGylated liposomes or metal nanoparticles (like quantum dots which has cadmium in their cores) even for the first time through a vaccination [10]. In a CARPA condition, anaphylatoxin induces mast cell to release lots of mediators which cause “CARPA cascade” of platelet-leukocyte aggregation, pulmonary microembolism, cytokine release, capillary leakage, bronchoconstriction (bronchospasm), pulmonary vasoconstriction, coronary vasoconstriction, systemic vasodilation, tachycardia, myocardial hypoxia, cardiac arrhythmia, and sudden death [10]. To prevent CARPA, antihistamines can be prescribed and quantification of sC5b-9 can be checked before a vaccination [10].
Through cell-mediated or pseudoallergic immunotoxicity, cadmium coupled with mercury in the influenza vaccine (of Lot Number: A14720017), could have caused loss of life of lots of vaccinee within 7 days of influenza vaccination. In the Republic of Korea, a 17-year-old man died within three days of influenza vaccination (Lot Number: A14720007), a 77-year-old woman died within a day (Lot Number: A14720016), and more than 100 vaccinees died within 7 days of influenza vaccination by the end of the November 2020 [25]. Because Korean authorities encourage flu vaccinations even in the heightened public anxiety, it can be postulated that more than 1,531 vaccinees could coincidentally die within 7 days of influenza vaccination in the 2020-2021 season, when considering the fact that 1,531 persons (among 6,680,000 flu vaccinated persons) coincidentally died in the 2019-2020 season [4].
As a “more delayed” form of immune-mediated side effects associated with vaccination “more than a week,” there are 9 cases of interstitial pneumonia after influenza vaccinations [5]. When considering the fact that these nine cases were associated with autoimmune or inflammatory allergy even 36 days and 45 days after influenza vaccinations, we can speculate that these cases of immune-related interstitial pneumonia must have been underestimated in the number of occurrences (or frequency). These cases showed 190-330% positive reactions to Drug Lymphocyte Stimulation Test (DLST), and pathologically showed lymphocyte alveolitis, granulation tissue plugs at periphery or sub-pleural lesions. These cases of interstitial pneumonia were cured by systemic intravenous methylprednisolone and oral prednisolone [5]. Some medical conditions including asthma, autism, multiple sclerosis, Guillain-Barre syndrome, transverse myelitis, Sudden Infant Death (SID), Alzheimer’s disease, and inflammatory bowel disease were suggested to be vaccination sequelae of delayed form of immune-mediated side effects, but no demonstrable scientific or clinical evidences of causality were found [20].
Interpretations of the findings in the current study should be done in the context of factual potential limitations. First, in the current study, there was neither control group nor MELISA. We did not expect that there would be so much inflammatory changes after influenza vaccination as seen in figures 1 & 2 and we falsely presumed that 1:4 diluted-vaccine injected group would serve the role of a saline injected control group. In the next study, saline-injected control group and MELISA would be necessary. Second, because the concentrations of mercury and cadmium were analyzed based on only one vial of influenza vaccine (of Lot Number: A14720017), there can be a problem of generalizability of the result if expanding the results from one specific batch of influenza vaccine (of Lot Number: A14720017) to all influenza vaccinees. Third, we neither participated in the autopsy of the deceased persons after influenza vaccinations nor we studied the histological findings of their lungs or hearts. So, we could not confirm any pathological changes as seen in Figures 1 and 2 in the deceased. However, what this study seeks to focus on and sheds a light on is that results of the current study may provide some guidance to future autopsies to identify whether there are lesions associated with immunotoxicity caused by vaccinees whether cellular-type hypersensitivity or CARPA. Fourth, the current study is too small in a scale that it may not provide a rationale for mandating a check for the presence of any metals in every vaccine, let alone influenza vaccinees. But, considering the fact that mercury of 1.77 ppb and a newly found cadmium of 0.12 ppb were detected in the influenza vaccine, other vaccinees (probably, including COVID-19 vaccinees) are recommended to be checked for a presence of any kinds of metals. Fifth, other inflammatory form of acute lymphocytic myocarditis or lymphocytic inflammatory cardiomyopathy was not observed in the current study. Thus, further evaluations are necessary to study whether there are cases of vaccine-associated myocarditis and inflammatory cardiomyopathy which can cause deteriorations of cardiac function and sudden death [26]. Sixth, further hemocompatibility study will be needed to identify the possible causality of cell-mediated immunity or CARPA [27].
In conclusion, one of the cost-free influenza vaccinees (Lot Number: A14720017), which were distributed by the Government of the Republic of Korea, were studied to contain cadmium of 0.12 ppb and mercury of 1.77 ppb. In the flu vaccine-injected mice, metal-induced immunotoxicity of delayed-type hypersensitivity (Type IV cell-mediated hypersensitivity) or of CARPA caused diffuse damages in lung parenchyma, intra-alveolar hemorrhage, edema, and infiltration of inflammatory cells. This metal-induced immunotoxicity could be responsible for some of the deaths of 100 persons who fortuitously died within 7 days of influenza vaccination by the November 2020 and of the deaths of 1,521 persons who coincidentally died within 7 days of influenza vaccinations during the 2019-2020 season in the Republic of Korea, and of the deaths who accidentally died within one week after influenza vaccination—23.2persons/100,000 vaccinees of an age of over 75 and 11.3 persons/100,000 vaccinees of an age between 65 and 75 in the United States [4] and the results may be helpful for the causal identification of some deaths of COVID-19 vaccinees.
Contributors
MH Jeon, JS Oh, KY Jeon participated in the designing of the study, organ harvesting of the experimental mice, data preparation for the Method 1, draft preparation, and proofreading. KY Jang was responsible for the data preparation by the Method 2, statistical data analysis, figure preparations, and proofreading. All authors interpreted the data and approved the final version of the article. All had access to the raw data.
Acknowledgments
Prof. Hyun-Jin Tae, D.V.M., PhD, Dept of Histology, Veterinary Medicine, JBNU, helped to prepare the H&E stains, gave advices and discussions for the histological features of the lungs, and provided essential assessments for all the harvested organs of brains, hearts, lungs, livers, and kidneys.
- The Medical Letter. Influenza Vaccine for 2020-2021. Medical Letter on Drugs and Therapeutics. 2020;62. https://bit.ly/2MFsbX1
- CDC. 2019-2020 U.S. Flu Season: Preliminary In-Season Burden Estimates. https://bit.ly/39BY23U
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD). Vaccine Effectiveness: How Well Do the Flu Vaccines Work? January 3, 2020. https://bit.ly/36wKxR7
- Jung J. Epidemiologic Evaluation and Risk Communication Regarding the Recent Reports of Sudden Death after Influenza Vaccination in the COVID-19 Pandemic. J Korean Med Sci. 2020 Oct 26;35(41):e378. doi: 10.3346/jkms.2020.35.e378. PMID: 33107233; PMCID: PMC7590648.
- Hibino M, Kondo T. Interstitial Pneumonia Associated with the Influenza Vaccine: A Report of Two Cases. Intern Med. 2017;56(2):197-201. doi: 10.2169/internalmedicine.56.7239. Epub 2017 Jan 15. PMID: 28090052; PMCID: PMC5337467.
- Seidenari S, Giusti F, Pepe P, Mantovani L. Contact sensitization in 1094 children undergoing patch testing over a 7-year period. Pediatr Dermatol. 2005 Jan-Feb;22(1):1-5. doi: 10.1111/j.1525-1470.2005.22100.x. PMID: 15660887.
- Vera Stejskal. Allergy and Autoimmunity Caused by Metals: A Unifying Concept. May 2015. doi: 10.1002/9781118663721.ch5, In book: Vaccines and Autoimmunity. p. 57-64. https://bit.ly/3j2KFwV
- Jeon KY. COVID-19 Vaccine-Safety First, Alleged “Greater Good” Last. American J Epidemiol Public Health. 2020 Sep 28;4(4):012-016. doi: 10.37871/ajeph.id39
- Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles: perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol. 2009 Aug 1;238(3):280-8. doi: 10.1016/j.taap.2009.04.010. Epub 2009 Apr 18. PMID: 19379767; PMCID: PMC2709954.
- Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol. 2014 Oct;61(2):163-73. doi: 10.1016/j.molimm.2014.06.038. Epub 2014 Aug 12. PMID: 25124145.
- Agilent Technologies. Inductively coupled plasma mass spectrometry (ICP-MS). Agilent atomic spectroscopy solutions for Metal Analysis in Food and Agriculture. https://bit.ly/3j9fdNl
- Nogueira R, Melo N, Novais E Bastos H, Martins N, Delgado L, Morais A, C Mota P. Hypersensitivity pneumonitis: Antigen diversity and disease implications. Pulmonology. 2019 Mar-Apr;25(2):97-108. doi: 10.1016/j.pulmoe.2018.07.003. Epub 2018 Aug 17. PMID: 30126802.
- Baum A, Ajithdoss D, Copin R, Zhou A, Lanza K, Negron N, Ni M, Wei Y, Mohammadi K, Musser B, Atwal GS, Oyejide A, Goez-Gazi Y, Dutton J, Clemmons E, Staples HM, Bartley C, Klaffke B, Alfson K, Gazi M, Gonzalez O, Dick E Jr, Carrion R Jr, Pessaint L, Porto M, Cook A, Brown R, Ali V, Greenhouse J, Taylor T, Andersen H, Lewis MG, Stahl N, Murphy AJ, Yancopoulos GD, Kyratsous CA. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science. 2020 Nov 27;370(6520):1110-1115. doi: 10.1126/science.abe2402. Epub 2020 Oct 9. PMID: 33037066.
- Hughes KT, Beasley MB. Pulmonary Manifestations of Acute Lung Injury: More Than Just Diffuse Alveolar Damage. Arch Pathol Lab Med. 2017 Jul;141(7):916-922. doi: 10.5858/arpa.2016-0342-RA. Epub 2016 Sep 21. PMID: 27652982.
- Su JR, Moro PL, Ng CS, Lewis PW, Said MA, Cano MV. Anaphylaxis after vaccination reported to the Vaccine Adverse Event Reporting System, 1990-2016. J Allergy Clin Immunol. 2019 Apr;143(4):1465-1473. doi: 10.1016/j.jaci.2018.12.1003. Epub 2019 Jan 14. PMID: 30654049; PMCID: PMC6580415.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: What does the evidence show? Vaccine. 2015 Jun 26;33(29):3288-92. doi: 10.1016/j.vaccine.2015.05.023. Epub 2015 May 23. PMID: 26004568; PMCID: PMC4599698.
- Lizarus Ross, Michael Klopas, Harvard Pilgrim Health Care. Electronic Support for Public Health-Vaccine Adverse Event Reporting System (ESP: VAERS). The Agency for Healthcare Research and Quality (AHRQ). Grant ID: R18 HS 017045. https://bit.ly/3ajz3S4
- Fritsche PJ, Helbling A, Ballmer-Weber BK. Vaccine hypersensitivity--update and overview. Swiss Med Wkly. 2010 May 1;140(17-18):238-46. PMID: 20349363.
- Lisa Schnirring. CIDRAP News. FDA authorizes Moderna COVID vaccine for emergency use. December 19, 2020. FDA authorizes Moderna COVID vaccine for emergency use | CIDRAP (umn.edu).
- Carey JB, Allshire A, van Pelt FN. Immune modulation by cadmium and lead in the acute reporter antigen-popliteal lymph node assay. Toxicol Sci. 2006 May;91(1):113-22. doi: 10.1093/toxsci/kfj142. Epub 2006 Feb 22. PMID: 16495351.
- Manousek J, Stejskal V, Kubena P, Jarkovsky J, Nemec P, Lokaj P, Dostalova L, Zadakova A, Pavlusova M, Benesova K, Kala P, Miklik R, Spinar J, Parenica J. Delayed-Type Hypersensitivity to Metals of Environmental Burden in Patients with Takotsubo Syndrome - Is There a Clinical Relevance? PLoS One. 2016 Nov 8;11(11):e0164786. doi: 10.1371/journal.pone.0164786. PMID: 27824862; PMCID: PMC5100929.
- Oxford Vaccine Group. Vaccine ingredients. Vaccine Knowledge Project. August 30, 2019. https://bit.ly/3pCFXIF
- Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, Moghadamnia AA. Cadmium toxicity and treatment: An update. Caspian J Intern Med. 2017 Summer;8(3):135-145. doi: 10.22088/cjim.8.3.135. PMID: 28932363; PMCID: PMC5596182.
- Nguyen KC, Rippstein P, Tayabali AF, Willmore WG. Mitochondrial Toxicity of Cadmium Telluride Quantum Dot Nanoparticles in Mammalian Hepatocytes. Toxicol Sci. 2015 Jul;146(1):31-42. doi: 10.1093/toxsci/kfv068. Epub 2015 Mar 25. PMID: 25809595; PMCID: PMC4476459.
- Yonhap News. Death toll among flu vaccine recipients approaching 100: agency. The Korean Herald. November 7, 2020. https://bit.ly/3jc2oSv
- Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, Kelle S, Klingel K, Maatz H, Parwani AS, Spillmann F, Starling RC, Tsutsui H, Seferovic P, Van Linthout S. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2020 Oct 12:1–25. doi: 10.1038/s41569-020-00435-x. Epub ahead of print. PMID: 33046850; PMCID: PMC7548534.
- Singh D, Dilnawaz F, Sahoo SK. Challenges of moving theranostic nanomedicine into the clinic. Nanomedicine (Lond). 2020 Jan;15(2):111-114. doi: 10.2217/nnm-2019-0401. Epub 2020 Jan 6. PMID: 31903854.

Sign up for Article Alerts