Correlation between Ct and Histological Findings in Non-Neoplastic Interstitial Lung Diseases with UIP-Pattern and NSIP-Pattern
Giuseppe Guzzardi*, Michela Barini, Paolo Irico, Silvia Riva and Alessandro Carriero
Radiology Dept,“Maggiore della Carita” Hospital - University of Eastern Piedmont-Corso Mazzini 18, 28100 Novara, Italy
*Address for Correspondence: Giuseppe Guzzardi, Radiology Dept. – “Maggiore della Carita” Hospital – University of Eastern Piedmont-Corso Mazzini 18, 28100 Novara - Italy, Tel: +0321-3733831; Fax: +0321-3732739; Email: barinimichela@gmail.com
Submitted: 21 August 2017; Approved: 13 September 2017; Published: 15 September 2017
Citation this article: Guzzardi G, Barini M, Irico P, Riva S, Carriero A. Correlation between Ct and Histological Findings in Non-Neoplastic Interstitial Lung Diseases with UIP-Pattern and NSIP-Pattern. Sci J Pulm Respir Med. 2017;1(1): 011-018.
Copyright: © 2017 Guzzardi G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: ILD; UIP; NSIP; Pulmonary Biopsy; Interstitial Lung Disease
Download Fulltext PDF
Purpose: The aim of this study was to verify the correlations between Computed Tomography (CT) and histological findings in subjects with Interstitial Lung Disease (ILD) with a Usual Interstitial Pneumonia (UIP) or Non-Specific Interstitial Pneumonia (NSIP) pattern in order to obtain a level of reliable diagnostic confidence that would make it possible to avoid the need for a lung biopsy.
Materials and Methods: This retrospective study involved 11 patients with a CT and bioptic diagnosis of ILD (six males and five females aged 58-77 years) histologically divided into nine with a UIP pattern (six with idiopathic pulmonary fibrosis, two with chronic hypersensivity pneumonitis, and one with rheumatoid arthritis), and two with a NSIP pattern (one with idiopathic NSIP and one with scleroderma NSIP). The CT images of each subject were reviewed in order to evaluate the spatial distribution and type of lesions (e.g. reticulations, septal thickening, honeycombing, ground glass opacities and bronchiectasis), and the findings were then compared with the bioptic findings in order to see whether there were any correlations.
Results: In the subjects with a UIP pattern, the closest CT/biopsy correlations were reticulations and septal thickening (observed in 100% of the patients); the correlations of honeycombing (55%) and ground glass opacities (20%) were respectively moderate and poor. In the subjects with a NSIP pattern, the major finding was ground glass opacities.
Conclusions: In the case of patients with a correct clinical diagnosis whose radiological picture indicates “possible UIP”, a confirmatory biopsy does not seem to be indicated. In patients with an NSIP pattern, a biopsy seems to be useful as this may be the initial manifestation of a UIP pattern.
Introduction
Idiopathic Interstitial Pneumonias (IIPs) include a heterogeneous group of non-neoplastic lung diseases in which the pulmonary interstitial is altered by various patterns of fibrosis and inflammation [1]. These alterations also usually involve the epithelial and endothelial linings of the peripheral airways, air spaces and blood vessels [1,2]. The recent classification by the American Thoracic Society (ATS)/European Respiratory Society (ERS) from 2013, divides IIPs into: (1) chronic fibro sing IIPs, including Idiopathic Pulmonary Fibrosis (IPF) and Idiopathic Nonspecific Interstitial Pneumonia (NSIP); (2) smoking-related IIPs, including Respiratory Bronchiolitis–Associated Interstitial Lung Disease (RB-ILD) and Desquamative Interstitial Pneumonia (DIP); (3) acute or sub-acute IIPs, including Cryptogenic Organizing Pneumonia (COP) and acute interstitial pneumonia; and (4) rare IIPs, including lymphoid interstitial pneumonia and idiopathic pleuroparenchymal fibro elastics. The ATS/ERS 2013 update on IIPs also proposed a classification based on disease behavior because the IIPs represent a heterogeneous group of diseases with different prognoses [3].
UIP Pattern
Most ILDs are inflammatory in nature, and the most frequent of these is Usual Interstitial Pneumonia (UIP) [4]; about half of the patients with UIP have the clinically idiopathic form called Idiopathic Pulmonary Fibrosis (IPF).
UIP has two fundamental histopathological signs [4]:
• Spatial heterogeneity: a patchy distribution of fibrosis with areas of dense parenchymal scarring alternating with less affected or normal areas;
• Temporal heterogeneity: areas of dense collagenous fibrosis (also known as “old fibrosis”) alternating with areas of new fibrosis with fibroblastic foci.
UIP pattern in patients with IPF
IPF is the most common of the IIPs and is a progressive, chronic, and fibro sing lung disease of uncertain cause characterized by the histopathological pattern of Usual Interstitial Pneumonia (UIP). The prognosis is poor in most cases, with median survival ranging from 2.5 to 3.5 years. 3–5; however, progression can be variable, and although most patients have rapid progression, some patients remain fairly stable [5].
The UIP pattern is the substrate most frequently associated with IPF, a progressive ILD with a fibrotic evolution limited to the lungs that mainly occurs in older male patients (> 60 years), smokers or ex-smokers [4-7]. A diagnosis of IPF requires the exclusion of other forms of ILD associated with systemic diseases or environmental and/or drug exposure [4-6].
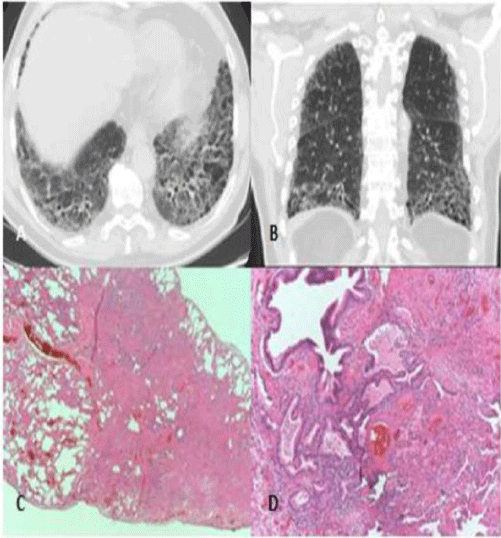
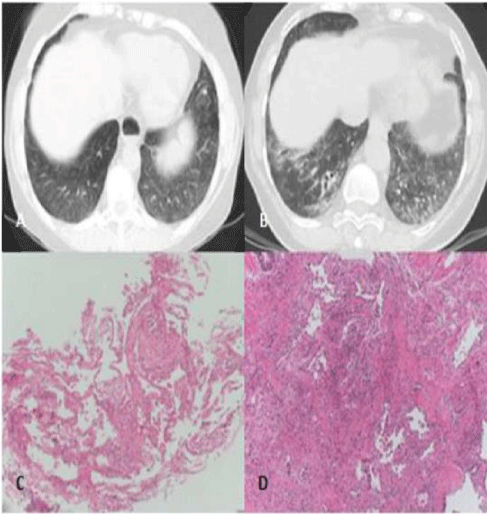
CT manifestations of UIP pattern in IPF: 1) a distorted parenchymal architecture with reticular opacities and thickening of the inter lobularsepta; 2) bronchiectasis and traction bronchiectasis; 3) pseudo-cystic air spaces with a sub-pleural cobblestone-like distribution (known as honeycombing); and4) ground glass opacities [1]. These anomalies may be variously present and have a mainly basal and sub-pleural distribution [1,8]. If present, the ground glass opacities are generally limited and confined to basal sub-pleural areas of fibrosis unless the patients are seen during the course of an IPF exacerbation [4,10].
Histological characteristics of IPF: 1) a patchy sub-pleural and para-septal fibrosis, prevalently in the lower lobes, 2) fibroblastic proliferation (fibroblastic foci): interstitial swelling in the inter face between the fibrotic and normal parenchyma; these areas of “new fibrosis” coexist with the “old fibrosis” consisting of dense collagen deposits, and represent the temporal heterogeneity of the UIP pattern, 3) alveolar collapse caused by dense fibrosis and the formation of cystic spaces lined by type II hyperplastic pneumocytesor bronchiolar epithelium (honeycombing). Honeycombing is frequent even in the absence of the macroscopic honeycombing revealed by CT [4,12].
UIP pattern in patients with CHP
CHP (or extrinsic allergic alveolitis) is a form of ILDs caused by an anomalous immunological response to inhaled, mainly organic antigens in predisposed subjects [4,9,13]. The disease usually goesunrecognised but, depending on the frequency and intensity of antigen exposure, may be classified as acute, sub-acute or chronic, although the three forms may overlap both clinically and radio logically [9,14].
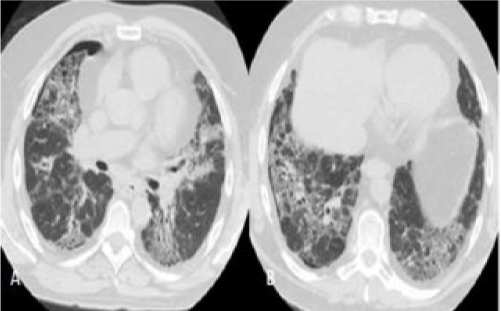
CT manifestations of CHP: 1)a patchy or para-hilar distribution of fibrosis without a sub-pleural dominance of IPF; however, it may be prevalently sub-pleural in some cases [17], 2) honeycombing involving the lower lobes and, in 80% of cases, the simultaneous involvement of the upper lobes (predominant honeycombing in the upper lobes is a more characteristic finding of the UIP pattern in CHP [4,16]), 3)areas of air trapping that create a mosaic appearance (also called “mosaic attenuation”) that is most clearly seen in scans recorded during maximum expiration and is due to underlying constrictive bronchiolitis [4,18]. However, in some cases, CHP may manifest itself with a UIP pattern that is totally indistinguishable from the alterations observed in IPF [4].
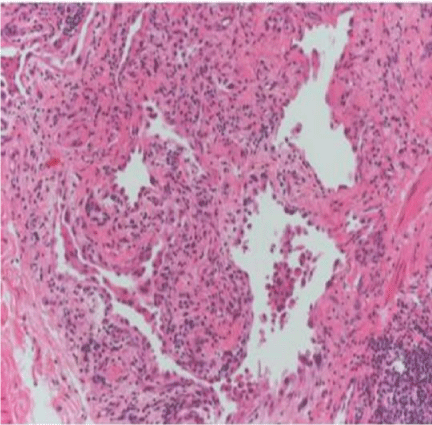
Histological characteristics of CHP pattern: 1) the predominance of a UIP pattern in the upper lobes; 2) giant cells or interstitial (not intra-alveolar) granulomas; 3) “bridging” fibrosis between the centrolobar and perilobar areas or between two adjacent centrolobar areas; and 4) the presence of inflammatory bronchiolitis with lymphoplasmacytic infiltration.
UIP pattern in patients with Rheumatic diseases
The Rheumatic Diseases (RDs) that are most frequently associated with pulmonary involvement are scleroderma, rheumatoid arthritis (RA), Systemic Lupuserythematosus (SLE), Sjögren’s syndrome, polymyositis/dermatomyositis, all of which are potentially capable of generating widespread pulmonary fibrosis [21]. RA is the most common RD throughout the world, and is second only to scleroderma in the genesis of ILDs [4]. High titers of rheumatoid factor and cigarette smoking are acknowledged risk factors for the development of ILDs in RA patients [22]. RA can have a UIP or NSIP CT and histological pattern, but it is the RD that most frequently has a bioptic UIP pattern [4,23].
CT manifestations of pulmonary involvement in RDs: 1) fibrosis as UIP or NSIP pattern but, when it has the former, the prognosis seems to be better than in the case of UIP pattern IPF [4], 2)reticular opacities, 3) lobular distortion, 4) traction bronchiectasis or bronchiolectasis, 5) honeycombing: if is present, RDs may imitate IPF perfectly, particularly if there is no evidence of other pulmonary alterations secondary to the RD [4],however, in comparison with IPF, honeycombing is less represented and less severe; furthermore, areas of honeycombing in the anterior part of the upper lobe are more frequent in RDs than in IPF [23].
Generally, other radiological findings can suggest the diagnosis of UIP pattern in patients with RDs reference, such as a dilated “open” esophagus in scleroderma, rheumatoid nodules in RA, pleural or pericardial effusion in SLE and/or RA, follicular bronchiolitis/lymphocytic interstitial pneumonia in RA and/or Sjögren’s syndrome [16].
Histological characteristics of pulmonary involvement in RDs: 1) lymphoid hyperplasia, which is visible at low magnification, and inflammation (more represented in the UIP pattern of RDs than the UIP pattern of IPF) [4], 2) honeycombing, emphysema, fibroblastic foci (less represented in the UIP pattern of RDs than the UIP pattern of IPF) [4,24], 3) pleural fibrosis.
NSIP Pattern
The concept of NSIP emerged when it was discovered that a sub-group of subjects with an ILD of unknown etiology whose lung biopsies did not reveal the diagnostic characteristics of any other well-characterized interstitial disease [12].
NSIP is less common than UIP, but is still one of the most common interstitial pneumonias. Its incidence is nowadays unknown, with different reports ranging from 14 to 36 % of all IIPs [15-20].
It may be cellular or fibrotic depending on whether its etiology is idiopathic or secondarily associated with vascular collagenopathy, hypersensitivity pneumonitis, drug-induced toxicity, infections or immunodeficiency.
The patients presenting an NSIP pattern have an average age of 40-50 years, and are therefore younger than those with fibrotic UIP.
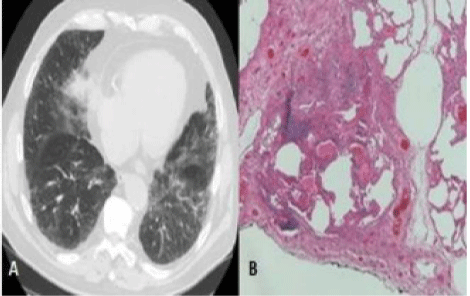
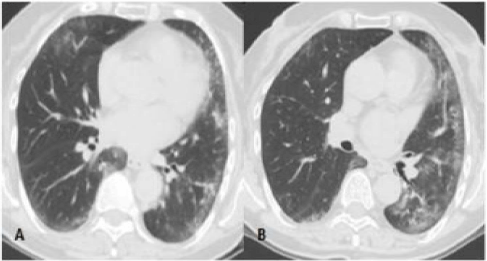
CT manifestations of NSIP pattern: 1) predominance of ground glass opacities, 2) traction bronchiectasis/bronchiolectasis: sign of fibrotic NSIP [11], 3) reticular thickening [1], 4) honeycombing (it’s not frequent, tends to be limited, and is less extensive than in the case of UIP) [11]. The alterations are bilateral, symmetrical and sub-pleural [25]. However, in the case of fibrotic NSIP, a diagnostic lung biopsy may be necessary because fibrotic NSIP can be very similar to UIP in its early stages [11].
NSIP may be cellular or fibrotic [1]. Cellular NSIP is much less frequent than fibrotic NSIP but, in both cases, the histological pattern is characterized by the homogeneity of spatial and temporal alterations.
Histological characteristics of cellular NSIP pattern: 1) chronic mild to moderate interstitial inflammation; 2) hyperplasia of the type II pneumocystis, 3) absence of dense fibrosis.
Histological characteristics of NSIP fibrotic pattern: 1) chronic mild to moderate interstitial inflammation; 2) interstitial and septal fibrosis, but less extensive than in the case of UIP and temporally homogeneous, 3) few or no fibroblastic foci [1,11].
Materials and Methods
The study population was selected from a histopathologic database containing patients who had under come to our observation in the period between January 2015 and January 2017. Inclusion criteria for entry to the study were patients who 1) had a multi detector CT examination performed and 2) had undergone diagnostic surgical lung biopsy (at our hospital or elsewhere) and had a histopathologic diagnosis of UIP or fibrotic NSIP within 3 months of the CT.
This pilot study retrospectively recruited 11 patients (six males and five females aged58-77 years) come to our observation in the period between January 2015 and January 2017.
The CT images of each patient were carefully reviewed with particular attention being given to the type and spatial distribution of the lesions: reticulations, septal thickening, honeycombing, ground glass opacities, thickened lesions and bronchiectasis.
The histological diagnosis were UIP in nine cases (six cases of IPF, two of CHP, and one of RA with a fibrotic evolution), and NSIP in two (one idiopathic and one scleroderma).
Subsequently, there view and bioptic findings relating to each subject were compared in order to verify their correlations.
The purpose of our study was to identify the correlations between histological findings and CT images in subjects with ILD with a UIP-pattern or NSIP pattern in order to obtain a level of reliable diagnostic confidence that would make it possible to avoid the need for a lung biopsy.
Results
None of the patients had a CT or bioptic picture of pulmonary exacerbation at the time they entered the study. The review of the CT images initially involved identifying the spatial localization of the parenchymal alterations (their apicobasalgradient and their uni- or bilateral nature), and then identifying and quantifying the type of lesion by assigning a score to each one [Table 1].
UIP pattern
As shown in table 1, all of the subjects characterized by a UIP pattern due to IPF or RA had bilateral and symmetrical pulmonary lesions distributed with an increase in gapicobasal gradient, except for patient UIP #1, whose lesions were prevalently (but not exclusively) unilateral. In the patients whose UIP pattern was due to CHP, the apicobasal gradient was less maintained because the upper and lower lobes were both involved; furthermore, the alterations in patient UIP #8 had a widespread patchy distribution. In line with previously published data, the differences in the spatial distribution of the lesions proved to be a CT criterion distinguishing a UIP pattern due to IPF from the UIP patterns of a different nature [1,4,11,17,23].
Table 1 also shows that the main alterations in all of our patients were prevalently sub-pleural reticular alterations and septal thickening associated with traction bronchiectasis and bronchiolectasis; the only patient who also had peri-hilaralterations was patient UIP #8. Honeycombing was less frequent: it was observed in only five (55%) of the nine patients, and was quantitatively variable. However, this is not surprising insofar as it has been previously demonstrated that there is a form of microscopic honeycombing that can be seen in biopsy specimens, and is presumably an initial manifestation of macroscopic honeycombing [4,12].
The finding of ground glass opacities was also scarcely and variably represented.
Once again, it was observed in only five (55%) of the nine patients, two of whom did not show honeycombing. Ground glass opacities are considered a sign of air space disease and may be present in patients with a UIP pattern, it typically increase in the case of exacerbations, and are due to an overlapping pulmonary infection (i.e. an acute IPF exacerbation) [4,11].
The spatial distribution of the alterations, the prevalence of reticulations, septal thickening and traction bronchiectasis, and the relative absence of ground glass opacities therefore seem to be consistent with a CT UIP pattern.
Each of the nine patients underwent a double trans-thoracic biopsy at the site in which the CT-revealed lesions were most representative. All of the samples showed various degrees of fibrosis depending on the patient, and the histological results corresponded to the CT findings.
The biopsies revealed microscopic distortion of parenchymal architecture mainly caused by collagenous fibrosis of the interstitium and inter lobar septa. Particularly in the patients with IPF, this fibrosis was heterogeneous spatially (with dense areas of collagen and fibrosis alternating with relatively normal areas) and temporally, with collagenous areas of old fibrosis and are as of new fibrosis and abundant fibroblastic foci. The subjects with IPF showed the highest percentage of fibroblastic foci, whereas the percentage was clearly less in the subjects with CHP and RA. The entity of fibroblastic foci is important for distinguishing a UIP pattern due to IPF from a UIP pattern due to other conditions.
Furthermore, a recent study by Walsh et al. has shown that the entity of the focicorrelates with the entity of bronchiectasis, and both are considered to be predictors of mortality in subjects with IPF [26].
The biopsies also revealed a quantitative correlation with the CT findings insofar as the biopsies performed at the sites of reticulations and septal thickening classified as“+++” in the CT images (i.e. widely represented) revealed an abundance of interstitial and septal fibrosis.
The patchy appearance was less represented in the patients with CHP or RA, but their biopsies nevertheless revealed the presence of ancillary findings that allowed the differential diagnosis of the UIP patterns. The two patients with CHP had granulomatous-like, plurinucleate giant cells in the interstitium, a finding that favoured the interpretation of a UIP pattern due to CHP. Furthermore, patient UIP #8 was a farm worker, and would therefore have come into contact with numerous antigens. On the other hand, patient UIP #7 was a teacher and apparently not subject to antigen exposure, and so the diagnosis made on the basis of the combined CT and bioptic findings was UIP due to CHP caused by a currently unknown antigen.
In the RA patient with lung disease, the inflammatory lymphoid infiltrate was more marked than in the case IPF with some follicular aggregates, a finding that suggested UIP due to RA.
The macroscopic honeycombing observed in some patients was bioptically confirmed by the finding of epithelium-lined cystic spaces in bronchiolar metaplasia and hypertrophic type II pneumocytes. In the patients without any signs of macroscopic honey combing, the biopsies showed microscopic honeycombing that is apparently not revealed by CT.
It is worth mentioning that the histological counterpart of ground glass opacities was only found in the case of patient UIP #2, who showed thickening of the inter-alveolarsepta. We believe that there may be two reasons for this: 1) that the bioptic samples of the remaining patients did not include any of the relatively few ground glass areas; or 2) that they were histologically “hidden” by other much more representative alterations. It can therefore be concluded that, in the case of a UIP pattern, there is a real correlation between the CT images of ground glass opacities and histological findings only when the opacities are highly represented and are not obscured by other adjacent findings such as reticulations, thickening and honeycombing.
NSIP Pattern
As shown in table 1, the pulmonary lesions in the two subjects with an NSIP pattern were distributed with an increasing apicobasal gradient, and were bilateral and symmetrical, although patient NSIP #2 showed the relative prevalence of the left side. They both showed mainly ground glass opacities that were particularly clear in sub-pleural regions. Among the ground glass alterations in the lower lobes, patient NSIP #2 also showed some sub-pleural reticulations and traction bronchiolectasis, butto a lesser extent than in the case of the UIP pattern. In the same patient, it seemed to be possible to see some sparing of the immediately sub-pleural portions of the lungs.
Neither of them showed sub-pleural pseudo cystic lesions due to macroscopic honeycombing, but patient NSIP #1 had an open esophagus as ancillary finding. Our review of the spatial distribution and prevalent type of CT alterations, such as the abundance of ground glass areas and the relative scarcity of reticulations/septal thickening and honeycombing, seemed to match published descriptions of the NSIP pattern, including one with a suspected fibrotic evolution.
Both patients underwent double trans-thoracic biopsy sampling of the sites in which the CT lesions were most representative. Microscopy did not reveal any of the marked fibrotic distortion of the parenchymal architecture typical of the UIP pattern. The samples of both patients showed that the predominant alteration was inter-alveolar septal fibrosis accompanied by moderate lymphoid infiltration. At the time of biopsy, there were no signs of exacerbation, capillary congestion or endo-alveolar exudate/transudate, and so the ground glass opacities revealed by CT seemed to be due to the inter-alveolar septal fibrosis. Patient NSIP #2 showed mild collagenous fibrosis of the interstitium and inter-lobar septa, which explains the more fibrotic CT picture. However, neither of the patients had fibroblastic foci of new fibrosis, and therefore lacked the temporal histological heterogeneity of the UIP pattern.
Further confirmation that ground glass opacities indicating air space disease may underlie inter-alveolar septal fibrosis is the fact that patient NSIP #2 developed an exacerbation of NSIP two months later. The CT examination performed at the time showed areas in the middle and lower fields with fibrotic alterations, and an increase in reticular/septal thickening and ground glass opacities. These new alterations overlapped and the obscured the preceding areas of NSIP. A follow-up CT scan one year later showed a return to the pre-exacerbation situation with new evidence of the previous areas of fibrosis. It can be reasonably concluded that, in the absence of signs of an exacerbation, the ground glass opacities are attributable to inter-alveolar septal fibrosis. However, in the presence of reticular thickening and/or ground glass opacities due to fibrosis, CT alone is not capable of differentiating the contribution of fibrosis from that of the other component because, as in our case, the may co-exist.
Discussion
The results of this study indicate that the main correlations between CT and biopsy findings in patients with a UIP pattern are the alterations that appear as reticular opacities and septal thickening. These two alterations were confirmed by a subsequent biopsy in all nine patients with a UIP pattern, and identified as collagenous fibrosis of the interstitium and inter-lobar septa. CT therefore seems to be sensitive in detecting such fibrotic alterations. Within the fibrosis, one element highlighted by the biopsies is the finding of fibroblastic foci, the presence and number of which distinguished a UIP pattern due to IPF from one of a different nature.
There was a weaker correlation with the honeycombing alterations visible in the CT scans of five of the patients with a UIP pattern (55%) insofar as the biopsies showed there is a form of microscopic honeycombing that cannot detected by means of CT. The absence of honeycombing on a CT scan should therefore not exclude the suspicion of UIP. On the other hand, radiological and histological evidence of honeycombing should not give rise to the error of presuming that the UIP pattern is due to IPF insofar as honeycombing can be the consequence of non-IPF fibrosis.
Particular consideration needs to be given to the finding of ground glass opacities in the context of the two patterns. Five of our nine patients with a UIP pattern (55%) showed ground glass opacities, but these correlated with histological findings only in the case of the patient in whom they were most widespread (1/5 = 20%). As mentioned above, this may have been due to bioptic sampling bias or because the opacities were hidden by other, more widespread lesions during the histological analysis. This is supported by the fact that there was a correlation between the CT and histological findings (intra-alveolar septum fibrosis) in the two patients with an NSIP pattern, in both of whom ground glass opacities were extensively represented and the other alterations (reticulations, septal thickening and honeycombing) were substantially absent.
It was also found that CT was not very specific for this finding as it cannot differentiate the various components involved the ground glass appearance. In the absence of signs of exacerbation, diagnosis may be aided by ancillary findings such as reticulations and, particularly, bronchiectasis/bronchiolectasis within the area of the ground glass opacities, which should give rise to the suspicion of fibrosis. Inpatients with exacerbations, it is not possible to distinguish ground glass opacities due to fibrosis from those due to intra-alveolar inflammation, and so subsequent follow-up is essential. On the basis of our findings, the gold standard when interpreting a fibrotic pattern requires a multidisciplinary clinical, radiological and histological approach, which is essential for correctly defining the nature of a UIP or NSIP pattern. However, in the case of patients with a correct clinical diagnosis whose radiological picture indicates “possible UIP”, a confirmatory biopsy does not seem to be indicated. This is in line with previously findings showing that CT alone is sufficient for making an accurate diagnosis of IPF in 70-100% of patients with a UIP pattern in an appropriate clinical context [9,11], and diagnostic accuracy increases to 90-100% when all of the elements of UIP are present, particularly honeycombing, which is the major predictor of [9,27]. On the other hand, a biopsy is necessary in cases in which the clinical diagnosis is doubtful and/or the radiographic findings are not decisive, as in the case of patients with an NSIP pattern insofar as this may be the initial form of a UIP pattern. In such cases a biopsy is indicated above all a means of searching for ancillary histological findings that may be capable of guiding the differential diagnosis of the ILDs that present a UIP or NSIP pattern.
Conclusions
The findings of this pilot study indicate a correlation between CT and bioptic findings in patients with a UIP pattern, especially in the case of reticular alterations and septal thickening; consequently, in patients with a correct clinical diagnosis whose radiological picture indicates “possible UIP”, a confirmatory lung biopsy does not seem to be indicated. In patients with an NSIP pattern, it can be said that, even in the case of a correct clinical diagnosis, a lung biopsy seems to be useful insofar as an NSIP pattern can be the initial manifestation of a UIP pattern.
- Wittram C, Mark EJ, McLoud TC. CT-Histologic Correlation of the ATS/ERS 2002 Classification of Idiopathic Interstitial Pneumonias. RadioGraphics. 2003; 23: 1057-1071. https://goo.gl/McNMbQ
- Cushley MJ, Davison AG, du Bois RM. The diagnosis, assessment and treatment of diffuse parenchymal lung disease in adults.Thorax. 1999; 54: 1-28. https://goo.gl/2ctrtH
- Sverzellati N, Lynch DA, Hansell DM, Johkoh T, King TE Jr, Travis WD. American Thoracic Society-European Respiratory Society Classification of the IdiopathicInterstitial Pneumonias: Advances in Knowledge since 2002. Radiographics. 2015; 35: 1849-1871. https://goo.gl/fX2wZh
- Smith M, Dalurzo M, Panse P, Parish J, Lesli K. Usual interstitial pneumonia-pattern fibrosis in surgical lung biopsies. Clinical, radiological and histopathological clues to aetiology. J ClinPathol. 2013; 66: 896-903. https://goo.gl/SzXMN1
- Kusmirek JE, Martin MD, Kanne JP. Imaging of Idiopathic Pulmonary Fibrosis. RadiolClin North Am. 2016; 54: 997-1014. https://goo.gl/8itWv8
- Gruden J, Prasad P, Leslie KO, Tazelaar HD, Colby TV. UIP diagnosed at surgical lung biopsy, 2000-2009: HRCT patterns and proposed classification system. AJR Am J Roentgenol. 2013; 200: 458-467. https://goo.gl/CUZjRM
- MacDonald SL, Rubens MB, Hansell DM, Copley SJ, Desai SR, Du Bois RM, et al. Nonspecific interstitial pneumonia and usual interstitial pneumonia: comparative appearances at and diagnostic accuracy of thin-section CT. Radiology. 2001; 221: 600-605. https://goo.gl/VgaMi7
- Wuyts WA, Cavazza A, Rossi G, Bonella F, Sverzellati N, Spagnolo P. Differential diagnosis of usual interstitial pneumonia: when is it truly idiopathic? Eur Respir Rev. 2014; 23: 308-319. https://goo.gl/CCL2S5
- Glazer SC, Rose CS, Lynch DA. Clinical and radiologic manifestations of hypersensitivity pneumonitis. J Thorac Imaging. 2002; 17: 261-272. https://goo.gl/mmdLhk
- Costabel U, Bonella F, Guzamn J. Chronic hypersensitivity pneumonitis. Clin Chest Med. 2012; 33: 151-163. https://goo.gl/SCELRg
- Vourlekis JS, Schwarz MI, Cherniack RM, Curran Everett D, Cool CD, Tuder RM, et al. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med. 2004; 116: 662-668. https://goo.gl/9yRCh1
- Hwang JH, Misumi S, Sahin H, Brown KK, Newell JD, Lynch DA. Computed tomographic features of idiopathic fibrosing interstitial pneumonia: comparison with pulmonary fibrosis related to collagen vascular disease. J Comput Assist Tomogr. 2009; 33: 410-415. https://goo.gl/Q8z3Tx
- Lynch DA, Newell JD, Logan PM, King TE JR, Muller NL. Can CT distinguish hypersensitivity pneumonitis from idiopathic pulmonary fibrosis? AJR Am J Roentgenol. 1995; 165: 807-11. https://goo.gl/a43G1V
- Silva CI, Chrug A, Muller NL. Hypersensitivity pneumonitis: spectrum of high-resolution CT and pathologic findings. AJR Am J Roentgenol. 2007; 188: 334-44. https://goo.gl/hCR3bm
- Palmucci S, Roccasalva F, Puglisi S, Torrisi SE, Vindigni V, Antonellamauro L. Clinical and radiological features of idiopathic interstitial pneumonias (IIPs): a pictorial review. Insights Imaging. 2014; 5:347-364. https://goo.gl/G8jey
- Akashi T, Takemuta T, Ando N, Eishi Y, Kitagava M, Takizawa M, et al. Histopathologic analysis of sixteen autopsy cases of chronic hypersensitivity pneumonitis and comparison with idiopathic pulmonary fibrosis/usual interstitial pneumonia. Am J ClinPathol. 2009; 131: 405–415. https://goo.gl/hj4ZBY
- Myers JL. Hypersensitivity pneumonia: the role of lung biopsy in diagnosis and management. Mod Pathol. 2012; 25: S58–67. https://goo.gl/Ai7vze
- Takemura T, Akashi T, Ohtani Y, Inase N, Yoshizawa Y. Pathology of hypersensitivity pneumonitis. CurrOpinPulm Med. 2008; 14: 440–454. https://goo.gl/tY1ji3
- Churg A, Muller NL, Flint J, Wright JL. Chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2006; 30: 201–208. https://goo.gl/V4Ah1e
- WenBinXu, Yi Xiao, HongRui Liu, MingWei Qin, WenJieZheng, JuHong Shi. Nonspecific interstitial pneumonia: clinical associations and outcomes. BMC Pulm Med. 2014; 14: 175. https://goo.gl/LYQC66
- Churg A, Sin DD, Everett D, Brown K,Cool C. Pathologic patterns and survival in chronic hypersensitivity pneumonitis . Am J SurgPathol. 2009; 33: 1765–1770. https://goo.gl/YHXpRb
- Takemura T, Akashi T, Kamiya H, Ikushima A, Ando T, Oritsu M, et al. Pathological differentiation of chronic hypersensitivity pneumonitis from idiopathic pulmonary fibrosis/usual interstitial pneumonia. Histopathology. 2012; 61: 102–35. https://goo.gl/WnwLy4
- Schenider F, Gruden J, Tazelaar HD, Lesliko KO.Pleuropulmonary pathology in patients with rheumatic disease. Arch Pathol Lab Med. 2012; 136: 1242–1252. https://goo.gl/xMmdLc
- Saag KG, Kolluri S, Koehnke RK, Georgou Ta,Rachow JW,Hunninghake GW. Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnormalities. Arthritis Rheum.1996; 39: 1711–1719. https://goo.gl/ZEpq2x
- Aquino SL, Webb NM, Golden J. Bronchiolitis obliterans associated with rheumatoid arthritis: findings on HRCT and dynamic expiratory CT. J Comput Assist Tomogr. 1994; 18: 555-558. https://goo.gl/QhuJpG
- Walsh SL, Wells AU, Sverzellati N, Deveraj A, von der Thusen J, Yousem SA, et al. Relationship between fibroblastic foci profusion and high resolution CT morphology in fibrotic lung disease. BMC Med. 2015; 13: 241. https://goo.gl/3h8Yf8
- Kim EJ, Collard HR, King TE Jr. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009; 136:1397–405. https://goo.gl/ACBhax
- Lee HK, Kim DS, Yoo B, Seo JB, Rho JY, Colby TV, et al. Histopathologic pattern and clinical features of rheumatoid arthritisassociated interstitial lung disease. Chest. 2005; 127: 2019–2027. https://goo.gl/LtzXDs
- Katzenstein Al, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis: histologic features and clinicsignificance. Am J Surg Pathol. 1994; 18: 136-147. https://goo.gl/zkC6UK
- Robbins SL, Cotran RZ. Pathologic basis of disease. Vol.2. In: Elsevier Masson, eds. The Lung. 2005: 711-772.
- Webb WR, Higgins CB. Thoracic imaging – Pulmonary Cardiovascular Radiology. In: Lippincott Williams & Wilkins, eds. Idiopathic Interstitial Pneumonias. 2011; 197: 433-455. https://goo.gl/FNDoLn
- Walsh SL Wells AU, Sverzellati N, Deveraj A, von der Thusen J, Yousem SA, Colby TV, et al. Relationship between fibroblastic foci profusion and high resolution CT morphology in fibrotic lung disease. BMC Med. 2015; 13: 241. https://goo.gl/wtsCwK

Sign up for Article Alerts