Pulmonary Function Test Abnormalities in Obese Non-Asthmatic Children and Adolescents
Angela Webb1, Roopa Siddaiah2, Melodi Pirzada1, Shahidul Islam3, Christina Valsamis1 and Claudia Halaby1*
1Pediatric Pulmonary Medicine, NYU Winthrop Hospital. Mineola, NY, USA
2Pediatric Pulmonary Medicine, Penn State Milton S Hershey Medical Center. Hershey, PA, USA
3Department of Biostatistics. NYU Winthrop Hospital. Mineola, NY, USA
*Address for Correspondence: Claudia Halaby, Pediatric Pulmonary Medicine, NYU Winthrop Hospital. Mineola, NY, USA, Tel: +516.663.3832; Fax: 516.663.33826; Email: chalaby@nyuwinthrop.org
Submitted: 31 August 2017; Approved: 15 September 2017; Published: 18 September 2017
Citation this article: Webb A, Siddaiah R, Pirzada M, Islam S, Halaby C, et al. Pulmonary Function Test Abnormalities in Obese Non-Asthmatic Children and Adolescents. Sci J Pulm Respir Med. 2017;1(1): 019-023.
Copyright: © 2017 Halaby C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Background: Obesity in children is a growing concern. There is little information on the effects of obesity on pulmonary function in non- asthmatic children.
Objectives: Identify abnormalities of Pulmonary Function Testing (PFT’s) in a cohort of non-asthmatic obese children and if present note the correlation between the degree of abnormality and the severity of obesity.
Method: A cohort of 35 subjects underwent standardized PFT’s [spirometer, static and dynamic lung volumes, and Diffusion Capacity for Carbon Monoxide (DLCO)]. Measurements were reported as percent predicted value for age, gender, height and ethnicity.
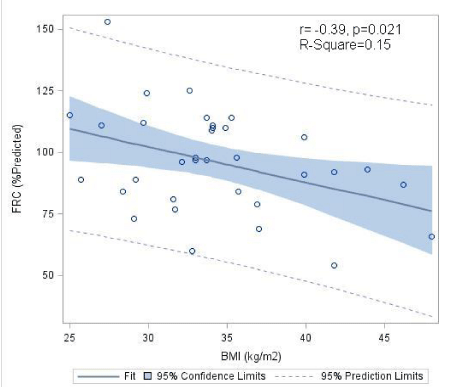
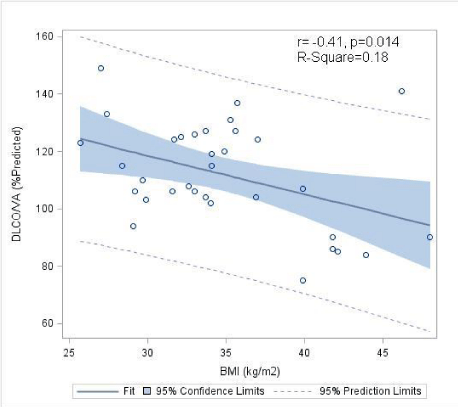
Results: 14 females and 21 males between 10 and 20 years of age were enrolled. The median BMI was 34.5 Kg/m2. . Isolated PFT abnormalities or abnormalities suggestive of lower airway obstructive defect or restrictive defect were noted in 20 patients (57%). Spiro metric measurements were generally unremarkable, with the exception of decreased in FEF25-75% in 9 subjects (25%), without direct correlation with BMI. Changes in static lung volumes suggestive of reduced respiratory system compliance was observed, with 16 subjects (45%) exhibiting a decrease in ERV and 6 subjects (17%) with decreased in FRC. DLCO/VA was increased in 12 participants (36.3%). A negative correlation between BMI and FRC (Rho -0.39, p-value 0.021) and BMI and DLCO/VA (Rho -0.42, p-value 0.015) was found.
Conclusion: In our cohort of obese, non-asthmatic children we observed PFT abnormalities suggestive of decreased respiratory system compliance and gas diffusion impairment that correlated with increasing BMI.
Introduction
Obesity in children is a growing problem [1]. Obesity has more than doubled in children and tripled in adolescents in the past 30 years. The percentage of children, ages 6–11 years, in the United States who were obese increased from 7% in 1980 to nearly 18% in 2010. Similarly, the percentage of adolescents, ages 12–19 years, who were obese increased from 5% to 18% over the same period. In 2010, more than one third of children and adolescents were overweight or obese [2].
Obesity in children, as in adults, can lead to various health problems, such as insulin resistance, diabetes, cardiovascular disease, musculoskeletal issues, and decreased exercise tolerance. In addition, obesity has been identified as a major risk factor for the development of asthma and is associated with profound changes in pulmonary physiology resulting in development of sleep-disordered breathing, and altered susceptibility to pulmonary infection [3].
There is a paucity of information on the relationship between obesity and lung function in children and adolescents. Data on Pulmonary Function Tests (PFTs) in obese children with and without asthma is scarce.
The most consistently reported effect of obesity on PFT in adults is a reduction in the Functional Residual Capacity (FRC). Obesity produces a “stiffening” of the entire respiratory system, likely due to a combination of effects on lung and chest wall compliance [4]. The reduction in lung compliance appears to be exponentially related to Body Mass Index (BMI) [5].
The effects of obesity on airway flow rates are controversial. Forced Expiratory Volume in the first second (FEV1) and Forced Vital Capacity (FVC) tend to decrease with increasing BMI. The FEV1-to-FVC ratio is typically well preserved or increased indicating that both FEV1 and FVC are affected to the same extent [6].
While studies on the Diffusing Capacity of The Lung for Carbon Monoxide (DLCO) in obese patients have been conflicting, Enache I .et al. found that DLCO was unaltered or reduced in obese subjects [7].
The primary objective of our study is to identify abnormalities of PFTs in non-asthmatic obese children. We hypothesized the PFTs abnormalities seen in obese adults also exist in children and adolescents and that these pulmonary function abnormalities are correlated to the degree of obesity.
Methods and Materials
Thirty five children from 10 to 20 years of age were recruited from the obesity clinic and CHANGE program (weight loss intervention program) at Winthrop University Hospital. Subjects participating in our study had a BMI (Weight (kg) / (Height (m))2) > 95th percentile for age and gender. Participants had no prior or current physician diagnosis of asthma based on typical asthma symptoms, such as recurrent wheeze, cough, and shortness of breath resolving with or without an inhaled bronchodilator.
Subjects with contraindication for performing PFTs, such as hemoptysis, pneumothorax, and recent eye, thoracic or abdominal surgeries were excluded from the study. Children with obesity due to a genetic syndrome, known cardiovascular disease, neuromuscular disorders, musculoskeletal deformities, or other restrictive pulmonary processes were not considered.
The local Institutional Review Board approved the study. Informed consent and assent from each subject and their parents were obtained at the time of enrollment.
Potential participants were evaluated for cardiopulmonary disease prior to enrollment. Weight and height were measured with a calibrated weighing scale and stadiometer following standard anthropometric methods.
PFTs were performed by the pediatric pulmonary department using Med graphic Ultima PFX equipment according to the American Thoracic Society/European Respiratory Society guidelines [8]. Spiro metric measurements (FVC, FEV1, FEV1/FVC, PEF, FEF25-75%) static lung volumes (ERV, FRC, RV), and dynamic lung volumes (TLC, VC) were recorded. Lung volumes were determined using nitrogen washout technique. All measurements were reported using a normal range of 80% to 120% of predicted value for age, gender, height, and ethnicity. Weng scale for FRC and Weng and NHANES III scale were utilized for the remaining measurements. DLCO was performed with single breath technique and results were corrected for alveolar volume (DLCO/VA); 75% to 120% of predicted value using Weng scale was considered within normal limits.
Statistical Analysis
Descriptive statistics are presented as mean ± SD. The Kolmogorov-Smirnov test was used to evaluate normality of PFT variables across whole sample and by gender. The variables were then compared between genders using two independent sample t-tests. Pearson’s correlation coefficients with 95% confidence intervals were calculated using Fisher’s Z-transformed data. Priori analyses of covariance (ANCOVA) models were developed using gender and BMI as the indicator variables.
This was an exploratory study that is limited in terms of statistical power due to its small sample size. As a result, no formal power calculation was carried out.
All calculations were performed using SAS 9.3 (SAS Institute, Inc)
Results
Of the 35 participants in the study, 14 were females and 21 were males. Twenty one participants were Caucasian (60%), 6 were African American (17%), and 8 were Hispanic (23%). Ethnic distribution of participants in the study was similar to the ethnic distribution in the county where the study was conducted.
Their median BMI was 34.5 Kg/m2(Interquartile Range (IQR) 29.9-37). The demographic characteristics of the study group are presented in Table 1.
Spiro metric measurements were in general normal, with the exception of an isolated decrease in FEF25-75% in 9 patients (25%), with abnormal values ranging from 50% to 78% of the normal predicted value, without direct correlation with BMI.
Abnormalities of lung volumes were more frequently seen. In terms of static lung volumes, 16 subjects (45%) had reduced ERV (with values ranging from 33% to 74% of the normal predicted value) and 6 (17%) had decreased FRC (ranging from 54% to 77% of the normal predicted value), suggestive of decreased lung compliance. An increase in RV was observed in 12 subjects (34%), of these, 2 (5.7%) also had an increase in FRC and TLC suggestive of a lower airway obstructive defect.
Dynamic lung volumes of subjects included in the study were generally within normal limits for the predicted values. The only exceptions were 3 subjects that had an increase in TLC (range 125-138% of predicted value) and 2 subjects that had a decrease in TLC associated with reduced FRC and RV, suggestive of restrictive lung defect.
DLCO/VA was measured in 33 subjects. It was increased in 12 participants (36.3%) with abnormal values ranging between 123% and 149% of the normal predicted value. None of the participants in the study had a decrease in DLCO/VA.
A comparison of the mean values of PFT measurements overall and by gender is shown in Table 2. There were no statistically significant differences in the Spiro metric measurements or in dynamic and static lung volumes between males and females. Males had a higher DLCO/VA than females (p = 0.035). There were no statistical differences in values of PFT’s between children younger and older than 15 years of age (data not shown).
The correlation between the PFT variables and BMI is shown in Table 3. A statically significant negative correlation between BMI and FRC was found (Rho -0.39, p-value 0.021) (Figure1). Similarly, there was a statistically significant negative correlation between BMI and DLCO/VA (Rho -0.41, p-value 0.014) (Figure2).
Discussion
In our cohort of non-asthmatic, obese children, 57% of the participants had PFT abnormalities.
In terms of static lung volumes, the most prominent observed changes were decreases in FRC (17% of the participants) and ERV (45% of the participants). The decrease in FRC was negatively correlated with the BMI. Studies in obese adults found similar lung volume abnormalities [9,10]. These changes suggest a reduction in lung compliance that may be the result of increased pulmonary blood volume and early closure of dependent airways [11]. The reduction of chest wall compliance due to increased adipose tissue surrounding the ribs, diaphragm, and abdomen also contributes to decreased overall respiratory compliance [12].
Other studies have had similar conclusions. Li, AM. et al. [13] studied a cohort of 64 children, ages 10 to 14 years, with primary obesity and found a negative correlation between FRC and BMI, but was not noted to be statistically significant as in our study.
In addition, 12 subjects (35%) in our study, had an elevated RV, suggestive of air trapping. The RV elevation is likely a result of the diminished ERV, which could be explained by the narrowing effect on small airways secondary to obesity. These changes were previously observed by Inselma LS et al [14].
As noted in adult studies [15], the majority of the children in the study had normal dynamic lung volumes (including VC and TLC).
We did observe a restrictive lung disease pattern (decreased TLC, RV, and FRC) in 2 subjects. These abnormal measurements were less likely due to an intrinsic lung disease and more likely due to obesity, since these children had no respiratory symptoms, had normal DLCO/VA, but had higher BMIs (41.8Kg/m2and 48Kg/m2). This restrictive defect can be explained by increased adipose tissue surrounding the ribs, diaphragm, and abdomen resulting in chest wall restriction.
The most frequent spirometric measurement change observed in our study was a decrease in FEF25-75% in 25% of the subjects. This flow limitation is suggestive of small airway obstruction. A reduction in airway caliber in obesity may be attributed to a reduction in FRC [16,17]. However, some studies have suggested that the narrowing of the airways may not be due entirely to the reduced lung volumes, since differences between obese and non-obese may persist even after adjustment for lung volumes [9]. Additional studies have shown that obesity is associated with an increased risk of airway hyper responsiveness [18,19], suggesting that a pro-inflammatory state associated with obesity can be responsible for this flow limitation.
Previous studies in obese, non-asthmatic children [13,14] found a decrease in DLCO without correlation with BMI. These abnormalities were explained by the authors as a result of structural changes in the interstitium of the lung resulting from lipid deposition and/or decreased alveolar surface area.
In our study, similar to studies in obese adults [20], we observed an increase in the ratio of DLCO to alveolar volume in 36% of our obese children. The increase in DLCO/VA was negatively correlated with BMI. This increase in DLCO/VA observed in obese patients, is believed to be due to an increase in pulmonary blood volume [15]. On the other hand, a low to normal DLCO or DLCO/VA and the negative correlation with BMI in some of the obese children in our study, may represent a loss of capillary bed (as seen with atelectasis) [15] or alterations in the alveolar-capillary membrane due to lipid deposition [13].
Our study has some limitations. We acknowledge that we have investigated a relatively small number of subjects, however our group was ethnically diverse (reflecting the ethnic distribution of the county where the study was conducted), and was homogeneous in terms of age and BMI distribution. All PFT measurements were reported as percent of the normal predictive value for the age, height, gender, and ethnicity. For this reason, we did not include a control group. Despite these limitations, our findings add relevant clinical data to the literature. Our study is one of the few prospective cross sectional studies in non-asthmatic, obese children that includes measurements of static and dynamic lung volumes, flow rates and DLCO, and then correlates these values with the severity of obesity. To better understand the natural history of these abnormalities, larger longitudinal studies focused on assessing the changes in lung function in relation to BMI variations over time are warranted.
Conclusion
In our cohort of obese, non-asthmatic children we observed PFT abnormalities suggestive of decreased lung and chest wall compliance (FRC) and gas diffusion impairment (DLCO) that correlate with increased BMI.
Our findings also support the use of BMI to predict the effect of obesity in pulmonary function. BMI is a simple tool that is widely available in pediatric health care settings to classify the severity of obesity. Our study is one of the few to date that has found a statistically significant relationship between BMI and abnormalities in lung volumes and DLCO in obese children.
Larger longitudinal studies are needed to support these findings and expand our current knowledge regarding the effects of obesity on pulmonary function in children.
- van de Griendt EJ, van der Baan-Slootweg OH, van Essen-Zandvliet EE, van der Palen J, Tamminga-Smeulders CL, Benninga MA, et al. Gain in lung function after weight reduction in severely obese children. Arch Dis Child. 2012; 97: 1039-1042. https://goo.gl/9ENtFF
- Statistics NCfH. Health, United States, 2011: Special feature on socioeconomic status and health. USA: Statistics NCfH; 2012. https://goo.gl/wyaZvg
- Suratt BT, Ubags NDJ, Rastogi D, Tantisira KG, Marsland BJ, Petrache I, et al. An official American Thoracic Society workshop report: Obesity and metabolism, an emerging frontier in lung health and disease. Ann Am Thorac Soc. 2017; 14: 1050-1059. https://goo.gl/ZbxJZk
- Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996; 109: 144-151. https://goo.gl/fm37UY
- Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998; 87: 654-660. https://goo.gl/X2gbd6
- Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010; 108: 206-211. https://goo.gl/WPeCmw
- Enache I, Oswald-Mammosser M, Scarfone S, Simon C, Schlienger JL, Geny B, et al. Impact of altered alveolar volume on the diffusing capacity of the lung for carbon monoxide in obesity. Respiration. 2011; 81: 217–222. https://goo.gl/o6w5yH
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardization of spirometry. Eur Respir J. 2005; 26: 319-338. https://goo.gl/sCMqLX
- King GG, Brown NJ, Diba C, Thorpe CW, Muñoz P, Marks GB, et al. The effects of body weight on airway caliber. Eur Respir J.2005; 25: 896–901. https://goo.gl/fEM6e2
- Jones RL, Nzekwu MMU. The effects of body mass index on lung Volumes. Chest. 2006; 130: 827–833. https://goo.gl/paUWEN
- Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001; 321: 249-279. https://goo.gl/9uo2tQ
- Ashburn DD, DeAntonio A, Redd MJ. Pulmonary system and obesity. Crit care clin. 2010; 26: 597-602. https://goo.gl/qzqug1
- Li AM, Chan D, Wong E, Yin J, Nelson EAS, Fok TF. The effects of obesity on pulmonary function. Arch Dis Child. 2003; 88: 361-363. https://goo.gl/1UfFvu
- Inselma LS, Milanese A, Deurloo A. Effect of obesity on pulmonary function in children. Pediatr Pulmonol. 1993; 16: 130-137.
- Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006; 13: 203-210. https://goo.gl/FxzV1j
- Zerah F, Harf A, Perlemuter L, Lorino L, Lobn rino AM, Atlan G. Effects of obesity on respiratory resistance. Chest. 1993; 103: 1470-1476. https://goo.gl/GaESYA
- Rubinstein I, Zamel N, DuBarry L, Hoffstein V. Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med. 1990; 112: 828-832. https://goo.gl/gAVW9H
- Chinn S, Jarvis D, Burney P: European community Respiratory Health survey. Relation of bronchial responsiveness to body mass index in the ECRHS. European Community respiratory health survey. Thorax. 2002; 57: 1028-1033. https://goo.gl/EQ4GDi
- Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of methacolline airway hyperresponsiveness in men: The Normative Aging Study. Thorax. 2002; 57: 581-585. https://goo.gl/uA2o7R
- Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983; 128: 501-506. https://goo.gl/UP8dnQ

Sign up for Article Alerts