Fragment Specific Classification and Fixation of Fractures of the Distal Radius
Ahmed Fathy Mohammed Sadek*
Assistant Professor of Orthopaedic Surgery and Traumatology, Orthopaedic Surgery Department, Faculty of Medicine, Minia University
*Address for Correspondence: Ahmed Fathy Mohammed Sadek, Assistant Professor of Orthopaedic Surgery and Traumatology, Orthopaedic Surgery Department, Faculty of Medicine, Minia University, Egypt, Tel: +002-010-111-506-66; E-mail: sadek_orthop@yahoo.com
Submitted: 08 July 2017; Approved: 10 September 2017; Published: 15 September 2017
Citation this article: Mohammed Sadek AF. Fragment Specific Classification and Fixation of Fractures of the Distal Radius. Int J Sports Sci Med. 2017;1(2): 034-043.
Copyright: © 2017 Mohammed Sadek AF. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: APL: Abductor Pollicis Longus; CRPS: Complex Regional Pain Syndrome; CRPS: Complex Regional Pain Syndrome; DRFs: Distal Radius Fractures; DRUJ: Distal Radio-Ulnar Joint; EPB: Extensor Pollicis Brevis; EPL: Extensor Pollicis Longus; FCR: Flexor Carpi Radialis; FVLP: Fixed Angle Volar Locked Plate; LTLI: Luno-Triquetral Ligament Injuries; PL: Palmaris Longus; RCJ: Radio-Carpal Joint; RPP: Radial Pin Plate; SLLI: Scapho-Lunate Ligament Injuries; TFCC: Triangular Fibrocartilage Complex; UPP: Ulnar Pin Plate; VCA: Volar Cortical Angle; VLP: Volar Locked Plate; VVLP: Variable angle Volar Locked Plate
Download Fulltext PDF
Introduction
Distal Radius Fractures [DRFs], which are coined to the term (pilon radiale), are the most common upper extremity fractures constituting 17-18% of all emergency fractures. The intra-articular variant stands for 50% of DRFs [1]. Unfortunately, DRFs are usually associated with other bony or soft tissue injuries in variable percentages according to the magnitude of trauma and the bone quality. For example, ulnar styloid fracture is associated with DRFs in 50-70% of cases [2]. In addition, it has been estimated that DRFs could be associated with capsular tears [2.4%], Triangular Fibro Cartilage Complex [TFCC] tears [40-60%], Scapho-Lunate Ligament Injuries [SLLI] [2-40%], Luno-Triquetral Ligament Injuries [LTLI] [20-68%] and cartilage lesions [2-30%] [3-5]. DRFs show trimodal pattern of occurrence being common at young adults (high energy trauma), after 60 years, and in postmenopausal osteoporotic women (low energy trauma) [6]. The clinical objectives of treatment of DRFs include: restoration of distal radius configuration through anatomic stable reduction, restoration of articular congruity of the radiocarpal and distal radioulnar articulations, maintenance of reduction through stable fixation, and finally allowing early active rehabilitation. Early active motion initiates some potential benefits comprising: minimizing stiffness, negating osteopenia of the distal fracture fragment, and enhancing cartilage repair. In addition there are radiological objectives aiming at restoration of distal radius alignment including: radial height loss < 5 mm, radial inclination > 15°, radiocarpal and radioulnar articular step-off < 2mm, maintaining sagittal tilt of the distal radial articular surface between 20° volar and 15° dorsal tilt [7]. Some authors have shown that articular surface step-off by > 1-2 mm will result in deleterious radiocarpal arthritic changes in 90% of patients within a follow-up period of 6-7 years [8]. Other authors suggest that the ability of intra-articular fracture remodeling becomes very limited when the joint step-off exceeds the thickness of the articular cartilage [6]. On the other hand, it is well established that coronal displacement of the DRFs will negatively affect the Distal Radioulnar Joint [DRUJ] function particularly in pronation/supination. Similarly, radial collapse will result in ulnocarpal impaction. Accordingly, DRFs with complex fragmentation patterns, extensive articular comminution, and meta-diaphyseal bone loss pose unique challenges [7,9]. Cast immobilization has been used satisfactorily in cases of undisplaced fractures or displaced stable fractures after reduction. In addition, it represents an appealing treatment option for elderly, unfit, and low demand patients. However, in the young, active, or high demand patients who have high expectations of regaining their normal activities, surgery might be mandatory to achieve the previously mentioned clinical and radiological objectives. Various surgical techniques have been proposed for such fractures including: Closed reduction and percutaneous k-wires fixation whether intra-focal [10], inter-focal, combined, or intra-focal cross-pinning [11]. External fixators whether fixed bridging, mobile bridging employing the concept of ligamentotaxis, or non-bridging radio-radial external fixators have been used extensively [12]. In addition, external fixator augmenting K-wires fixation for the control of intra-articular fragments has been shown great stability in such situations [13]. Other methods such as distraction bridge plate fixation [14,15], open reduction and internal fixation using single dorsal plating system [16] or single volar plating system including the four generations of the radial volar plates; non-locking volar plates, Fixed Angle Volar Locking Plates [FVLP], Variable Angle Volar Locking Plates [VVLP], and finally anatomical locking plates applying the double tired subchondral support principle of distal plate screws [17,18] have been added to the armamentarium. Moreover, intramedullary devices including micronail and dorsal nail plate have been used for DRFs with minimal articular involvement [6]. Bio-absorbable implants have been considered one of the up to date technological advents in DRFs fixation. The Inion OTPS Hand System (Inion Inc, Oklahoma City, OK) is bio-absorbable distal radius plate which is commercially available. This system is contoured after application in hot water, biodegradable within 2 years and accepts polyaxial locking screws up to 20° [6]. The Tri-Med Fragment Specific Fixation [FSF] system was first introduced by Medoff and Kopylov in 1998 for tailored fixation of complex multi fragmentary intra-articular DRFs [19]. Such fractures comprise very small and distal fragments in amenable for traditional plate and screws fixation [7,20]. This has been augmented by the arthroscopic assisted fixation which has shown great evolutions in the last decade starting from the standard wet technique [3,4] to the dry technique introduced by Del Pinal [5,21]. Despite the multitude of surgical techniques and the diversity of implants used for fixation there is no level I evidence supporting certain type of treatment for DRFs [22]. Since its introduction in 1998, FSF system proved to stand the test of time as a versatile tool that could be used efficiently for DRFs fixation particularly in complex multi fragmentary intra-articular patterns that is in amenable for traditional single plating system fixation. This review will focus some highlights on the Fragment Specific Classification [FSC] in addition to relevant anatomy, biomechanics, indications, techniques and approaches of the FSF system. Finally an overview of the literature regarding the outcome and complications associated with its use will be exhibited.
Relevant anatomy and Biomechanics
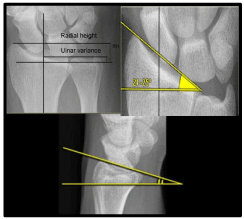
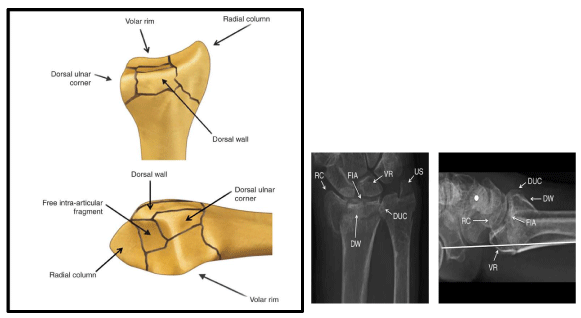
It is well known that the distal radius has a quadrilateral cross section with well-defined anatomic features including the styloid process, the dorsal (Lister’s) tubercle, and four surfaces: anterior, posterior, lateral, and medial. The volar cortex is thicker than the dorsal cortex with both being thinner in females [23]. The volar cortex is thicker towards the ulnar side. There is no difference in cortical thickness between medial and lateral surfaces [24]. The scaphoid fossa, lunate fossa, and sigmoid notch are three concave articular surfaces. The scaphoid and lunate fossae are separated by a dorso-volar ridge which defines the scaphoid and lunate facets. The volar lip of the lunate facet projects distally 3mm more than the scaphoid facet with being only 5 mm thick representing an extreme difficulty in fixation in cases of fractures. The styloid process is conical and projects 10–12 mm beyond the articular surface of the scaphoid and lunate facets constituting the radial height. In addition, it projects anterior to the coronal plane of the radial diaphysis by 15°. The dorsal tubercle lies 5-10 mm proximal to the dorsal articular surface of the distal radius [25]. There is well established ulnar variance [0 ± 2 mm] which affects the amount of load transmitted to the distal radius and the TFCC. The ridge separating the DRUJ and Radio Carpal Joint [RCJ] bounding the distal part of the ulnar notch represents the radial attachment point for the TFCC. The distal radial articular surface has an average radial inclination of 22° [21-25] and volar tilt of 11° [2-20]. On the other hand, the sigmoid notch has distal and medial inclination averaging 22° [Figure 1].
New insights have been laid on the anatomy of the distal radius putting special emphasis on three important concepts namely: the watershed line, dorsal tubercle analysis and the three column theory [25].
Watershed line concept
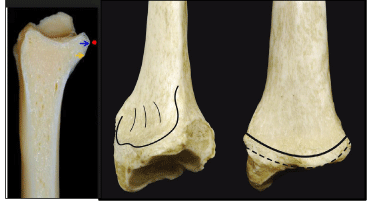
Nelson was the 1st to introduce the term watershed line of the distal radius describing the most distal fibrous zone bordered by the distal volar articular margin of the distal radius (distally) and the pronator quadratus line [distal attachment line of the pronator quadrates] (proximally). The area in between these two lines measures approximately 3-5 mm. This line represents the most distal allowable limit for application of volarly placed implants, otherwise impingement of the Flexor Pollicis Longus [FPL] and finger flexor tendons ensues [Figure 2]. In a biomechanical study, it was demonstrated that the contact pressure between the Flexor Tendons Particularly [FPL]. With the volar distal margin of the Volar Locked Plate [VLP] is significantly increased if this margin bypasses the watershed line [26-28].
Dorsal tubercle analysis
Extensive morph metric measurements have been performed regarding the length and height of the Lister’s tubercle in cadavers and CT scans. It was found that its mean height and length were (3.6 mm& 18.3 mm) in cadavers and (3.3 mm& 13.2 mm) in CT scans respectively. On the ulnar side, the height between the bottom of the groove and the tip of the tubercle was twice the height in the cadaver study (7 mm) than in the CT scan study (3.4 mm) [29,30]. Gases et al. found that the pronator quadrates line is separated from the tip of the dorsal tubercle by about 22 mm which forms a mainstay in determination of the length of the distally placed screws to avoid irritation of the EPL or other extensor tendons [31].
Wrist Columns and Volar Cortical Angle [VCA]
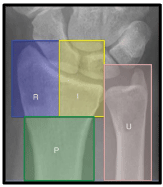
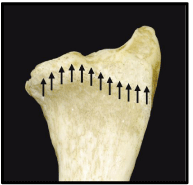
The three column concept introduced by Rikli and Regazzoni in 1996 divides the distal radius and ulna into radial, intermediate and ulnar columns [32]. The radial column encompasses the radial styloid process and the scaphoid fossa. It provides a platform to support the carpus and prevent radial translation while the wrist is loaded in ulnar deviation. In addition, it serves as an anchor for the radio-scapho-capitate and long radio-lunate ligaments preventing ulnar translation of the carpus. Moreover, it is the insertion site for the brachioradialis which is the main deforming force of this column in cases of DRFs. The intermediate column includes the lunate and the sigmoid fossae. The volar lip of the lunate facet gives origin to the short radio-lunate ligaments which prevent volar subluxation or dislocation of the carpus in addition to the volar distal radio-ulnar ligament [7]. The volar cortex of the intermediate column extends distally more than the radial column which should be taken into consideration while applying any hardware to the volar distal radius and in plate manufacturing [25].The dorsal wall of the intermediate column gives attachment to the dorsal radio-carpal ligaments and serves as a dorsal support for the carpus preventing dorsal subluxation or dislocation when the radiocarpal joint is loaded [7]. The radial column, intermediate column and the supporting radial metadiaphysis [pedestal] serve mainly for load transmission from the carpus to the forearm. Finally, the ulnar column encompasses the ulnar head and the TFCC which maintain the DRUJ and forearm rotation [26] [Figure 3,4]. The stability of the DRUJ relies mainly on the bony architecture of the ulnar head and the sigmoid notch in addition to the volar and dorsal distal radio-ulnar ligaments attached to the volar rim and dorso-ulnar corner of the lunate facet respectively. The distal oblique bundle, a ligamentous structure within the distal interosseous membrane, inserts onto the dorsal inferior rim of the sigmoid notch and is a secondary stabilizer of the DRUJ [7]. The ulnar volar cortical angle of distal radius was found to be more than the radial volar cortical angle in cadaveric studies [35° versus 25°]. This difference was less in CT studies. In addition females have higher VCA than males [25] [Figure 5].
Putnam et al showed that every 10 N grip force is transmitted to axial force in the distal radial metaphysis of about 26-52 N depending on hand position and radius length [33]. It has been demonstrated biomechanically that dorsal radial tilt by 45° results in increased ulnar load from 21% to 67% [34]. A load exceeding 2500 N is required to break the radius. On the other hand, loads causing fixation system failure range from 55-825 N and are directly related to the type of hardware used and its inherent characteristics [35]. Anatomically, the main distal fracture line of DRFs has been well documented to be dorsally at 7.9 ± 2.7 mm and palmarly 11.7 ± 3.9 mm proximal to the dorsal/palmar apex of the lunate fossa, running obliquely from palmar proximal to dorsal distal [36].
Classification of DRFs and Fragment Specific Classification [FSC]
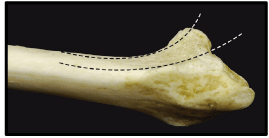
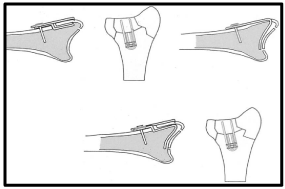
Ideal fracture classification should facilitate diagnosis, guide decision making, standardize treatment, help define expected outcomes, and serve as a research tool. More than 10 classification systems have been proposed for DRFs starting from Gartland and Werley [37], Frykman [38], the AO [39], Melone [40], till Fernandez [41]. In addition, a CT based classification was introduced dividing intra-articular DRFs into 5 types: (1) intra-articular fracture with displaced dorso-ulnar fragment, (2) dorsal split with dorsal dislocation, (3) palmar split with palmar dislocation, (4) complex DRF with metaphyseal comminution, and (5) destruction of the articular surfaces [42]. However, all classification systems have shown inter-observer and intra-observer unreliability [43]. The concept of intra-articular Fragment Specific Classification [FSC] was introduced by Medoff and Kopylov in 1998 [19]. This classification system describes five major distal radial articular fragments: the radial column, the dorsal wall, the dorso-ulnar corner, the volar rim, and the impacted intra-articular fragment [Figure 6].
To define the type of the fracture, adequate radiological evaluation should be done both pre-and post- traction. Standard x-ray views are required in the form of anteroposterior [A/P], lateral, and oblique views. In addition, scaphoid views and long forearm views may be required. The contralateral uninjured limb should be x- rayed. Lateral x-ray with 10° cephalic tilt, 10° tilt [A/P] and 45° oblique pronation views are very important projections to demonstrate clear view of the volar and dorsal articular rims. Computed tomography and three dimensional CT are sometimes required to delineate the fracture lines of complex comminuted intra-articular fractures. This modality provided great help to the upper limb surgeon as a tool for pre-operative planning [44].
Fragment Specific Fixation [FSF]
1.6.1. Principle: Fragment specific fixation has been introduced by Medoff and Kopylov in 1998 for management of complex intra-articular DRFs that are in amenable for treatment with single plating system. This technique has been introduced to fix every single fracture fragment with low profile small plates, pins or combination of both to achieve anatomical reduction with biomechanical stability allowing early rehabilitation. All of this could be achieved via minimal incisions and fixation devices which coapt with easier rehabilitation.
Medoff has outlined the basic principles for the FSF procedures including [19] :
• Application of pins, wire-forms, clamps, small contoured pin plates, small contoured low profile locking plates on the specific components of the fracture aiming at restoration of distal radius geometry.
• The fixation implants are symbiotic and multiplanar [by application of more than one plate in orthogonal planes with an angle of 50°-70°] creating rigid load sharing construct with small flexible implants having spring like behaviour.
• Fixation of distal fragments is based on the strong ipsilateral bone proximally.
• Hardware should allow for gliding motion of tendons.
• The aim of these implants is neutralizing the deforming dorsal and volar forces to the carpus.
• The exposure should cause minimal soft tissue disruption.
• The fracture should be stable enough to allow early range of motion.
• Application of cancellous bone graft or bone graft substitutes to fill the bony voids if needed.
• Volar arthrotomy should be avoided whenever possible and if needed this should be done through limited dorsal approach or via arthroscopy [45].
Implants used for FSF: When fixing small bone fragments, screw hole involves more than 30% of the fragment diameter, concentrating stress and decreasing bone strength to about 50% of that of intact bone, and this may result in iatrogenic comminution [46]. In such situations, the FSF system provides high modularity to fix the specific fractured columns, thus facilitating the management of complex multi fragmentary DRFs [47].
Implants used for FSF could be divided into 4 main categories [20]
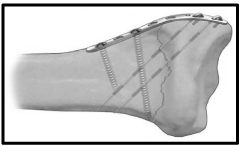
The pin plates [Figure 7] [48]: These are 2mm low profile pre-contoured titanium plates that have the criteria of incorporating 0.045 inch k-wires [pins] fixation with 2.3 mm cortical screw fixation to the proximal intact ipsilateral radial diaphysis. Each k- wire is used to stabilize the fracture fragment to the contralateral intact cortex. It is, then, bent into adjacent hole in the plate thus providing two point fixation construct thus achieving optimum stability by combining the versatility of the K-wires added to the rigidity of plate and screws. In addition, the pin-plate provides buttress to the fractured column fragment. Pin plates are applied to fix the radial column or intermediate column fractures via radial column pin plate or ulnar column pin plate [RPP, UPP] respectively. These plates are manufactured into different sizes [RPP 3,5,7], [UPP 3,5] [20].
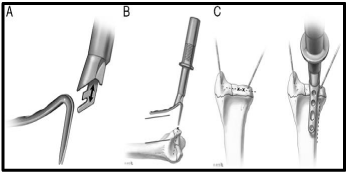
Hook plates [Figure 8]: Volar rim hook plate was introduced for fixation of the volar marginal rim or volar ulnar [lunate facet] fragment [49]. It has a characteristic narrow size with fixed angle hook allowing fixation of very distal volar rim fractures in osteoporotic patients. However, it is contraindicated in open fractures, inadequate soft tissue coverage, pediatric patients, fractures with metaphyseal voids, or very small fracture fragments. The tines of the hook plate are inserted just adjacent or distal to the watershed line while the plate is parallel to the radial shaft in the sagittal and coronal planes. The hook plate is inserted via special jig and inserter after temporary fixation of the fracture fragment with 0.045 inch k-wires. The proximal part of the plate is fixed to radial diaphysis with 2.3 mm cortical screws or pegs. Dorsal ulnar hook plates and dorsal radial hook plates were added to the category of hook plates to stabilize the dorso-ulnar corner and dorsal cortical rim fragments respectively [50,51].
Wire-forms and clamps [Figure 9]: These are pre-bent wires that can be applied to the dorso-ulnar corner, dorsal rim, volar rim, or intra-articular fragment fractures. They are fixed to the intact adjacent ipsilateral cortical bone using 2.3 mm cortical screws with one or two square washers to achieve maximal stabilization of the wire-form U-shaped proximal loop. They are classified into [20]:
• The small fragment clamp which provides stabilization of the dorsal cortical wall fragment that provides pinch-type grip with extra-osseous and endosteal wire form. This type comprises two subtypes:
1. Outer [dorsal] wire-form to fix dorsal rim fragment.
2. Inner wire-form in case there is severe comminution to prevent collapse of the dorsal cortical rim.
• Buttress pin where intra-articular fragments are stabilized by providing peripheral cortical reconstruction around fragment and adding endosteal buttress. They support intra-articular fragment or any structural bone graft used for its support and prevent collapse of the articular surface.
• Small fragment clamp/ buttress pin combines function of small fragment clamp and buttress pin into single device to provide simultaneous stabilization of dorsal wall fragment and intra-articular component.
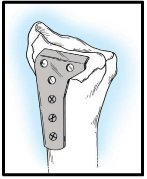
Volar buttress plates [Figure10]: These are 2 mm, 2.4 mm, or 2.7 mm L- or T-shaped low profile titanium plates that are used for buttressing the volar rim fractures. They are fixed to the pedestal supporting the radial column by cortical screws. The indication of such forms of plates is DRF that has very distal comminution in amenable for any type of fixation and just can be buttressed, in addition to those distal fractures that are associated with volar subluxation of the radio carpal joint and require volar support to maintain reduction [20]. Application of all screws through the distal limb of the plate usually is unnecessary. More recently, 2 mm, 2.4 mm, 2.7 mm locked plating systems have been added to the FSF system. Following the principles of Rikli and Regazzoni [32], by applying more than one low profile plate in orthogonal planes with an angle of 50°-70° , a multiplanar, load-sharing construct will result that anatomically restores the articular surface while providing enough stability to allow immediate motion after surgery [7].
Approaches used in FSF [52]
FSF could be performed through single or combined surgical approaches. Each approach could be employed for addressing certain fragment or fragments of the fracture while being minimally invasive. These approaches include: the distal Henry approach, the trans-Flexor Carpi Radialis [FCR] approach, the extended FCR approach, the modified Henry approach, the direct lateral approach to the 1st extensor compartment, the volar ulnar approach, the dual approach through a single volar incision, or the universal dorsal approach.
The distal Henry approach [52]: is indicated for fixation of DRFs particularly, volar rim and sometimes radial styloid fractures. A 5cm longitudinal incision is made just lateral to the FCR tendon extending distally to the distal volar wrist crease. Care should be taken to avoid injury of the palmar cutaneous branch of the median nerve that arises 3-5 cm proximal to the distal wrist crease and passes just lateral to the Palmaris Longus [PL] tendon. The superficial dissection proceeds between the FCR tendon and the radial artery. The FCR tendon is retracted medially protecting the median nerve while the radial artery with its surrounding fat is retracted laterally. Then, the FPL tendon and the finger flexor tendons are retracted medially. The pronator quadratus is now apparent in the field where it is incised in an L-shaped fashion both distally and radially to expose the distal radius. This approach provides access to distal volar radius, volar wrist capsule and scaphoid.
The trans-FCR approach [52]: Is exactly the same as the distal Henry approach with the only exception is that the superficial surgical dissection proceeds through the FCR tendon sheath.
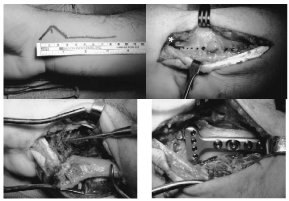
The extended FCR approach [53] [Figure 11]: This approach is versatile and very beneficial in gaining access to the volar aspect of the distal radius with more extension to the lateral and dorsal radial surfaces to address the DRFs with dorsal instability or comminution, intra-articular fractures, and relatively old fractures with nascent callus. The FCR approach is extended by: (1) releasing the radial septum, (2) mobilizing the proximal radial fragment into pronation, and (3) using the fracture plane for exposure or what is known as intraoral technique of reduction. This is achieved by mobilizing the proximal fragment in a trap door manner to have good view of the articular surface from inside the fracture site. From this point, the intra-articular fracture fragments are manipulated, reduced and molded against the carpus which acts as a template for reduction. In addition, bone graft can be placed into the voids of the fracture site.
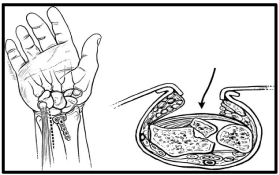
The volar ulnar approach [Figure 12] [54]: is indicated for FSF of the volar lunate facet fragment or the sigmoid notch fragment of the distal radius. A 5-cm longitudinal incision is made beginning proximally at the midpoint between the Flexor Carpi Ulnaris [FCU] and the PL and ending at the distal volar wrist crease. The deep interval is between the ulnar neurovascular bundle medially and the carpal tunnel contents laterally. The flexor tendons provide an excellent buffer to avoid pressure on the median nerve. The ulnar nerve and artery, which are more superficial, are identified and retracted ulnarly using right-angle retractor. The surgeon could be confronted by the median-ulnar nerve connections in the forearm and hand if this approach is extended e.g. Martin-Gruber, Marrinaci, and Riche-Cannieu connections [55].
Modified Henry approach [Figure 13] [56]: The skin incision is made lateral to the radial vessels with the plane is, now, between the radial vessels and the brachioradialis. Now the surgeon can proceed radially and dorsally to the 1st extensor compartment through the same incision. In addition, the brachioradialis is released and the styloid process is exposed after opening of the 1st extensor compartment with retraction of the Extensor Pollicis Brevis [EPB] and Abductor Pollicis Longus [APL] dorsally.
Direct lateral approach [52]: is indicated for exposure and fixation of radial column fractures. A direct straight lateral incision is centered over the 1st extensor compartment. The superficial radial nerve branches are identified and protected. Mostly, the superficial radial nerve emerges from below the brachioradialis tendon approximately 8-9 cm proximal to the radial styloid and on average divides into four branches [the surgeon should be careful for the possible high (5 cm proximal to the radial styloid) or low bifurcation of this nerve]. This nerve travels between the brachioradialis and extensor carpi radialis longus. The 1st extensor compartment is incised and the APL and EPB are retracted dorsally. An important step is the identification of the brachioradialis insertion which is routinely located about 17 mm proximal to the tip of the radial styloid and represents the floor of 1st extensor compartment. This tendon should be subperiosteally released to obviate its deforming force on the distal radius thus facilitating reduction maneuvers [52].
Dual approach from a single incision [57]: is indicated when the comminution is mainly volar with inability to approach the volar lunate fossa fragment through the distal or modified Henry approach. A straight midline volar incision is made extending to the distal volar wrist crease. After subcutaneous dissection, a lateral channel is approached lateral to the median nerve and medial to the FCR to access the distal radius. A medial channel is performed just medial to the median nerve and lateral to PL to expose the volar lunate fossa fragment and the sigmoid notch.
Universal Dorsal [trans-EPL] approach [52]: is utilized for exposing and fixing the dorsal rim and dorso-ulnar corner fractures. A standard straight 5 cm dorsal incision is performed targeting the 3rd dorsal compartment exposing more of the meta-diaphysis than the carpus. The EPL is identified and retracted laterally. The dorsal radius is exposed by subperiosteal dissection of the 2nd through 4th compartments, making a retinacular flap based medially. Care must be taken in the subperiosteal elevation because the dorsal fracture fragments are frequently bound to the undersurface of the retinaculum and need to be dissected free. Neurectomy of the posterior interosseous nerve is preferably performed at this time. The retinacular flap is used to cover the hardware used to minimize attrition of the tendons of the extensor compartments. When there is need for combined volar and dorsal approaches, the combined modified Henry and dorsal wrist approaches enable the surgeon to visualize 270° of the whole distal radius circumference [56].
Arthroscopic guided DRFs fixation [5]: Arthroscopically assisted DRFs fixation has gained uprising curve in the last decade due to its supreme role in the guidance of closed reduction, limited open reduction, or assessment and management of associated carpal injuries. Arthroscopy affords direct visualization of the radial styloid, scaphoid fossa, lunatefossa, TFCC, and the volar radiocarpal ligaments. Traction of 5 to 10 lb. provides adequate visualization and fluid flow. A small joint arthroscope with a diameter of 2.7 mm or smaller 30° lens is necessary. This could be efficiently performed through the 3-4 portal as a working portal and 6R portal as a viewing portal with fixed platform not interfering with the reduction. The use of wet arthroscopy was controversial in such situations due to the fear of fluid extravasation and compartment syndrome. That is why the dry wrist arthroscopy technique has been introduced. Del Piñal has proposed a few tips for adequate arthroscopic technique including keeping the scope sheath valve open, using suction power only when needed, using neurosurgical patties or a syringe 5-10 ml of saline attached to the side valve of the sheath and emphasizing that the joint should be irrigated when needed.
Medoff has illustrated the sequence of FSF as follows [20]:
• The initial step should be restoration of the radial column length and inclination with maintenance of the articular congruity with the intermediate column via initial fixation by trans-styloid pin/s with subsequent unloading of the lunate facet.
• The corner stone of fixation is the fixation of the volar lunate facet fragment which constitutes the basic step for progressing into stable fixation. In addition, this step is crucial for prevention of volar carpal subluxation or failure of fixation [57-60]. In case screws are placed from anterior, the length of the screws should not exceed 75% of the A/P diameter of the distal radius to facilitate dorsal reduction [7].
• The reduction and fixation of the dorsal comminution is done from ulnar to radial direction.
• The dorso-ulnar corner, which is usually displaced dorsally, proximally and ulnarly, is reduced and fixed using k-wires, ulnar pin plate, dorsal wire-form, ulnar hook plate or anatomically shaped 2.4 mm L-plate.
• Free intra-articular and dorsal wall fragments are reduced and fixed using wire-forms or buttress pins.
• The radial column is definitively fixed with RPP.
• If the ulnar column is involved to the degree that instability of the DRUJ is jeopardized, it should be stabilized to achieve articular congruity, uneventful pronation-supination motion and prevent chronic ulnar sided wrist pain. This could be achieved via fixing the ulnar styloid fracture by tension band wiring, mini screws, cannulated screws, bone sutures, anchors or k-wires. If there is associated ulnar head or neck fracture, this could be stabilized by anatomical low profile distal ulna plate.
• According to the personality of the fracture, fixation may be a subset of the previous steps.
In a modification of what Medoff has originally set as basic sequence of fixation, Rhee, Medoff and Shin have recently proposed an algorithm for surgical fixation of multifragmentary DRFs starting by fixing the intermediate column to the pedestal in stepwise fashion, addressing volar rim, dorso-ulnar corner, free intra-articular fragments, and dorsal wall fragments, in that order. The radial column is then, reconstructed onto the pedestal and buttressed against the intermediate column. As a final step, the ulnar column is checked for stability and if instability was found it is managed according to the underlying cause; fixing ulnar styloid fragment, DRUJ stabilization or TFCC repair [7].
Outcomes of [FSF]: Multifragmentary DRFs have always posed a major challenge for orthopaedic surgeons. For instance, the volar ulnar fragment is a key stone for the procedure of FSF which entails fixation using at least 2 screws to prevent rotation or displacement [18]. The 1st, 2nd, and even 3rd generation radial volar plates have shown high incidence of fixation failure when dealing with the volar lunate facet fragment. In addition, due to the common dorsal tilting nature of most DRFs, the application of volar plating seemed biomechanically disadvantageous [58,59]. Harness, et al. emphasized the role of FSF particularly regarding the volar lunate facet fragment in achieving stability of fixation with later on easier and more predictable rehabilitation [60]. Biomechanical studies have proven more biomechanical stability of the FSF systems compared to FVLP in stabilizing the dorso-ulnar corner fragment where no significant differences regarding cyclic load to failure were found. Moreover, improved stiffness characteristics regarding ulnar sided fixed fragments were found in the FSF system. In addition they can withstand normal physiological loads that allow early motion [61]. First generation dorsal plating systems with its notably high profile have fallen out of favour due to their reported complications namely; tendon attrition, rupture, the need for implant removal (25-33%), and loss of fracture reduction due to volar collapse. The recent advent of low profile pre-contoured dorsal plating systems has produced pronouncedly better results with minimal complications [6]. However, the introduction of the Acumed dorsal rim plate as part of FSF system proved anatomically and biomechanically more favourable with the combination of 2.3 mm and 3.5 mm pegs and screws [62]. External fixation devices are mainly used as adjuncts to other forms of fixation to provide ligamentotaxis in addition to neutralizing the forces at the fracture site whether bending, compression or torsion. Nevertheless, this is commonly associated with disabling complications (27%) in the form of stiffness, Complex Regional Pain Syndrome [CRPS], pin site infection or sensory nerves irritation. FSF has been shown to provide greater stability in comminuted fractures type C3 according to AO classification when compared to external fixator augmented with 0.062 inch K-wires while comparable stability was exhibited in type C2 [13]. In an interesting cadaveric study, an innovative non-bridging radial-radial external fixator construct was applied to the distal radius to address 3-part or 4-part intra-articular fractures [Fragment Specific Fixator; South Bay Hand Surgery LLC, Torrance, CA]. They achieved inter-fragmentary fixation with applying the concept of fixed angle plates [63]. The routine intra-operative fluoroscopy proved to be less accurate to assess DRFs articular step-off < 1-2 mm and gapping of < 2 mm when applying FSF systems [64]. That is why the complementary role of arthroscopy to FSF has gained uprising curve in the last decade with the introduction of the dry arthroscopic technique that has been more and more refined by Del Pinal due to its supreme role in the guidance of closed reduction, assessment of associated injuries of TFCC, SLL, LTL, capsular tears and cartilage loose flaps [5]. Rikli and Regazzoni concept of two column fixation entailing that two low profile titanium plates applied for FSF in two orthogonal planes, with 50°-70° angle in between, has proven to provide more stiffness and stability than the traditional AO volar plate and Pi plate or external fixation systems [32]. Radial pin plate has proven very beneficial in maintaining the radial height and inclination in osteoporotic patients [47]. Most of the authors reported excellent functional and radiological outcomes after FSF in types C1, C2 and C3 according to the AO classification with 96% incidence of return to normal activities [7,19,44,46,65].
Complications
As any technique used for fixation of DRFs, FSF system has its merits and demerits. One of the drawbacks of FSF is the high incidence of hardware removal from the dorsal surface of the radius once union is achieved due to tendon irritation which has been reported to be as high as 5.8% [20]. Neurological complications in the form of temporary median nerve paraesthesia, superficial radial nerve and dorsal cutaneous branch of the ulnar nerve irritation, and CRPS type II have been reported to be complicating the FSF [45,65]. Secondary osteoarthritis and tenosynovitis have been estimated to occur in 15 % of patients treated with FSF system [65,66]. Extensor tendon tendinitis and EPL ruptures have been reported in different studies [66-68]. Failure of radial column fixation and loss of reduction of fracture fragments have been also reported [66-68]. Pin migration, loss of reduction in cases of osteoporotic patients which may necessitate the use of well configured bone graft or bone graft substitute also have been documented [66,67]. Ulnar column a vascular necrosis, DRUJ instability and wrist stiffness have also been reported [62,64,69]. Other complications that are common to all methods of fixation of DRFs have been also reported in the form of infection, malunion, non-union, intra-articular pins or screws placement and compartment syndrome [7].
Conclusion
Fragment specific fixation system provides the orthopaedic surgeons with a versatile, biomechanically rigid load sharing construct combining the rigidity of plate and screws and the versatility of K-wires that enable them to successfully manage the most complex multifragmentary intra-articular DRFs with a tailored step-wise strategy.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006; 37: 691-697. https://goo.gl/iNYruD
- McKay SD, MacDermid JC, Roth JH, Richards RS. Assessment of complications of distal radius fractures and development of a complication checklist. J Hand Surg Am. 2001; 26: 916-922. https://goo.gl/Dz5WiQ
- Doi K, Hattori Y, Otsuka K, Abe Y, Yamamoto H. Intra–articular fractures of the distal aspects of the radius: arthroscopically assisted reduction compared with open reduction and internal fixation. J Bone Joint Surg Am. 1999; 81: 1093-1110. https://goo.gl/uNnSWV
- Varitimidis SE, Basdekis GK, Dailiana ZH, Hantes ME, Bargiotas K, Malizos K. Treatment of intra-articular fractures of the distal radius: fluoroscopic or arthroscopic reduction?. J Bone Joint Surg Br. 2008; 90: 778-785. https://goo.gl/Js9fJg
- Del Pinal F. Technicaltips for (dry) arthroscopicreduction and internal fixation of distal radius fractures. J Hand Surg Am. 2011; 36: 1694-1705. https://goo.gl/Qau4RF
- Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007; 74: 233-246. https://goo.gl/MosNEJ
- Rhee PC, Medoff RJ, Shin AY. Complex distal radius fractures: an anatomic algorithm for surgical management. J Am Acad Orthop Surg. 2017; 25: 77-88. https://goo.gl/iDxKAp
- Trumble TE, Schmitt SR, Vedder NB. Factors affecting functional outcome of displaced intra-articular distal radius fractures. J Hand Surg Am. 1994; 19: 325-340. https://goo.gl/8APhmK
- Ipaktchi K, Livermore M, Lyons C, Banegas R. Current concepts in the treatment of distal radial fractures. Orthopedics. 2013; 36: 778-784. https://goo.gl/sGp76Q
- Kapandji AI. Treatment of non–articular distal radial fractures by intrafocal pinning with arum pins. In: Saffer P., Cooney WP (eds): Fractures of the distal radius. Philadelphia, JB Lippincott 1995; 71-83.
- Maire N, Lebailly F, Zemirline A, Hariri A, Facca S, Liverneaux P. Prospective continuous study comparing intrafocal cross-pinning HK2 (®) with a locking plate in distal radius fracture fixation. Chir Main. 2013; 32: 17-24. https://goo.gl/7nNXYw
- Hayes AJ, Duffy PJ, McQueen MM. Bridging and non-bridging external fixation in the treatment of unstable fractures of the distal radius: a retrospective study of 588 patients. Acta Orthop. 2008; 79: 540-547. https://goo.gl/fzZZEF
- Dodds SD, Cornelissen S, Jossan S, Wolfe SW. A biomechanical comparison of fragment-specific fixation and augmented external fixation for intra-articular distal radius fractures. J Hand Surg Am. 2002; 27: 953-964. https://goo.gl/e7aXdC
- Ruch DS, Ginn TA, Yang CC, Smith BP, Rushing J, Hanel DP. Use of a distraction plate for distal radial fractures with metaphyseal and diaphyseal comminution. J Bone Joint Surg Am. 2005; 87: 945-954. https://goo.gl/FWUF7Z
- Wolf JC, Weil WM, Hanel DP, Trumble TE. A biomechanic comparison of an internal radiocarpal-spanning 2.4-mm locking plate and external fixation in a model of distal radius fractures. J Hand Surg Am. 2006; 31: 1578-1586. https://goo.gl/FbwRDp
- Obert L, Rey PB, Uhring J, Gasse N, Rochet S, Lepage D, et al. Fixation of distal radius fractures in adults: a review. Orthop Traumatol Surg Res. 2013; 99: 216-234. https://goo.gl/MszG1Y
- Zhang X, Hu C, Yu K, Bai J, Tian D, Xu Y, et al. Volarlockingplate (VLP) versusnon-lockingplate (NLP) in the treatment of die-punchfractures of the distalradius, an observational study. Int J Surg. 2016; 34: 142-147.
- Inagaki K, Kawasaki K. Distal radius fractures-design of locking mechanism in plate system and recent surgical procedures. J Orthop Science. 2016; 21: 258-262. https://goo.gl/oDKdZr
- Medoff RJ, Kopylov P. Immediate internal fixation and motion of comminuted distal radius fractures using a new fragment specific fixation system. Orthop Trans. 1998; 22: 165. https://goo.gl/wNamCW
- Benson LS, Medoff RJ. Fragment-specific fixation of distal radius fractures. In: Slutsky D.J, Osterman AL editors. Fractures and injuries of the distal radius and carpus. Philadelphia: W.B. Saunders; 2009.
- Del Pinal F, Garcia Bernal FJ, Pisani D, Regalado J, Ayala H, Studer A. Dry arthroscopy of the wrist: surgical technique. J Hand Surg Am. 2007; 32: 119-123. https://goo.gl/DCxBZc
- Chen NC, Jupiter JB. Management of distal radial fractures. J Bone Joint Surg Am. 2007; 89: 2051-2062. https://goo.gl/EFtkyz
- Herzberg G et al. Anatomie du radius distal. Cahiers d’enseignement de la SOFCOT. Paris, Expansion scientifique Publications. 1998; 14-27.
- Mueller TL, van Lenthe GH, Stauber M, Gratzke C, Eckstein F, Muller R. Regional, age and gender differences in architectural measures of bone quality and their correlation to bone mechanical competence in the human radius of an elderly population. Bone. 2009; 45: 882-891. https://goo.gl/tt6aUr
- Obert L, Loisel F, Gasse N, Lepage D. Distal radius anatomy applied to the treatment of wrist fractures by plate: a review of recent literature. SICOT J. 2015; 1: 14. https://goo.gl/ebNkEQ
- Nelson D. Anatomy notes and their clinical significance for the volar approach By David L. 2013. https://goo.gl/gk9VTa
- Imatani J, Akita K, Yamaguchi K, Shimizu H, Kondou H, Ozaki T. An anatomical study of the watershed line on the volar, distal aspect of the radius: implications for plate placement and avoidance of tendon ruptures. J Hand Surg Am. 2012; 37:1550-1554. https://goo.gl/YMt3UG
- Tanaka Y, Aoki M, Izumi T, Fujimiya M, Yamashita T, Imai T. Effect of distal radius volar plate position on contact pressure between the flexor pollicis longus tendon and the distal plate edge. J Hand Surg Am. 2011; 36: 1790-1797. https://goo.gl/nTqZ8f
- Clement H, Pichler W, Nelson D, Hausleitner L, Tesch NP, Grechenig W. Morphometric analysis of lister’s tubercle and its consequences on volar plate fixation of distal radius fractures. J Hand Surg Am. 2008; 33: 1716-1719. https://goo.gl/UC4p6n
- Pichler W, Windisch G, Schaffler G, Rienmüller R, Grechenig W. Computer tomography aided 3D analysis of the distal dorsal radius surface and the effects on volar plate osteosynthesis. J Hand Surg Eur. 2009; 34: 598-602. https://goo.gl/DufWVW
- Gasse N, Lepage D, Pem R, Bernard C, Lerais JM, Garbuio P, et al. Anatomical and radiological study applied to distal radius surgery. Surg Radiol Anat. 2011; 33: 485-490. https://goo.gl/X7ZPby
- Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function: A preliminary report of 20 cases. J Bone Joint Surg Br. 1996; 784: 588-592. https://goo.gl/aSGbTT
- Putnam MD, Meyer NJ, Nelson EW, Gesensway D, Lewis JL. Distal radial metaphyseal forces in an extrinsic grip model: implications for post-fracture rehabilitation. J Hand Surg Am. 2000; 25: 469-475. https://goo.gl/dFN61X
- Short WH, Palmer AK, Werner FW, Murphy DJ. A biomechanical study of distal radial fractures. J Hand Surg Am. 1987; 12: 529-534. https://goo.gl/xxf7ei
- Augat P, Iida H, Jiang Y, Diao E, Genant HK. Distal radius fractures: mechanisms of injury and strength prediction by bone mineral assessment. J Orthop Res. 1998; 16: 629-635. https://goo.gl/DykASb
- Baumach SF, DallAra E, Weninger P, Antoni A, Traxler H, Dorr M, et al. Assessment of a novel biomechanical fracture model for distal radius fractures. BMC Musculoskelet Disord. 2012; 13: 252. https://goo.gl/r4pQJk
- Gartland JJ, Werley CW. Evaluation of healed Colles’ fractures. J Bone Joint Surg Am. 1951; 33: 895-907. https://goo.gl/HpfSQx
- Frykman GK. Fracture of the distal radius including sequelae: shoulder–hand–finger syndrome–disturbance in the distal radioulnar joint and impairment of nerve function: A clinical and experimental study. Acta orthop scand. 1967; 108: 1-155. https://goo.gl/kPo1KN
- Muller ME, Nazarian S, Koch P, Schatzker J. The comprehensive classification of fractures of long bones. Berlin, Springer-Verlag. 1990. https://goo.gl/y8xRJR
- Melone CP Jr. Distal radius fractures: Patterns of articular fragmentation. Orthop Clin North Am. 1993; 24: 239-253. https://goo.gl/qjbhE9
- Fernandez DL, Wolf SW. Distal radius fractures. In: Green DP, Hotchkiss RN, Pederson WC, Wolfe, SW (eds): Green’s operative hand surg. Philadelphia, Elsevier Churchill Livingstone. 2005; 645-710.
- Mader K, Pennig D. The treatment of severely comminuted intraarticular fractures of the distal radius. Strategies Trauma Limb Reconstr. 2006; 1: 2-17. https://goo.gl/SE4W4P
- Mlynarek RA, Lawton JN. Fragment-specific internal fixation of distal radius fractures. In: Distal radius fractures. 2016; 71-87. https://goo.gl/2NWx5d
- Medoff RJ. Essential radiographic evaluation for distal radius fractures. Hand Clin 2005; 21: 279-288. https://goo.gl/jNoKZi
- Saw N, Roberts C, Cutbush K, Hodder M, Couzens G, Ross M. Early experience with the TriMed fragment-specific fracture fixation system in intraarticular distal radius fractures. J Hand Surg Eur. 2008; 33: 53-58. https://goo.gl/rmbCSn
- Tencer A. Biomechanics of fixation and fractures. In: Buchholz, et al., ed. Rockwood and Green, Fractures in Adults. 6th Ed Philadelphia: Lippincott Williams & Wilkins; 2006: 67-96.
- Gavaskar AS, Muthukumar S, Chowdary N. Fragment-specific fixation for complex intra-articular fractures of the distal radius: results of a prospective single-center trial. J Hand Surg Eur. 2012; 37: 765–771. https://goo.gl/78119T
- Schnall SB, Kim BJ, Abramo A, Kopylov P. Fixation of distal radius fractures using a fragment-specific system. Clin Orthop Relat Res. 2006: 445: 51-57. https://goo.gl/N4ojWT
- Bakker AJ, Shin AY. Fragment-specific volar hook plate for volar marginal rim fractures. Tech Hand Up Extrem Surg. 2014; 18: 56-60. https://goo.gl/38WJNY
- OShaughnessy MA, Shin AY, Kakar S. Stabilization of volar ulnar rim fractures of the distal radius: current techniques and review of the literature. J Wrist Surg. 2016; 5:113-119. https://goo.gl/pQFc9g
- Hiroyuki O, Tomonori B, Kentaro F, Osamu O, Atsuhiko M, Hideki T, et al. Difficulty in fixation of the volar lunate facet fragment in distal radius fracture. Orthopedics. 2017; 2017: 1-5. https://goo.gl/S8NTSK
- Ilyas AM. Surgical approaches to the distal radius. Hand (N Y). 2011; 6:8-17. https://goo.gl/e69RqB
- Orbay JL, Badia A, Indriago IR, Infante A, Khouri RK, Gonzalez E, Fernandez DL. The extended flexor carpi radialis approach: a new perspective for the distal radius fracture. Tech Hand Up Extrem Surg. 2001; 5: 204-211. https://goo.gl/Lvwtj7
- Tordjman D, Hinds RM, Ayalon O, Yang SS, Capo JT. Volar-ulnar approach for fixation of the volar lunate facet fragment in distal radius fractures: a technical tip. J Hand Surg Am. 2016; 41: 491-500. https://goo.gl/TqD27d
- Dogan NU, Uysal II, Seker M. The communications between the median and ulnar nerves in upper limb. Neuroanatomy. 2009; 8: 15-19. https://goo.gl/4Wei8z
- Schumer ED, Leslie BM. Fragment-specific fixation of distal radius fractures using the TriMed device. Tech Hand Up Extrem Surg. 2005; 9: 74-83. https://goo.gl/GDXAgX
- Li Z, Zhang Z, Yu S, et al. Treatment of the distal fracture in radioulnar based on the volar wrist dual channel approach and postoperative x-ray diagnosis. Australas Phys Eng Sci Med. 2015; 38: 695-707. https://goo.gl/Z42gQx
- Beck JD, Harness NG, Spencer HT. Volar plate fixation failure for volar shearing distal radius fractures with small lunate facet fragments. J Hand Surg. 2014; 39: 670-678. https://goo.gl/eRmwAm
- Kawasaki K, Nemoto T, Inagaki K, Tomita K, Ueno Y. Variable-angle locking plate with or without double-tiered subchondral support procedure in the treatment of intraarticular distal radius fracture. J Orthop Traumatol. 2014; 15: 271-274. https://goo.gl/ozaxiU
- Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004; 86:1900-1908. https://goo.gl/F7913J
- Taylor KF, Parks BG, Segalman KA. Biomechanical stability of a fixed-angle volar plate versus fragment-specific fixation system: cyclic testing in a C2-type distal radius cadaver fracture model. J Hand Surg Am. 2006; 31: 373-381. https://goo.gl/3aiJWD
- Geissler WB. Management distal radius and distal ulnar fractures with fragment specific plate. J Wrist Surg. 2013; 2:190-194. https://goo.gl/4ehtWb
- Slutsky DJ. External fixation of distal radius fractures. J Hand Surg Am. 2007; 32: 1624-1637. https://goo.gl/2ZYVgk
- Thiart M, Ikram A, Lamberts RP. How well can step-off and gapdistances be reduced when treating intra-articular distal radius fractures with fragment specific fixation when using fluoroscopy. Orthop Traumatol Surg Res. 2016; 102:1001-1004. https://goo.gl/v4vb2C
- Landgren M, Abramo A, Geijer M, Kopylov P, Tagil M. Fragment-specificfixation versus volar lockingplates in primarilynon-reducible or secondarily redisplaced distal radius fractures: a randomized controlled study. J Hand Surg Am. 2017; 42:156-165. https://goo.gl/erFHt9
- De Smet A, Lamouille J, Vostrel P, Loret M, Hoffmeyer P, Beaulieu JY. Dorsal approach and internal fixation of impacted intra-articular distal radius fractures with 2.4 mm locking plates. Hand Surg Rehabil. 2016; 35: 203-209. https://goo.gl/r6c6Ta
- Chang HC, Poh SY, Seah SC, Chua DT, Cha BK, Low CO. Fragment-specific fracture fixation and double-column plating of unstable distal radial fractures using AO mini-fragment implants and Kirschner wires. Injury. 2007; 38: 1259-1267. https://goo.gl/dJCLdB
- Abdelgaid SM, Abdelazeem ME, Alsharkawy W. Fragment-specific fixation of complex distal radial fractures with small intraarticular fragments; volar locked plate augmented with Kirschner wires. Curr Orthop Pract. 2015; 26: 306-313. https://goo.gl/X4LkkQ
- Benson LS, Minihane KP, Stern LD, Eller E, Seshadri R. The outcome of intra-articular distal radius fractures treated with fragment-specific fixation. J Hand Surg Am. 2006; 31: 1333-1339. https://goo.gl/K6zfV2

Sign up for Article Alerts