The Role of Muscle Tissue and Resistance Training in Cardiometabolic Health
Ismael Galancho-Reina1, Antonio Jesus Sanchez-Oliver2, Pedro Jose Gonzalez-Matarin3*, Javier Butragueno4, Borja Bandera-Merchan5, Walter Suarez-Carmona2, Felipe Isidro-Donate6, Francisco Jose Tinahones5 and Manuel Macias-Gonzalez5
1Exercise and obesity group, SEEDO, Spain
2Department of Sports, Faculty of Sport Sciences. University of Pablo Olavide, and Department of Physical Education and Sports, Faculty of Educational Sciences. University of Sevilla, Sevilla (Spain)
3Department of Physical Education, Faculty of Educational Sciences. University of Almeria, Almeria, (Spain)
4LFE Research Group, Department of Health and Human Performance, Technical University of Madrid, Spain
5Unidad de Gestión Clínica Endocrinología y Nutrición. Instituto de Investigación Biomédica de Málaga (IBIMA), Complejo Hospitalario de Málaga (Virgen de la Victoria)/Universidad de Málaga (Spain). CIBER Pathophysiology of obesity and nutrition (CB06/03), Spain
6Department of Physical Education. Generalitat de Catalunya, Barcelona (Spain) and International Institute of Physical Exercise and Health, Alicante (Spain)
*Address for Correspondence: Pedro Jose Gonzalez Matarin, Area de Educación Física y Deportiva. Edificio Científico-Técnico III (CITEIII) Planta Baja, Despacho 0.30 Universidad de Almería. Ctra. De Sacramento s/n, 04120 La Cañada de San Urbano, Almería, Spain, ORCID ID: orcid.org/0000-0003-1935-6261; Tel: +349-500-15464; E-mail: pjgonzalezmatarin@hotmail.com
Submitted: 21 December 2018; Approved: 19 January 2019; Published: 21 January 2019
Citation this article: Reina IG, Sanchez-Oliver AJ, Gonzalez-Matarin PJ, Butragueno J, Merchan BB, et al. The Role of Muscle Tissue and Resistance Training in Cardiometabolic Health. Int J Sports Sci Med. 2019;3(1): 001-012.
Copyright: © 2019 Reina IG, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
In this review our aim is to discuss the potential benefits of resistance training in healthy subjects and patients with cardio-metabolic disease. In the last decades, evidence about the pivotal role of muscle tissue and proper muscle functionality in health and disease have been accumulating. Sarcopenia and muscle wasting have erected as a first-order risk predictor, and strength and muscle mass now constitute good markers of functionality and quality of life. Therefore, aside of its evident mechanic and aesthetic considerations, muscle tissue deploys a wide range of endocrine and metabolic functions, which are essential for health optimization and disease prevention. As follows, strategies directed to improve muscle quality and quantity, as it is Resistance Training regimens, should be prioritized and included in clinical guidelines and general health advice.
Introduction
Importance of muscle tissue in health
There is extensive evidence that physical activity and exercise are essential to improving health, either by preventing a host of non-communicable diseases related to a sedentary lifestyle [1] or attenuating, with proper planning and programming of this physical activity, a large number of these diseases once contracted [2].
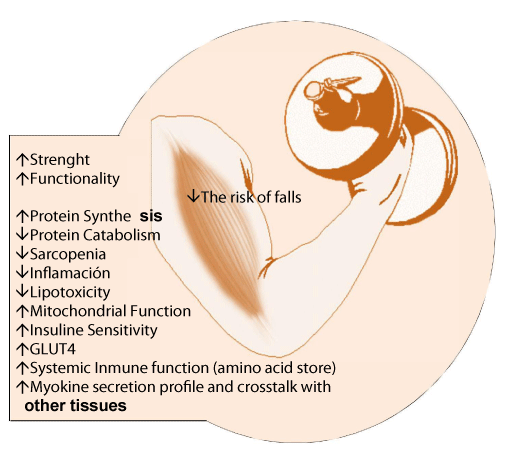
The role of muscle tissue in these two areas is undeniable. Muscle plays a central role in metabolic health and in the metabolism of the body’s proteins, serving as the main reservoir of amino acids to maintain protein synthesis in vital tissues and organs in the absence of the absorption of amino acids from the intestine and providing hepatic gluconeogenic precursors. Altered muscle metabolism contributes to the onset of disturbances. The prevention of many common pathological conditions and existing chronic diseases involves the preservation of muscle mass as well as its correct functioning. Thus, maintaining adequate muscle mass, muscle quality and optimal levels of strength is fundamental [3,4]. Recent studies show a population with lower levels of muscle strength and reduced myofibrillary proteins which affect muscle size and generate risk of functional disability [5], indicating that the development of muscle tissue and strength levels are closely linked to health (Figure 1) [5].
The protein content of certain tissues and organs, such as the brain, heart and liver, are essential for the survival of the species. These tissues and organs depend on a relatively constant supply of amino acids through the blood to serve as precursors for the synthesis of new proteins, thereby balancing the persistent rate of protein breakdown that occurs in all tissues, ensuring the proper functioning of the same [6].
In the absence of nutrient intake, muscle protein serves as the primary storehouse for replacing these blood amino acids absorbed by other vital tissues and organs [7]. In the fasting state, amino acids serve not only as precursors for protein synthesis, but also as precursors of hepatic gluconeogenesis [8]. Consequently, the protein mass of essential tissues and organs, as well as the necessary concentration of plasma glucose vital for survival can be kept relatively constant despite the absence of nutritional intake; however, the muscle mass must be adequate for supplying the required amino acids.
Different studies [9] have shown that the depletion of muscle mass is incompatible with life and that there is a strong association between muscle breakdown and survival in different diseases. It has even been proven that lack of muscle development along with increased adipose tissue is a major cause of a large number of diseases and conditions such as diabetes, cancer and obesity [1,10,11], with strength training being one of the key tools to prevent these diseases and improve muscle development [12].
It has been proven that developing muscle tissue through exercise and physical activity may improve the physiological responses necessary for recovery in certain disorders/diseases, increasing the accelerated synthesis of acute-phase proteins in the liver and the synthesis of proteins involved in immune function [6]. Likewise, it has been observed that regular participation in strength training programs can minimize musculoskeletal disorders, improving health and well-being [5,12,13]. In an increasingly aging and sedentary population, where activities requiring a strength component have decreased, a decrease in strength and muscle development has been observed [5], with the main consequence being muscle atrophy, which is directly associated with the contractile capacity of the muscle. This compromised muscle function has been identified as a predictor of hospitalization, disability and even death [14]. Therefore, skeletal muscle plays a crucial role in the performance of daily activities, the maintenance of our health and in the prevention of diseases [15,16].
Regular strength training attenuates the decrease in muscle mass occurring with age [5,17]. For this reason, this type of exercise has gradually been introduced in the prevention of different chronic diseases with very satisfactory results [1].
Strength training in health
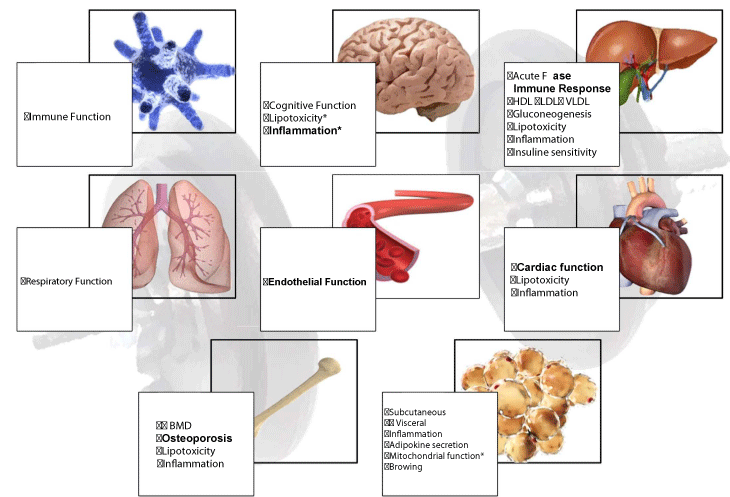
Aerobic endurance exercise is often prescribed to reduce existing illnesses (Figure 2) (cardiovascular disease, diabetes, etc.) and appears to have shown beneficial effects in reducing chronic inflammation [18]. Strength training, independent of aerobic endurance exercise, significantly reduced the risk of developing metabolic syndrome, although the combination of strength training and resistance exercise (concurrency) was associated with a lower risk of developing metabolic syndrome (25%) compared to sedentary behavior [19], with a substantial improvement in different anthropometric parameters, cardiorespiratory fitness and metabolic factors in overweight and obese subjects (Figure 2) [20]. Although both types of training seem to exert positive functions in terms of prevention and/or treatment of various pathologies, strength training is essential and even a priority in some of these because it increases or maintains muscle mass, which in turn maintains an active metabolism, improves glucose homeostasis, exerts hormonal regulation functions to control diverse biological processes, such as the inflammatory process, in an autocrine, paracrine and endocrine form (Figure 2) [21].
Strength training has a favorable effect on body composition, as it decreases fat mass including abdominal fat, increases HDL, lowers LDL, lowers plasma glucose concentration and reduces systolic and diastolic blood pressure (Figure 2) [22]. It also improves insulin sensitivity, improves glucose tolerance, and prevents the sarcopenia and osteoporosis often seen in the elderly and middle-aged population. In fact, it has been proven that in untrained people, strength training with light loads may increase their strength and muscle development [23].
There is also extensive scientific evidence on the benefits of training on the Hormonal Environment (HGH, IGF-1, testosterone, etc.), improvements in Insulin Resistance (IR), and development of increased insulin-independent GLUT4 translocation improving the anabolic process [24-26].
Several studies have shown that proper strength training planning could maintain or even increase Bone Mineral Density (BMD) in the hip and neck of the femur in overweight and obese elderly people. Strength training has also been observed to have a positive effect on the BMD of women with problems in the lumbar region, the femur and the radius [27,28]. Therefore, strength training should be a central component of public health promotion programs, along with aerobic exercise [29]. Based on the above observations, it is understood that strength training is essential in preventing and treating obesity and its comorbidities [26]. Indeed, authors including Hunter and colleagues have shown how increased muscle mass and strength training improve intra-abdominal fat loss and increases total daily energy expenditure, a key factor in maintaining weight loss [30,31]. These findings cause us to rethink weight loss treatments and improved fitness, with the combination of strength and cardiovascular training appearing to optimize the results [29,32,33].
Strength training and sarcopenia
It has been found that as we grow older muscle mass decreases resulting in a loss of strength of one to two percent annually [34]. This loss of muscle is considered one of the most dramatic effects of aging in terms of quality of life in the elderly. It is also associated with metabolic alterations, which worsen the problem, and an increased mortality rate [24,35]. For this reason, sarcopenia is considered an independent condition (Cao & Morley, 2016).
Both sarcopenia and obesity pose a health risk. They can present together, or in combination, exponentially increasing the risk for overall metabolic health and inducing an earlier onset of a possible functional disability [36,37]. The combination of sarcopenia and obesity is commonly referred to as sarcopenic obesity or sarcobesity. Simply reducing the prevalence of sarcopenia in the US by 10% could reduce health costs by $1.1 million [36]. For this reason, experts on the subject have shown the need to include weight training in adolescents to prevent this strength deficiency that is generated in the future [13,38].
Inflammatory processes have been shown to escalate the process of age-related loss of muscle mass [39]. High levels of inflammatory cytokines are associated with an increased risk of loss of muscle mass and strength. This pro-inflammatory environment usually develops in obese and sedentary individuals who develop metabolic diseases [4]. Some studies have shown that elevated levels of Tumor Necrosis Factor Alpha (TNFα) may increase muscle catabolism by suppressing the Akt/mTOR pathway. In addition, they may antagonize the anabolic effect of IGF- 1, due to the development of resistance to growth hormone. Thus, the relationship between inflammation, muscle strength and muscle mass seems to have a pathogenic explanation based on the effect of inflammation on the balance between protein synthesis and protein catabolism at the muscular level [39].
As we know, sarcopenia is multifactorial and is influenced by sex, age, genetic background and lifestyle. This decrease in muscle mass has been found to appear when we grow older [5,40,41]. Furthermore, it has been observed that sarcopenia favors the functional impairment of physical capacities and IR, increasing considerably in the elderly population [42]. In the presence of IR, these insulin effects are dysfunctional and would facilitate accelerated muscle loss [41]. The balance between muscle hypertrophy and atrophy is altered by suppressing IGF-1 signaling, which leads to the reduction in activation of the PI3K/ PKB pathway and a decrease in protein synthesis, as well as in the expression of Atrogin-1 and MuRF1 [39] which contributes to the breakdown of muscle protein. In addition, increased adiposity and free fatty acid concentrations inhibit GH production and decrease plasma IGF-1 concentrations associated with decreased muscle mass, muscle strength and protein synthesis, as well as with increased cell death, which in turn leads to the accumulation of visceral fat and to the decline in lean body mass [40]. Furthermore, the progressive decrease in motor neuron function during aging can lead to denervation of muscle fibers, which can result in loss of muscle mass [40,43].
Strength training is probably the most effective measure to prevent and treat sarcopenia. Evidence suggests that, along with a good diet and healthy habits, this condition may be mitigated by regular physical activity, especially with strength training.
Different authors have recommended developing strength training or combined/concurrent routines between two to four days a week, alternatively [44] using full-body routines to achieve greater muscle involvement at the beginning, followed by more analytical planning where the exercises are performed by muscle groups [29,30,45]. The duration of each session should be individualized for each person depending on their level and abilities, but taking into account factors such as rest, number of repetitions, and series [46]. All these adaptations have been seen to be more easily developed if they are supervised by training specialists, which also improves adherence [47,48].
Combining strength training with high-protein diets may maintain and even increase lean body mass during weight loss interventions [49], with the combination of diet and exercise leading to improved health.
Strength training in low-grade systemic inflammation
In recent years, it has been shown that chronic low-grade systemic inflammation is the basis of many, if not all, typically Western diseases centered on the metabolic syndrome [50]. Different studies have shown how chronic inflammation is one of the key physiological mechanisms related to IR, type I diabetes mellitus and the metabolic syndrome [51,52]. In addition, systemic inflammation is intimately associated with the development of other serious diseases such as dyslipidemia, non-alcoholic hepatic steatosis, cancer, depression or cardiovascular disease [52]. It should also be noted that other currently very prevalent conditions, such as inflammatory bowel disease, asthma, rheumatoid arthritis and periodontal disease, among others, are also associated with chronic inflammation (Table 1) [53-56].
In this complex system, both adipose tissue and fat free mass play an important role. The adipose organ consists of several fat depots that exert different physiological functions and pathophysiological implications that can protect the body [4]. This tissue, currently considered an endocrine organ [57], produces a wide variety of adipokines and cytokines that influence inflammatory, procoagulant, antifibrinolytic and vasoactive cascades, suggesting a direct influence on inflammation [58,59], especially in circumstances of visceral obesity, where the secretory profile of adipose tissue is particularly altered, with an increase in cytokines with inflammatory activity. The most studied are leptin, resistin, Tumor Necrosis Factor Alpha (TNFα) and interleukins (IL-6, IL-1b, IL-18), although there are many more [60].
The major anti-inflammatory cytokines are IL-1 receptor antagonists (IL-1ra), Transforming Growth Factor Beta (TGF-β) and interleukins IL-4, IL-6, IL-10, IL-11 and IL-13 and adiponectin (61–64). Under normal physiological conditions, there is usually a balance between pro-inflammatory and anti-inflammatory adipokines, which serve as immunomodulators. However, in obesity, the anti-inflammatory response may be insufficient to counteract inflammatory activity giving way to chronic low-grade inflammation [4].
Physical exercise is as an effective tool to slow or block all the processes mentioned above [65-67]. As a result of strength training and muscle contraction alone, the tissue itself secretes cytokines with anti-inflammatory functions such as IL-1ra and IL-10 among others [4]. It has been shown that IL-1ra also increases after aerobic exercise and also after strength training [69]. The anti-inflammatory effects of exercise are also based on other mechanisms such as inhibition of monocytes and infiltration of macrophages into adipose tissue [67,70]. These new findings show that skeletal muscle is an organ that communicates with other organs such as adipose tissue, liver, pancreas, bones and brain, with physical inactivity and muscle deterioration being responsible for metabolic disorders or resistance to the effects of different myokines [4], showing how lack of physical activity is the precursor of a large network of diseases such as cardiovascular diseases, cancer, diabetes and obesity.
With reference to the above, it can be deduced that an acute strength training session does not improve chronic inflammation, but in the long term this situation could change. Strength training alters visceral fat and levels of several pro-inflammatory cytokines, accordingly weight training could be key in maximizing anti-inflammatory benefits if performed consistently, and reducing markers of inflammation in the absence of changes in body composition [26]. In fact, although C-reactive protein does not appear to change after an acute series of strength training in the long term [71], it does seem to affect its basal levels [72], improving the physiological and biological state of chronic low-grade inflammation.
A recent study examined the effects of strength training on the inflammatory state, hematological markers and physical fitness in elderly women with cognitive impairment. The result was that after 28 weeks of strength training, there was an increase in functional fitness and anti-inflammatory cytokine concentrations along with attenuation of inflammation and improved cognition in elderly women with cognitive impairment [73]. Independent of fat loss, strength exercise also increases muscle tone, and thereby increases production of myokines that favor fatty acid oxidation, hypertrophy or have anti-inflammatory effects such as FGF-21, LIF, BDNF, IL-1ra, IL-8, IL-15 [17,74] or the multifaceted IL-6 that is known to reduce the production of TNF-α and to increase anti-inflammatory cytokines [75]. IL-6 has always been considered a pro-inflammatory cytokine responsible for loss of insulin sensitivity, secreted by adipose tissue and macrophages, but when IL-6 synthesis is carried out by muscle tissue as a consequence of physical activity its effects are seemingly contradictory to this. IL-6 produced in muscle tissue as a result of muscle contraction during exercise increases lipolysis and oxidation of fatty acids and increases glucose uptake by activation of Phosphatidylinositol 3-Kinase (PI3K) and Akt [66,76,77].
The role of IL-6 may be different depending on its origin. In the case of adipocytes or classically activated M1 macrophages this would suppose the activation of the NF-kB pathway (nuclear factor kappa beta is a transcription factor that coordinates the immune inflammatory response and underlies metabolic diseases such as type 2 diabetes and obesity), in which case other pro-inflammatory cytokines such as TNF-α and IL-1Beta are emitted, while in the muscle or alternatively activated M2 macrophages this would be regulated by the intracellular increase of calcium and/or p38 MAPK without the presence of the above [76] exerting anti-inflammatory effects as we have seen, by inhibiting TNFαe inducing the synthesis of IL-ra or IL-10 among others, which are of anti - inflammatory origin, as well as increasing sensitivity to insulin and leptin [65,76]. In addition, some studies suggest that IL-6 is involved in satellite cell-mediated muscle hypertrophy, which in turn would improve the inflammation state. This expression of IL-6 appears to correlate with an increase in muscle tissue due to its inducing action on the proliferation of satellite cells, exerting a positive impact on the proliferative capacity of muscle stem cells [75,78,79].
Therefore, it is necessary to differentiate between the effects of chronic elevation of IL-6 (secreted by adipocytes or by immune cells infiltrated into adipose tissue) and the acute elevation that occurs with muscle contractions (released predominantly from muscle cells) [80]. Finally, we could conclude that the effect of long-term strength training has been shown to decrease chronic low-grade inflammation and improve muscle mass responses in relation to metabolic disorders that can be generated from a decrease in physical activity and an increase in muscle atrophy [81].
Strength training and improved insulin sensitivity
IR occurs when nutrient storage pathways, evolved to maximize energy efficiency, are exposed to a chronic energy surplus. The accumulation of ectopic lipids in the liver and skeletal muscle triggers pathways that alter insulin signaling, which reduces the uptake of muscle glucose and decreases the synthesis of hepatic glycogen [82-85]. Muscle IR, due to ectopic lipids, precedes IR in the liver and diverts ingested glucose into the liver, resulting in an increase in de novo liver lipogenesis and hyperlipidemia. Subsequent infiltration of macrophages into White Adipose Tissue (WAT) leads to increased lipolysis, which increases the synthesis of hepatic triglycerides and hyperlipidemia due to increased esterification of fatty acids. Macrophage-induced WAT lipolysis also stimulates hepatic gluconeogenesis, promoting fasting and postprandial hyperglycemia through increased fatty acid administration to the liver, resulting in increased hepatic acetyl-CoA content, a potent activator of pyruvate carboxylase and increased conversion of glycerol to glucose [86,87]. These substrate-regulated processes are mostly independent of insulin signaling in the liver, but are dependent on insulin signaling in WAT, which becomes defective with inflammation [88].
In this context, it has been observed that systemic inflammation contributes to the development of IR in people with obesity or metabolic disorders, while fat loss, mainly visceral fat, appears to reduce the infiltration of macrophages and the expression of factors related to inflammation in adipose tissue [89].
Hyperglycemia and hyperinsulinemia can cause metabolic alterations and exacerbate various diseases. Numerous studies show that exercise is a potent stimulator of signaling pathways to produce GLUT4 translocation to the cell membrane independently of the action of insulin. In addition, exercise-mediated IL-6 production plays an important role in improving insulin sensitivity [90,91]. Weight training has been reported to improve glucose stability versus aerobic exercise (Riddell et al. 2017), although it also could cause a moderate increase in blood glucose in some individuals [92]. In anaerobic exercise, as in the case of weight training, consisting of short bursts of intense activity, these glucose concentrations have shown a tendency to increase. Therefore, according to the decision tree shown by Riddell and colleagues in the consensus on physical exercise and diabetes, aerobic exercise and strength training activities should be combined, with strength training at the beginning to attenuate hypoglycemia [91].
Likewise, subjects with IR and lipotoxicity present an incomplete oxidation of fatty acids, increasing the concentration of lipid intermediates such as diacylglycerols and ceramides, which in turn amplify IR [90,93]. However, strength training has been shown to improve both the oxidative capacity of skeletal muscle and the degradation of intramuscular lipids in both type I and type II fibers, improving GLUT4 translocation [94-96]. Furthermore, the TNFα reduction associated with physical exercise, especially with strength training, involves an improvement in IR status, since TNFα is capable of causing a decrease in the auto phosphorylation of IR stimulated by the serine phosphorylation of insulin receptor substrate 1, suggesting an important role of TNFα in the development of IR. When TNFα activity is blocked, biochemically or genetically, the result is an improvement in insulin sensitivity [66]. Muscle contraction induces glucose uptake into skeletal muscle both independently and synergistically with insulin. Insulin stimulates glucose transport activity in skeletal muscle and a large part of this stimulation is associated with a GLUT4 translocation to the lipid bilayer, that is, the cell membrane, from intracellular compartments to the sarcolemma and T tubules, allowing glucose to enter the cell through facilitated diffusion [97,98].
The practice of controlled and planned physical activity consisting of aerobic exercise and especially strength training, or both combined, is associated with a reduction in glycosylated Hemoglobin (HbA1c) by 0.67% in patients with type 2 diabetes mellitus [99]. The overall reduction in HbA1c induced by exercise is similar to the reductions achieved by commonly used oral antidiabetic drugs such as metformin [100]. In fact, a recent meta-analysis has shown that non-pharmacological approaches such as physical exercise are superior to pharmacological interventions in the prevention of type 2 diabetes mellitus [101]. It can be asserted that muscle contraction increases glucose transport and represents an alternative signaling pathway to insulin. In addition, it can be said that Rac1 is activated during contraction in skeletal muscle and recent studies suggest that Rac1 is a regulator in glucose uptake [2]. Thus, physical exercise, especially strength training, has an insulin-sensitizing effect, having been shown to be a powerful weapon to prevent and treat IR and/or type 2 diabetes mellitus [102].
To conclude, both cardiovascular and strength training can increase insulin sensitivity and optimize glucose tolerance (Table I) [91,103], but above all strength training, as it induces an acute decrease in intramyocellular deposits of glycogen [104], intramuscular lipid degradation, GLUT-4 translocation, blocking of inflammatory cytokines such as TNFα and an increase in the ability of the muscle to utilize glucose [105].
Strength training and lipotoxicity
If we focus on the effects more closely related to physical fitness than on metabolic health, Intramuscular Triglycerides (IMTG) cause deleterious effects such as loss of the capacity to produce strength, poor physical condition or decreased mobility in older adults. In this study, Manini et al., found that after 30 days of unilateral leg immobilization, healthy young subjects experienced a 15-20% increase in IMTG in the thigh muscles. The increase in IMTG also represented a 4-6% loss of strength, again emphasizing that IMTG is more than an inert storage tank, as it may also play a role in the loss of strength related to inactivity [106].
There is sufficient evidence to show that small amounts of IMTG represent a readily available source of energy and that the problem described above will depend on many conditions, including where they are stored (intermuscular, intramuscular or beneath the fascia), the composition (it is not the same when the composition of the triglyceride is saturated or polyunsaturated fatty acids, for example) or the size/number of lipid droplets, since in trained people, as with brown adipose tissue, lipid droplets are smaller and more numerous. It will also depend on the physical condition, mitochondrial functionality and density and oxidative capacity, insulin sensitivity and other conditions of the subject in question, since it is not only the IMTGs themselves, but also the physiological conditions that lead to the accumulation of intermediates and metabolites. Thus emerged what authors such as Goodpaster and colleagues in 2001 called the “athlete’s paradox” in which trained people present a large amount of IMTG but without any metabolic alterations, since these IMTG arise as an adaptation to a great need, as well as capacity, to oxidize these energy sources [107]. Consequently, it could be suggested that, as with cardiovascular training or High-Intensity Interval Training (HIIT) [108] strength training, through the adaptations discussed regarding IMTG turnover, would improve insulin sensitivity or muscle oxidative capacity, reduce mitochondrial and endoplasmic reticulum stress, and improve strength and muscle mass (Table 1).
Conclusions
In this review, we have discussed the role of Resistance Training (RT) and muscle tissue on different conditions relative to health and disease. First, the muscle tissue as an organ acts as the main amino acid reservoir of the organism, which provides necessary precursors for protein synthesis and hepatic neoglucogenesis. Secondly, a qualitatively and quantitatively healthy amount of muscle has serious implications maintaining metabolism homeostasis, osteomuscular health, inflammation status, hormone regulation, mitochondrial oxidative potential and functional capacity in general. In that respect, RT as a training approach and by means of preserving and incrementing muscle mass quantity and quality could well have a pivotal role in the prevention and management of diverse health and disease conditions, particularly those concerning cardiometabolic disorders. Under the scrutiny of the available evidence, we can affirm that RT has an important role improving the metabolic profile: betterment of lipid profile, improved glucose homeostasis, decreased systolic and diastolic arterial pressure, greater insulin sensitivity; and positively affecting osteomuscular parameter. Such as, an increase of bone mineral density, diminishing incidence and prevalence of osteoporosis and osteopenia; lessening the risk of developing sarcobesity and sarcopenia, conditions related to an increased in disability, risk of hospitalization and risk of all-cause premature death in general. Potentially, all of this would allow a great reduction in the costs of to health systems, being those private or public and curtailing low-grade inflammation status through anti-inflammatory cytokine secretion and downregulation of inflammatory molecular cascades.
To conclude, RT should be considered and included in every exercise program, mainstream public health recommendations and exercise guidelines. Due to its potential to achieve positive health outcomes, prevent chronic disease and diminish the risk of disability. Developing universal guidelines regarding RT and its proper appliance in the healthy and disease-affected population should be a public health priority that we must promptly deal with in order to face the rapid-expanding chronic disease burden.
Acknowledgment
This study was supported by “Centros de Investigación En Red” (CIBER, CB06/03/0018) of the “Instituto de Salud Carlos III” (ISCIII), and grants from ISCIII (PI11/01661, PI14/00696) and Co-financed by the European Regional Development Fund (FEDER). MMG was recipient of the Nicolas Monardes Programme from the “Servicio Andaluz de Salud, Junta de Andalucia”, Spain (C-0029-2014).
- Pedersen B, Saltin B. Exercise as medicine-evidence for prescribing exercise as therapy in 26 different chronic diseases. J Med Sci Sport. 2015; 25: 1-72. https://goo.gl/54WdiR
- Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology. 2013; 28: 330-358. https://goo.gl/4iMa3u
- Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L. Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc. 2016; 17: 789-796. https://goo.gl/JYLVtw
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012; 8: 457-465. https://goo.gl/7UvPsv
- Mitchell W, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012; 2: 26. https://goo.gl/GwEkXD
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006; 84: 475-482. https://goo.gl/m5qWQ1
- Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF. Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969; 48: 574-583. https://goo.gl/xDATg4
- Felig P. The glucose-alanine cycle. Metabolism. 1973; 22: 179-207. https://goo.gl/LzQTDt
- Kotler DP, Tierney AR, Wang J, Pierson RN. Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989; 50: 444-447. https://goo.gl/zoX5TV
- Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009; 15: 2920-2926. https://goo.gl/Vds7QY
- Scott D, Chandrasekara SD, Laslett LL, Cicuttini F, Ebeling PR, Jones G. Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5-10 years in community-dwelling older adults. Calcif Tissue Int. 2016; 99: 30-42. https://goo.gl/Es79m6
- Ciolac E, Rodrigues-da-Silva J. Resistance training as a tool for preventing and treating musculoskeletal disorders. Sport Med. 2016; 46: 1239-1248. https://goo.gl/BPXEzn
- Faigenbaum AD, MacDonald JP. Dynapenia: it’s not just for grown-ups anymore. Acta Paediatr. 2017; 106: 696-697. https://goo.gl/FGCu9E
- Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et als. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Geront A Biol Sci Med. 2006; 61: 72-77. https://goo.gl/mitKfj
- Adams SC, Segal RJ, McKenzie DC, Vallerand JR, Morielli AR, Mackey JR, et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized. Breast cancer Res. 2016. 158: 497-507. https://goo.gl/uFRC6E
- Gomes M, Martinez P, Pagan L, Damatto R, Cezar MDM, Lima ARR, et al. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017; 8: 20428-20440. https://goo.gl/Woha5K
- Phillips M, Patrizi R, Cheek D, Wooten J, Barbee J, Mitchell J. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sport Exerc. 2012; 44: 2099-2110. https://goo.gl/mwuPdL
- Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. NIH Public Access. 2010; 411: 785-793. https://goo.gl/22Ht3F
- Bakker EA, Lee D, Sui X, Artero EG, Ruiz JR, Eijsvogels TMH, et al. Association of resistance exercise, independent of and combined with aerobic exercise, with the incidence of metabolic syndrome. Mayo Clin Proc. 2017; 92: 1214-1222. https://goo.gl/iHPj8P
- Schwingshackl L, Dias S, Strasser B, Hoffmann G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/ obese subjects: a systematic review and network meta-analysis. PLoS One. 2013; 8: 12. https://goo.gl/iHPj8P
- Henningsen J, Rigbolt KTG, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010; 9: 2482-2496. https://goo.gl/Zn6qgt
- Candón LÁ, Sánchez OA, Galancho RI, Suárez CW, González JJA. Ejercicio físico, obesidad e inflamación. Rev Digit Educ física. 2016; 41: 65-82. https://goo.gl/u8LJfR
- Schoenfeld BJ, Wilson JM, Lowery RP, Krieger JW. Muscular adaptations in low-versus high-load resistance training: A meta-analysis. Eur J Sport Sci. 2016; 16: 1-10. https://goo.gl/NxTmND
- Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014; 2: 819-829. https://goo.gl/WxAvr3
- Kraemer W, Ratamess N. Hormonal responses and adaptations to resistance exercise and training. Sport Med. 2005; 35: 339-361. https://goo.gl/6dh6mp
- Strasser B, Arvandi M, Siebert U. Resistance training, visceral obesity and inflammatory response: a review of the evidence. Obes Rev. 2012; 13: 578-591. https://goo.gl/kV23Qa
- Beavers KM, Beavers DP, Martin SB, Marsh AP, Lyles MF, Lenchik L, et al. Change in bone mineral density during weight loss with resistance versus aerobic exercise training in older adults. J Gerontol A Biol Sci Med Sci. 2017; 72: 1582-1585. https://goo.gl/reSrvV
- Kelley G, Kelley K, Tran Z. Resistance training and bone mineral density in women: a meta-analysis of controlled trials. Am J Phy Med Rehabil. 2001; 80: 65-77. https://goo.gl/wGVkuS
- Benito PJ, Alvarez-Sanchez M, Díaz V, Morencos E, Peinado AB, Cupeiro R, et al. Cardiovascular fitness and energy expenditure response during a combined aerobic and circuit weight training protocol. PLoS One. 2016; 11: 11. https://goo.gl/XvYv6E
- Hunter G, Bryan D, Wetzstein C, Zuckerman PA, Bamman MM. Resistance training and intra-abdominal adipose tissue in older men and women. Med Sci Sport Exerc. 2002; 34: 1023-1028. https://goo.gl/r23nSH
- Hunter G, Wetzstein C, Fields D, Brown A, Bamman MM. Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol. 2000; 89: 977-984. https://goo.gl/Tz94
- Roubenoff R. Physical activity, inflammation, and muscle loss. Nutr Rev. 2007; 65: 208-212. https://goo.gl/D5nf5c
- Ziaaldini MM, Koltai E, Csende Z, Goto S, Boldogh I, Taylor AW, et al. Exercise training increases anabolic and attenuates catabolic and apoptotic processes in aged skeletal muscle of male rats. Exp Gerontol. 2015; 67: 9-14. https://goo.gl/XkY4y9
- Hughes V, Frontera W, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. 2002; 76: 473-481. https://goo.gl/E58mWp
- Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010; 1: 9-21. https://goo.gl/CTsSCj
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004; 52: 80-85. https://goo.gl/xcZrpE
- Waters D, Baumgartner R. Sarcopenia and obesity. Clin Geriatr Med. 2011; 27: 401-421. https://goo.gl/rNgdm3
- Faigenbaum A. Resistance exercise and youth: survival of the strongest. Pediatr Exerc Sci. 2017; 29: 14-18. https://goo.gl/t5EyB2
- Sakuma K, Aoi W, Yamaguchi A. The intriguing regulators of muscle mass in sarcopenia and muscular dystrophy. Front Aging Neurosci. 2014; 6: 230. https://goo.gl/VDPXoo
- Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab. 2015; 12: 22-26. https://goo.gl/NFgdwF
- Aghili R, Malek M, Valojerdi AE. Body composition in adults with newly diagnosed type 2 diabetes: effects of metformin. J Diabetes Metab. Disorder. 2014; 13: 88. https://goo.gl/mc8vaF
- Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International sarcopenia initiative. Age Ageing. 2014; 43: 748-759. https://goo.gl/4h9jYi
- Dodds R, Sayer AA. Sarcopenia. Arq Bras Endocrinol Metabol. 2014; 58: 464-469. https://goo.gl/aKiZjV
- Law T, Clark L, Clark B. Resistance exercise to prevent and manage sarcopenia and dynapenia. Annu Rev Gerontol Geriatr. 2016; 36: 205-228. https://goo.gl/wkyZFP
- Strasser B, Schobersberger W. Evidence for resistance training as a treatment therapy in obesity. J Obes. 2011; 482-564. https://goo.gl/F6X6VN
- Buchheit M. Monitoring training status with HR measures. Do all roads lead to Rome? Front Physiol. 2014; 5: 73. https://goo.gl/N6nigm
- Faulkner MS, Michaliszyn SF, Hepworth JT, Wheeler MD. Personalized exercise for adolescents with diabetes or obesity. Biol Res Nurs. 2014; 16: 46-54. https://goo.gl/hKH1eA
- Storer TW, Dolezal BA, Berenc MN, Timmins JE, Cooper CB. Effect of supervised, periodized exercise training vs. self-directed training on lean body mass and other fitness variables in health club members. J Strength Cond Res. 2014; 28: 1995-2006. https://goo.gl/4EKZEg
- Verreijen A, Engberink M, Memelink RG, van der Plas SE, Visser M, Weijs PJ, et al. Effect of a high protein diet and/or resistance exercise on the preservation of fat free mass during weight loss in overweight and obese older adults. Nutr J. 2017; 16: 10. https://goo.gl/mwPxrU
- Ruiz-Núñez B, Pruimboom L, Dijck-Brouwer DAJ, Muskiet FAJ. Lifestyle and nutritional imbalances associated with Western diseases: causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. J Nutr Biochem. 2013; 24: 1183-1201. https://goo.gl/1xKY3u
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004; 25: 4-7. https://goo.gl/61VqNj
- Saltiel A, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017; 127: 1-4. https://goo.gl/jUyAiT
- Akash MSH, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013; 114: 525-531. https://goo.gl/4KRytZ
- Lucas M, Chocano-Bedoya P, Shulze MB, Mirzaei F, O’Reilly ÉJ, Okereke OI, et al. Inflammatory dietary pattern and risk of depression among women. Brain Behav Immun. 2014; 36: 46-53. https://goo.gl/TeBTbe
- Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep. 2012; 32: 1-15. https://goo.gl/Wpytje
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012; 2: 1. https://goo.gl/VmqvQF
- Li Y, Su Y, Chen S, Zhang Y, Zhang Z, Liu C, et al. The effects of resistance exercise in patients with knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil. 2016; 30: 947-959. https://goo.gl/23fRJn
- Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001; 60: 329-339. https://goo.gl/BUh49s
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004; 92: 347-355. https://goo.gl/SKVUrB
- Ceperuelo-Mallafré V, Ejarque M, Serena C, Duran X, Montori-Grau M, Rodríguez MA, et al. Adipose tissue glycogen accumulation is associated with obesity-linked inflammation in humans. Mol Metab. 2016; 5: 5-18. https://goo.gl/HUnrj3
- Bing C. Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte. 2015; 4: 149-152. https://goo.gl/dw5FWV
- De Carvalho MH, Colaço AL, Fortes ZB. Cytokines, endothelial dysfunction, and insulin resistance. Arq Bras Endocrinol Metabol. 2006; 50: 304-312. https://goo.gl/Z19ySg
- de Luis DA, González Sagrado M, Conde R, Aller R, Izaola O, Castro MJ. Circulating adipocytokines in morbid obese patients, relation with cardiovascular risk factors and anthropometric parameters. Nutr Hosp. 2011; 26: 91-96. https://goo.gl/WGWfJ2
- de Luis DA, Aller R, Izaola O, Ovalle HF. The serum profile of adipokines in naïve patients with diabetes mellitus type 2 and obesity. J Clin Lab Anal. 2011; 25: 409-413. https://goo.gl/y81GJy
- Brandt C, Pedersen BK. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010. https://goo.gl/Ro6op3
- Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008. https://goo.gl/GeA9Am
- Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005; 98: 1154-1162. https://goo.gl/ve7mHf
- Perrier S, Darakhshan F, Hajduch E. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett. 2006; 580: 6289-6294. https://goo.gl/768sBT
- Izquierdo M, Ibañez J, Calbet JAL, Navarro-Amezqueta I, González-Izal M, Idoate F, et al. Cytokine and hormone responses to resistance training. Eur J Appl Physiol. 2009; 107: 397-409. https://goo.gl/co8uVd
- Gleeson M, Bishop N, Stensel D. Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011; 11: 607-615. https://goo.gl/K6n99t
- Peake JM, Nosaka K, Muthalib M, Suzuki K. Systemic inflammatory responses to maximal versus submaximal lengthening contractions of the elbow flexors. Exerc Immunol Rev. 2006; 12: 72-85. https://goo.gl/pfg9Bd
- Stewart L, Flynn M, Campbell W, Craig B, Robinson J, Timmerman K, et al. The influence of exercise training on inflammatory cytokines and C - reactive protein. Med Sci Sport Exerc. 2007; 39: 1714-1719. https://goo.gl/Hg37HP
- Chupel MU, Direito F, Furtado GE, Minuzzi LG, Pedrosa FM, Colado JC, et al. Strength training decreases inflammation and increases cognition and physical fitness in older women with cognitive impairment. Front Physiol. Frontiers. 2017; 8: 377. https://goo.gl/pyv8pb
- Nielsen AR, Pedersen BK. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl Physiol Nutr Metab. 2007; 32: 833-839. https://goo.gl/K32x2s
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-Alpha production in humans. FASEB J. 2003; 17: 884-886. https://goo.gl/F2QrWu
- Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, et al. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004; 320: 449-454. https://goo.gl/yimxJn
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008; 88: 1379-1406. https://goo.gl/p9duua
- McKay BR, De Lisio M, Johnston APW, O’Reilly CE, Phillips SM, Tarnopolsky MA, et al. Association of interleukin-6 signalling with the muscle stem cell response following muscle-lengthening contractions in humans. PLoS One. 2009; 4: 6. https://goo.gl/seFegY
- Ropelle ER, Flores MB, Cintra DE, Rocha GZ, Pauli JR, Morari J, et al. IL-6 and IL 10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKβ and ER stress inhibition. PLoS Biol. 2010; 8: 8. https://goo.gl/SZdcxo
- Muñoz-Cánoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013; 280: 4131-4148. https://goo.gl/XfqhYH
- Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 Is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008; 7: 33-44. https://goo.gl/Apng54
- Alam S, Mustafa G, Alam M, Ahmad N. Insulin resistance in development and progression of nonalcoholic fatty liver disease. World J Gastrointest Pathophysiol. 2016; 7: 211-217. https://goo.gl/cBtfGH
- Fotbolcu H, Zorlu E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J Gastroenterol. 2016; 22: 4079-4090. https://goo.gl/mZvM4F
- Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008; 94: 206-218. https://goo.gl/HnyGn8
- Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007; 21: 1443-1455. https://goo.gl/Fcucyj
- Kang YS. Obesity associated hypertension: new insights into mechanism. Electrolyte Blood Press. 2013; 11: 46-52. https://goo.gl/HDoXX8
- Libby P. History of Discovery: inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012; 32: 2045-2051. https://goo.gl/Qb7N29
- Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. American Society for Clinical Investigation. 2016; 126: 12-22. https://goo.gl/vtsb6F
- Bastarrachea R, López-Alvarenga J, Bolado-García V, Téllez-Mendoza J, Comuzzie A, Laviada-Molina H. Macrófagos, inflamación, tejido adiposo, obesidad y resistencia a la insulina. Gac Med Mex. Academia Nacional de Med. 2007; 143: 505-512. https://goo.gl/2fpnu6
- Petersen AMW, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol. 2006; 57: 43-51. https://goo.gl/5WWqar
- Riddell M, Gallen I, Smart C, Taplin CE, Peter Adolfsson, Alistair N Lumb, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017; 5: 377-390. https://goo.gl/h2nZ1G
- Turner D, Luzio S, Gray B, Dunseath G, Rees ED, Kilduff LP, et al. Impact of single and multiple sets of resistance exercise in type 1 diabetes. Scand J Med Sci Sports. 2015; 25: 99-109. https://goo.gl/9KC6Rv
- Summers SA, Goodpaster BH. CrossTalk proposal: intramyocellular ceramide accumulation does modulate insulin resistance. J Physiol. 2016; 594: 3167-3170. https://goo.gl/cBn2gB
- Bastard J-P, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006; 17: 4-12. https://goo.gl/7zDyJH
- Kim HJ, Lee JS, Kim CK. Effect of exercise training on muscle glucose transporter 4 protein and intramuscular lipid content in elderly men with impaired glucose tolerance. Eur J Appl Physiol. 2004; 93: 353-358. https://goo.gl/A72n9n
- Shepherd SO, Cocks M, Tipton KD, Witard OC, Ranasinghe AM, Barker TA, et al. Resistance training increases skeletal muscle oxidative capacity and net intramuscular triglyceride breakdown in type I and II fibres of sedentary males. Exp Physiol. 2014; 99: 894-908. https://goo.gl/wodSJ7
- Karlsson HKR, Chibalin AV, Koistinen HA, Yang J, Koumanov F, Wallberg-Henriksson H, et al. Kinetics of GLUT4 trafficking in rat and human skeletal muscle. Diabetes. 2009; 58: 847-854. https://goo.gl/PKTawf
- Wallberg-Henriksson H, Constable SH, Young DA, Holloszy JO. Glucose transport into rat skeletal muscle: interaction between exercise and insulin. J Appl Physiol. 1988; 65: 909-913. https://goo.gl/Ho2VV3
- Umpierre D, Ribeiro PAB, Kramer CK, Leitão CB, Zucatti ATN, Azevedo MJ, et al. Physical activity advice only or structured exercise training and association with HbA 1c levels in type 2 diabetes. JAMA. 2011; 305: 1790. https://goo.gl/9DzkUq
- Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012; 35: 446-454. https://goo.gl/4NwYXQ
- Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother. 2012; 46: 1453-1469. https://goo.gl/r7U2U8
- Lundell LS, Krook A. ContRac1ion-mediated glucose uptake: a central role for Rac1. Diabetes. 2013; 62: 1024-1025. https://goo.gl/iWdU2T
- Sylow L, Nielsen IL, Kleinert M, Møller LLV, Ploug T, Schjerling P, et al. Rac1 governs exercise-stimulated glucose uptake in skeletal muscle through regulation of GLUT4 translocation in mice. J Physiol. 2016; 594: 4997-5008. https://goo.gl/cuGyvM
- Koopman R, Manders RJF, Jonkers RAM, Hul GBJ, Kuipers H, van Loon LJC. Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Eur J Appl Physiol. 2006; 96: 525-534. https://goo.gl/sw3Uon
- Koopman R, Manders RJF, Zorenc AHG, Hul GBJ, Kuipers H, Keizer HA, et al. A single session of resistance exercise enhances insulin sensitivity for at least 24 h in healthy men. Eur J Appl Physiol. 2005; 94: 180-187. https://goo.gl/pz1TBk
- Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007; 85: 377-384. https://goo.gl/oy17MM
- Goodpaster B, He J, Watkins S, Kelley DS. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001; 86: 5755-5761. https://goo.gl/sZJeqm
- Shepherd SO, Cocks M, Tipton KD, Ranasinghe AM, Barker TA, Burniston JG, et al. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J Physiol. 2013; 591: 657-675. https://goo.gl/i1xem3

Sign up for Article Alerts