C-Fos Protein Immunoreactivity in Raphe Nuclei of Trained and Sedentary Rats
Fabio Cesar Prosdocimi3, Guilherme Aparecido Monteiro Duque da Fonseca2, Hélio Doyle Pereira da Silva2, Ana Luiza Cabrera Martimbianco3, Maria Inês Nogueira1 and Lucio Frigo3*
1Anatomy Department, Biomedical Sciences Institute, Universidade de São Paulo, São Paulo 05508-900 SP
2Dental research division, Univeritas-UnG, Guarulhos, 07023-070 SP
3Medicine Department, Universidade Metropolitana de Santos, 11045-002 SP
*Address for Correspondence: Lucio Frigo, Dental Research Division, Implantology Department, Univeritas – UnG (Universidade de Guarulhos), Address: Praca Teresa Cristina, 01, Guarulhos, SP, 00723-070, Brazil, Tel/Fax: +055-112-464-1684; E-mail: luciofrigo@uol.com.br
Submitted: 15 May 2019; Approved: 20 June 2019; Published: 24 June 2019
Citation this article: Prosdocimi FC, Duque da Fonseca GAM, da Silva HDP, Cabrera Martimbianco AL, Frigo L, et al. C-Fos Protein Immunoreactivity in Raphe Nuclei of Trained and Sedentary Rats. Int J Sports Sci Med. 2019;3(1): 042-046.
Copyright: © 2019 Prosdocimi FC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Raphe nuclei; c-Fos protein; Fatigue; Swimming-test
Download Fulltext PDF
Fatigue can be defined as an acute impairment of exercise performance. Many complex inter playing factors contribute to determine fatigue and in broad sense they can be separated as peripheral and central factors. Central Nervous System (CNS) adaptation responses to prolonged exercise could be considered in overall metabolic terms. Despite considering that brain function is not determined by a single neurotransmitter system, serotonin (5-HT) is the most extensive neurotransmitter studied. It is linked to fatigue due its well-known effects on sleep, lethargy, drowsiness and loss of motivation. The present study was designed to investigate the activation of Raphe nuclei neurons, the main source of 5-HT in the brain, through immunodetection of c-Fos protein in trained and sedentary rats. Sixteen male Wistar rats were divided randomly into 2 groups of eight animals, named T (trained - five weeks, 15’ - 60) and S (sedentary). After c-Fos immunocytochemistry, neuron counting was proceeded only if cell body was unequivocally immunostained and achieved by Image Pro-Pus computer program captured digital images. The examiner was unaware of the groups’ nature. In describing the c-Fos immunoreactive neurons (c-Fos-ir) in the rat brain. Evaluation of c-Fos-ir showed no significant differences in most of Raphe nuclei, except in most caudal part of Dorsal Raphe nuclei (p = 0,008) and Raphe Palidus nuclei (p = 0.006). Trained group c-Fos-ir neurons were more numerous than sedentary group. These regions may be related to excitatory respiratory nuclei modulation.
Abbreviations
In the past, there were many studies on the effects of supplementing calcium, iron, zinc and selenium after physical exercise, but little attention was paid to the role of trace element chromium in human life activities.
CNS: Central Nervous System; PF: Peripheral Factors; CF: Central Factors; CFS: Chronic Fatigue Syndrome; c-Fos-ir: c-Fos Immunoreactive; RLi: Rostral Linear; CLi: Caudal Linear; DR: Dorsal; MnR: Median; PMnR: Paramedian; PnR: Pontis; RMg: Magnus; RPa: Palidus; ROb: Obscurus
Introduction
Fatigue can be defined as an acute impairment of exercise performance, including an increase in perceived effort necessary to exert a desired force or power output and the eventual inability to produce that force or power output [1-3]. Many complex inter playing factors contribute to determine fatigue and in broad sense they can be separated as peripheral and central factors [1,2,4]. Peripheral Factors (PF) are related to muscle itself and motor plate. Muscle glycogen depletion, decrease of adenosine diphosphate rephosphorylation, disturbs in neuromuscular transmission, sarcolemma excitability and excitation-contraction coupling are the more cited factors [1,4] Central Factors (CF) comprehend the events occurring in the spinal cord and encephalon related to initiation, modulation and sustaining the motor drive. The Central Nervous System (CNS) adaptation responses to prolonged exercise could be considered in overall metabolic terms, like: altering blood glucose, the main nervous tissue fuel; raising body temperature (hyperthermia), reducing brain activity; increasing plasma concentration of Ammonia (NH3), affecting cerebral blood flow, astrocyte function and synaptic transmission [1,4-6]. In the other hand, the CNS adaptation responses could be restricted to a cluster of brain nucleus and/or neurotransmitter systems involved in motor control and mood. The compelling evidence of specifics neurological mechanisms can affect the magnitude of descending motor drive is a recent issue in fatigue theme [4]. Monoamine hypothesis of central fatigue stats that the balance of neurotransmitters (including serotonin, dopamine, and norepinephrine) influences thermoregulation, motor impulses, and sensations of fatigue. It is generally believed that the reduction of CNS drive to the motor neurons could be due a reduction in corticospinal impulses reaching motor neurons or a feedback inhibition mediated by sensory afferents from the muscle at spinal cord level [1,4]. In addition, Chronic Fatigue Syndrome (CFS), in which the major symptom is a chronic debilitating fatigue argues in favor of central fatigue, once these patients show normal isolated muscle function, metabolism and excitability-contraction coupling [1,4]. Recently much interest has been focused on the exercise-induced in neurotransmitter release during exercise as part of the explanation for central fatigue. Findings have let to the Serotonin-Fatigue Hypothesis [1,4]. Despite considering that brain function is not determined by a single neurotransmitter system, serotonin (5-HT) is the most extensive neurotransmitter studied. It is linked to fatigue due its well-known effects on sleep, lethargy, drowsiness and loss of motivation [1,4] The serotonin-fatigue hypothesis suggests that increased concentrations of brain 5-HT can impair CNS function during prolonged exercise causing deterioration in exercise performance [1,4]. Prolonged exercise led to an increase of plasma free fat acids displacing tryptophan from its binding sites on plasma albumin and increase consumption of blood branched-chain amino acids. The increase of free tryptophan in the blood and the lesser dispute for the blood-brain transporter cause a raise in tryptophan availability in brain that are metabolized in 5-HT by serotonin producing neurons [1,4]. In fact, since Barchas and Freedman [7] there have been accumulating evidences of changes in brain 5-HT following prolonged exercise in rat. Detection of 5-hidroxi-indole-acetic acid (5-HT metabolite) and 5-HT itself have detailed the brain localization of the activity increase, such as: midbrain, hippocampus, striatum and cerebrospinal [1,4]. In more recent years, a number of studies have attempted to modulate central serotonin levels by means of dietary supplementation or pharmacological interventions in human and animals analyzing motor activity and performance. Most of the results support the theory [1-3]. However, a more holistic theory emerges arguing in favor of a balance among different neurotransmitters [1-3]. In this perspective, the present study was designed to investigate the activation of Raphe nuclei neurons, the main source of 5-HT in the brain, through immunodetection of c-Fos protein in trained and sedentary rats.
Materials and Methods
Animals
All experiments were performed with the approval of, and in accordance with the regulations laid down by São Paulo University Bioethics Committee, protocol number-22/2002. Sixteen male Wistar rats were housed in standards conditions of temperature (22-25°C), relative humidity (40-60%) and 12/12h light/dark cycle with unrestricted access to food and water. Animals were provided by Central Animal House of Biomedical Science Institute of São Paulo University. All rats were placed in common box and divided randomly into 2 groups of eight animals, named T (trained) and S (sedentary).
Trained group was submitted to forced swim test and, sedentary group was not trained at all.
Forced swimming-test
Forced swimming-test protocol used was developed by Vieira [8]. T group animals were placed in water tanks, one for each rat. Water volume was enough for the animals do not reach the bottom, temperature was maintained between 30-32°C and the animals were dried after training sections. Forced swimming ranged from 15 to 60 min, Monday to Friday and from 1 to 5 weeks, according figure 1 and table 1.
To avoid exercise adaptation, progressive overloading was achieved by adding weight to animal’s tail. They range from 1% of the animal’s body weight to 5% maximum and were properly attached to animal’s tail.
c-Fos immunohistochemistry protocol
Animals were anesthetized 90 min after stimulus with pentobarbital (30 mg/ kg, i.p.) and transcardically perfused with 0.9% saline followed by cold 4% paraformaldehyde in 0.1 M borate fixative (1L), pH 9.5. Each brain were removed from the skull and cryoprotected in 0.1 M potassium phosphate buffer, pH 7.4 (KPBS) containing 20% sucrose for 2 h at 4°C, and then placed in 20% sucrose in KPBS overnight at 4°C. The brain were cut along frontal plane in 30 μm thick sections and five 1-in-5 series were collected in antifreeze solution. One series of sections was immunohistochemically stained using conventional avidin-biotin immunoperoxidase protocol. These sections were pretreated in 0.3% hydrogen peroxide solution diluted in KPBS. After KPBS rinses sections were incubated in primary c-Fos antiserum (Oncogene Science Inc., USA) at 1:10.000 over 48 hours at 4°C. Next, brain sections were rinsed in KPBS and incubated for 1h in goat anti-rabbit biotinylated secondary antibody (Vector Labs, Burlingame, CA, USA), followed by avidin-biotin peroxidase complex (ABC Kit Vector Labs.) in KPBS for 1h. Sections were incubated in diaminobenzidine tetrahydrocloride (DAB; Sigma) and 0.01% hydrogen peroxide dissolved in KPBS. Reaction was terminated after 2-4 min. with successive rinses in KPBS. Before cover slipping, DAB reaction product was intensified using osmio tetroxide procedure. Another section series was thionin stained (Nissl’s method) to provide cytoarchitectonic and tract references. The sections were mounted onto previously gelatin-coated slides and cover slipped with DPX (BDH, England).
Data analysis
Precise Raphe nuclei location was established matching the sequential sections, immunohistochemistry with thionin labeled sections and comparing it to “The Rat Brain in Stereotaxic Coordinates”30. Citoarchitecture studies defined a further parcellation of Raphe nuclei. From the upper (rostral) to lower (caudal) levels, it can found the following nucleus: Rostral Linear (RLi), Caudal Linear (CLi), Dorsal (DR), Median (MnR), Paramedian (PMnR), Pontis (PnR), Magnus (RMg), Palidus (RPa) and Obscurus (ROb) [9]. Neuron counting was proceeded only if cell body was unequivocally immunostained in Image Pro-Pus computer program captured digital images. The examiner was unaware of the groups’ nature. Statistical analysis was performed with Tuckey test and ANOVA. Statistical level of significance was set at p <0.05.
Results
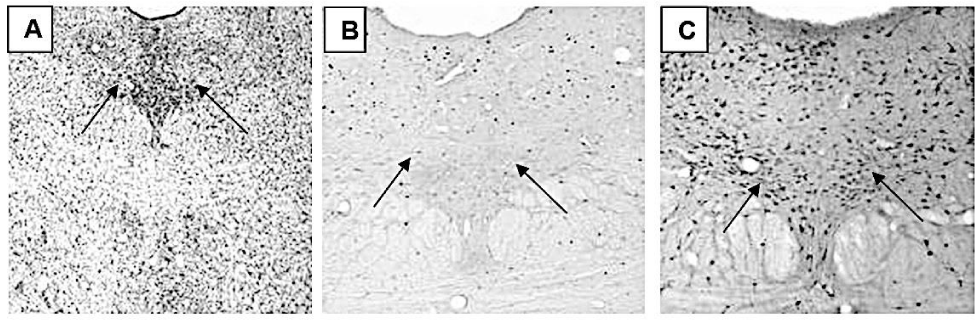
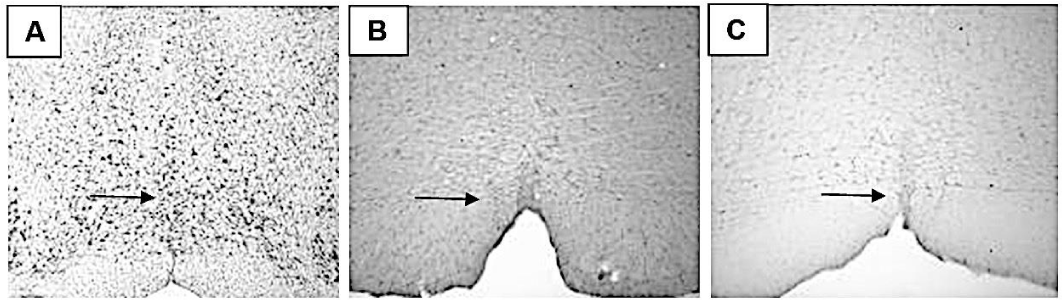
In describing the c-Fos immunoreactive (c-Fos-ir) neurons in rat brain, we followed the parcellation provided by Paxinos and Watson [10].
Immunoreactivity of c-Fos protein in MnR, PMnR, PnR, RMg, Rob, RLi, CLi nucleus were observed, but there was not statistical difference between trained and sedentary groups.
Statistical difference was found only in most caudal or posterior parts of DR (p = 0.008) and RPa nuclei (p = 0.006). Illustrated in figure 2, graphic 1 and figure 3, graphic 2.
Discussion
Methodological considerations
c-Fos protein is a gene product of the immediate-early c-Fos proto-oncogen. It is rapidly synthesized after different external stimulus and reaches maximum cell nuclear concentration after 60-90 minutes, lasting 2-5h.
c-Fos protein immunodetection indicates physiological cell activity and is very appropriate for central nervous system studies [11-14].
Animal adaptation to environment is absolutely important to reduce stress imposed by swimming task like water contact and housing displacement. It reduces the “background noise” induced non-intended stimulus. So, prior testing all groups were environment adapted for a week, by way of 30 minutes water contact in tanks with 1/3 of water level and experiments were realized in the same period of the days.
c-Fos immunoexpresion was mostly absent in Raphe nuclei in inefficient or mild external stimuli, so we excluded the basal immunoexpresion control group [12].
Biological considerations
The serotonin-fatigue hypothesis suggests that increased concentrations of brain 5-HT is one important contributor of CNS impairing during prolonged exercise causing deterioration in exercise performance. Ample research data support the association between 5-HT and fatigue. Different approaches had been used, like detection of 5-HT and 5-HT metabolites following prolonged exercise [7,15-17].
In more recent years, a number of studies have attempted to modulate central serotonin levels by means of dietary supplementation or pharmacological interventions in human and animals analyzing motor activity and performance. Most results support the theory that augmentation of serotonin releasing or, more recently, an imbalance between serotonin and dopamine lead to the sense of fatigue. Serotonin-containing neurons are located in median and paramedian zones of the brain stem and concentrated in neuronal cells cluster designated Raphe nuclei [18-20].
Besides concentrating serotonin-containing neurons Raphe nuclei may be aptly designated as a multiple neurotransmitter complex. Serotonin-containing neurons vary from 10% in PnR to 70% in DR in the total neuron number of each group [21].
Raphe nuclei physiological function is accessed by way of nucleus ablation/ stimulation or studying their hodology (axon projections). The first approach is less accurate once Ablation/stimulation easily outborders the nucleus limits. Hodology study approach is more precise but mostly theoretical. It is assumed that raphe nuclei participate in sleep/awake cycle, hyperreactivity, aggressive behavior, pain, motor somatic and visceral behavior, endocrine, learning and memory [22-24].
Raphe nuclei have widespread innervations to neuron cell groups that importantly contribute to motor control or to motoneurons directly. DR neurons, we found highly stimulated in Trained groups, innervates substantia nigra and caudatus-putamen. Substantia nigra is a group of dopamine-containing neurons that modulate the basal ganglia circuit complex, so important in motor output [25]. In addition, DR innervates all cortical cell layers in lateral parts of the cerebral cortex [26,27].
DR and PnR neurons innervate locus coeruleus, the main noradrenalin containing neurons [28] suggest that DR and PnR exerts a strong inhibitory influence in locus coeruleus and substantia nigra, important noradrenergic and dopaminergic centers [29]. These Raphe nuclei projections strongly support the serotoninergic theory of fatigue.
On the other hand, raphe nuclei descendant projections to medulla and spinal cord are related to the opposite effect of fatigue. Raphe-spinal pathway stimulation releases 5-HT onto ventral horn motoneurons, markedly enhancing their excitability. These projections are synaptic facilitatory to extensors, flexors, α and γ motoneurons, increasing motor responsiveness and are related to the activation of 5-HT2 receptors positively coupled to L-type Ca2+ ion channels [30-32]. Additionally, DR and RPa C-Fos-ir neuros may project to ventral respiratory column and could explain the difference between Sedentary and Trained in swimming test [33].
Raphe nuclei fos-ir of trained and sedentary groups plus hodologic descriptions of these nuclei points to an even complex situation. We argue that it is necessary to consider, to the central fatigue theme, not only the participation of others neurotransmitters, once we describe bigger activity of Raphe nuclei (supposedly serotoninergic) in trained groups. We suggest caution on serotonin-fatigue hypothesis because specific subsets (nucleus) of serotonin-producing neurons of Raphe nuclei are clearly motor facilitatory.
Conclusion
c-Fos immunoreactive neurons in trained group outnumbered sedentary group in all rafe nuclei.
- Meeusen R, Watson P, Hasegawa H, Roelands Bb, Piacentini MF. Central fatigue: the serotonin hypothesis and beyond. Sports Med. 2006; 36: 881-909. https://bit.ly/2XiXAT1
- Weir JP, Beck TW, Cramer JT, Housh TJ. Is fatigue all in your head? A critical review of the central governor model. Br J Sports Med. 2006; 40: 573-586. https://bit.ly/2XpQtIk
- Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997; 29: 45-57. https://bit.ly/2RpcKRd
- Cordeiro LMS, Rabelo PCR, Moraes MM, Teixeira-Coelho F, Coimbra CC, Wanner SP, et al. Physical exercise-induced fatigue: the role of serotonergic and dopaminergic systems. Braz J Med Biol Res. 2017; 50: e6432. https://bit.ly/2Ktc78x
- Dalsgaard MK, Secher NH. The brain at work: a cerebral metabolic manifestation of central fatigue? J Neurosci Res. 2007; 85: 3334-3339. https://bit.ly/31Pe04H
- Nybo L, Secher NH. Cerebral perturbations provoked by prolonged exercise. Prog Neurobiol. 2004; 72: 223-261. https://bit.ly/2ITrvb2
- Barchas JD, Freedman DY. Brain amines: response to physiological stress. Biochem Pharmacol. 1963; 12: 1232-1235. https://bit.ly/2XqtSLE
- Vieira C, De Lima TC, Carobrez Ade P, Lino-de-Oliveira C. Frequency of climbing behavior as a predictor of altered motor activity in rat forced swimming test. Neurosci Lett. 2008; 445: 170-173. https://bit.ly/2RtLPDz
- Taber E. Brodal A. Walberg F. The raphe nuclei of the brain stem in the cat. I. Normal topography and cytoarchitecture and general discussion. J Comp Neurol. 1977; 114: 161-187. https://bit.ly/2x3iff1
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. Academic Press; 1998. https://bit.ly/2L5hnP9
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends in Neuroscience. 1989; 12: 459-462. https://bit.ly/2IWSylL
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991; 14: 421-451. https://bit.ly/2WRQtwx
- Krukoff TL. Expression of c-fos in studies of central autonomic and sensory systems. Mol Neurobiol. 1993 Fall-Winter; 7: 247-263. https://bit.ly/2KwdinJ
- DiNardo LA, Travers JB. Distribution of fos-like immunoreactivity in the medullary reticular formation of the rat after gustatory elicited ingestion and rejection behaviors. J Neurosci. 1997; 17: 3826-3839. https://bit.ly/31PeAPV
- Chaouloff F, Elghozi JL, Guezennec Y, Laude D. Effects of conditioned running on plasma, liver and brain tryptophan and on brain 5-hydroxytryptamine metabolism of the rat. Br J Pharmacol. 1985; 86: 33-41. https://bit.ly/2FlExx6
- Chaouloff F, Kennett GA, Serrurrier B, Merino D, Curzon G. Amino acid analysis demonstrates that increased plasma free tryptophan causes the increase of brain tryptophan during exercise in the rat. J Neurochem. 1986; 46: 1647-1650. https://bit.ly/2IWQKJG
- Chaouloff F, Laude D, Elghozi JL. Physical exercise: evidence for differential consequences of tryptophan on 5-HT synthesis and metabolism in central serotonergic cell bodies and terminals. J Neural Transm. 1989; 78: 121-130. https://bit.ly/2WY5KvT
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992; 72: 165-229. https://bit.ly/2WY5NYB
- Dahlström A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964; 20: 398-399. https://bit.ly/2WVnDuU
- Björklund A, Falck B, Stenevi U. Classification of monoamine neurones in the rat mesencephalon: distribution of a new monoamine neurone system. Brain Res. 1971; 32: 269-285. https://bit.ly/2MYetOB
- Wiklund L, Björklund A. Mechanisms of regrowth in the bulbospinal serotonin system following 5,6-dihydroxytryptamine induced axotomy. II. Fluorescence histochemical observations. Brain Res. 1980; 191: 109-127. https://bit.ly/2WURPGS
- Holstege G, Kuypers HG. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res. 1982; 57: 145-175. https://bit.ly/2Fnt0xf
- Smith DA, Flynn JP. Afferent projections to quiet attack sites in cat hypothalamus. Brain Res. 1980; 194: 41-51. https://bit.ly/2IvGtFa
- Parent A. Identification of the pallidal and peripallidal cells projecting to the habenula in monkey. Neurosci Lett. 1979; 15: 159-164. https://bit.ly/2L6c6Hc
- Van der Kooy D, Hattori T. Dorsal raphe cells with collateral projections to the caudate-putamen and substantia nigra: a fluorescent retrograde double labeling study in the rat. Brain Res. 1980; 186: 1-7. https://bit.ly/2ZBHcKI
- Tohyama M, Shiosaka S, Sakanaka M, Takagi H, Senba E, Saitoh Y, et al. Detailed pathways of the raphe dorsalis neuron to the cerebral cortex with use of horseradish peroxidase-3,3',5,' tetramethyl benzidine reaction as a tool for the fiber tracing technique. Brain Res. 1980; 181: 433-439. https://bit.ly/2RqXhQs
- Lidov HG, Grzanna R, Molliver ME. The serotonin innervation of the cerebral cortex in the rat- an immunohistochemical analysis. Neuroscience. 1980; 5: 207-227. https://bit.ly/31M2t6n
- Dray F, Weliky I. Identification of O-methyloximes of ketosteroids by gas chromatography, thin-layer chromatography, mass spectra, and kinetic studies. Anal Biochem. 1970; 34: 387-402. https://bit.ly/31IOUEB
- Meeusen R, Watson P, Dvorak J. The brain and fatigue: new opportunities for nutritional interventions? J Sports Sci. 2006; 24: 773-782. https://bit.ly/2x53iJ9
- Kavanagh JJ, McFarland AJ, Taylor JL. Enhanced availability of serotonin increases activation of unfatigued muscle but exacerbates central fatigue during prolonged sustained contractions. J Physiol. 2019; 597: 319-332. https://bit.ly/2J1SSj6
- Claghorn GC, Fonseca IAT, Thompson Z, Barber C, Garland T Jr. Serotonin-mediated central fatigue underlies increased endurance capacity in mice from lines selectively bred for high voluntary wheel running. Physiol Behav. 2016; 161: 145-154. https://bit.ly/2WZMzBS
- Perrier JF. If serotonin does not exhaust you, it makes you stronger. J Physiol. 2019; 597: 5-6. https://bit.ly/2N09InV
- Morinaga R, Nakamuta N, Yamamoto Y. Serotonergic projections to the ventral respiratory column from raphe nuclei in rats. Neurosci Res. 2019; 143: 20-30. https://bit.ly/2LdnGjV




Sign up for Article Alerts