Development, Characterization and Isolation of Small Hepatocyte-Like Cells from Primary Culture of Cirrhotic Liver of Biliary Atresia
Takayoshi Tokiwa1*, Mariko Wakai1, Taisuke Yamazaki1 and Shin Enosawa2
1Department of Liver Cell Biology, Kohno Clinical Medicine Research Institute, Tokyo, Japan
2Division of Advanced Medical Sciences, National Center for Child Health and Development, Tokyo, Japan
*Address for Correspondence: Takayoshi Tokiwa, Department of Liver Cell Biology, Kohno Clinical Medicine Research Institute, Kita-Shinagawa, Shinagawa, 140-0001, Tokyo, Japan, Tel: 81-03-3474-1833; Fax: 81-03-3474-1833; E-mail: t.tokiwa@kcmi.or.jp
Submitted: 25 May 2017; Approved: 09 June 2017; Published: 03 July 2017
Citation this article: Tokiwa T, Wakai M, Yamazaki T, Enosawa S. Development, Characterization and Isolation of Small Hepatocyte-Like Cells from Primary Culture of Cirrhotic Liver of Biliary Atresia. Int J Stem Cell Res. 2017;3(1): 017-024.
Copyright: © 2017 Tokiwa T, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Biliary atresia; Liver; Progenitor cells; Primary culture
Download Fulltext PDF
Biliary atresia is a rare and serious liver disease in newborn infants and often leads to biliary cirrhosis. Small hepatocytes which show high growth potentials and express differentiated phenotypes may be ideal cells for in vivo therapies for biliary atresia. In this study, we sought to determine whether small hepatocyte-like cells can be isolated from non-parenchymal cell fractions of biliary atresia liver. When non-parenchymal cells were cultivated on collagen type I-coated or -uncoated dishes, clusters of small hepatocyte-like cells appeared in 1-2 weeks. Small hepatocyte-like cells were positive for the hepatic stem cell marker epithelial cell adhesion molecule in addition to the hepatocytic (albumin, CK18) and biliary (CK19) markers and exhibited the striking hepatocytic morphology. The clusters of small hepatocyte-like cells were surrounded by the fibroblast-like cells with a mesenchymal cell phenotype. We could successfully detach only the small hepatocyte-like cell clusters from the substrate using the proteolytic enzyme kit Liberase T-Flex. Small hepatocyte-like cells thus isolated grew in culture and expressed gene profiles similar to those previously reported for adult human small hepatocytes. Carbon tetrachloride-induced liver injury tended to be attenuated when small hepatocyte-like cells were transplanted into nude mice. This study may facilitate future autologous stem cell therapy to treat this obstinate disease.
Abbreviations
BA: Biliary Atresia; SHs: Small Hepatocytes; SHLCs: SH-Like Cells; NPC: Non-Parenchymal Cell; CPS: Carbamoyl Phosphate Synthetase; OTC: Ornithine Transcarbamylase; HBSS: Hanks Balanced Salt Solution; HEPES: N-2 Hydroxyethylpiperazine-N`-2-Ethanesulfonate; FBS: Fetal Bovine Serum; EGF: Epidermal Growth Factor; DMEM/F12: Dulbecco`S Modified Eagle`S Medium/F12; RT-PCR: Reverse Transcription Polymerase Chain Reaction; G3PDH: Glyceraldehyde-3-Phosphate Dehydrogenase; AAT: Α1 Anti-Trypsin; c/EBPα: CCAAT/Enhancer Binding Protein; CK: Cytokeratin; CYP: Cytochrome P450; EpCAM: Epithelial Cell Adhesion Molecule; TO: Tryptophan 2,3-Dioxygenase; α-SMA: α-Smooth Muscle Actin; HLA: Human Leukocyte Antigen; PAS: Periodic Acid- Schiff; ELISA: Enzyme-Linked Immunosorbent Assay; CCl4: Carbon Tetrachloride; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; LD: Lactate Dehydrogenase; EDTA: Ethylenediaminetetraacetic Acid.
Introduction
Biliary Atresia (BA) is a rare and serious liver disease in newborn infants and often leads to biliary cirrhosis [1]. If the Kasai operation [2] is not successful, liver transplantation is required. However, transplantation is restricted by the scarcity of organs and requires life-long immunosuppression. Therefore, alternative therapeutic approaches including hepatocyte transplantation or liver progenitor cell therapy have been developed [3,4]. Adult Human Small Hepatocytes (SHs) possess both growth potential in culture and hepatic characteristics [5]. SHs or SH-Like Cells (SHLCs) may be an ideal cell type for in vivo therapies for BA if they can also be isolated from livers of BA patients. Previously, we reported that clusters of SHLCs appeared when Non-Parenchymal Cell (NPC) fractions from cirrhotic liver of BA were subjected to primary culture [6]. However, the nature of SHLCs remained to be defined due to insufficient cellular characterization. In the present study, we investigated the appropriate culture conditions for developing SHLC clusters from NPCs of the BA liver. The characteristics of SHLCs and their isolation were also examined. This study may facilitate future autologous stem cell therapy to treat this obstinate disease.
Materials and Methods
Liver tissue
Liver specimens were obtained from patients with cirrhosis secondary to BA (n = 22 patients; age 4 to 60 months (mean, 12.8 months) at the National Center for Child Health and Development (Setagaya, Tokyo, Japan). Liver specimens from patients with Carbamoyl Phosphate Synthetase (CPS) or Ornithine Transcarbamylase (OTC) deficiency (n = 3 patients; age 5 to 10 months) were used as controls in this study because of the absence of cirrhosis in these tissues. Experimental procedures were performed according to the ethical guidelines of the 1975 Declaration of Helsinki, with informed consent from the patient’s parent and approval by the Human Studies Committee of the National Center for Child Health and Development and Kohno Clinical Medicine Research Institute, Tokyo, Japan. Histological examination showed that liver specimens with BA were all cirrhotic (data not shown).
Primary culture of NPCs
Liver cell suspensions were prepared using the collagenase perfusion method as described elsewhere [7-9]. The cell suspension was centrifuged once at 160 × g for 5 min, and the cell pellet fraction was centrifuged once at 50 × g for 1 min. The supernatant fraction, which was the NPC fraction, was resuspended in Hanks balanced salt solution (HBSS) and 10 mm N-2-Hydroxyethylpiperazine-N`-2-Ethanesulfonate (HEPES), pH 7.2. Panning to remove erythroid cells was performed as previously described [10]. Isolated NPCs were suspended in Williams’ E medium (Invitrogen, Tokyo, Japan) containing 10% Fetal Bovine Serum (FBS), 25 ng/ml Epidermal Growth Factor (EGF, Sigma-Aldrich, Tokyo, Japan), 0.1 μm insulin (Wako, Osaka, Japan), 1 μm dexamethasone (Sigma-Aldrich), and 1% penicillin-streptomycin and plated on type I collagen-coated or -uncoated dishes at high density (105 cells/cm2). Two days later, culture medium was switched to Dulbecco`S Modified Eagle`S Medium/F12 (DMEM/F12, Invitrogen) medium supplemented with 5% FBS and differentiation-inducing factors (Differentiation-Inducing Condition/Condition I), DMEM/F12 medium supplemented with only differentiation-inducing factors (differentiation-inducing condition/condition II), or DMEM/F12 medium supplemented with 5% FBS (differentiation-noninducing condition/condition III). Condition III was prepared for use as a control. Differentiation-inducing factors were 1 × insulin-transferrin-sodium selenite (Invitrogen), 10 ng/ml EGF (Sigma-Aldrich), 1 mm L-ascorbic acid phosphate magnesium salt (Wako), 10 mm nicotinamide (Sigma-Aldrich), and 1 μm dexamethasone (Sigma-Aldrich). To isolate and develop SHLCs a cell cluster appeared in the dish was encircled with a small cloning cylinder (Corning, Tokyo, Japan), incubated with the proteolytic enzyme kit Liberase T-Flex (The mixture of collagenase and thermolysin, Roche custom biotech, Tokyo, Japan) for 2 min, and then aspirated with a Pasteur pipette. The cells thus harvested were plated into a plastic dish (35 mm), and then grown in condition I. Cells were cultivated at 37°C in a fully humidified atmosphere containing 5% CO2.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Tokyo, Japan), treated with DNase (Qiagen), and used as a template for synthesis of cDNA using a first strand cDNA kit (ReverTraAce-α, Toyobo, Osaka, Japan). A 5-μl aliquot of cDNA was then amplified with PCR, which was carried out in a total volume of 50 μl and included 0.2 mm dNTPs, 0.25 μmol/L primers, and 1.25 U Taq DNA polymerase (MBI Fermentas, Amherst, NY). A program consisting of 25-40 cycles was used for all PCRs and consisted of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 2 min. Glyceraldehyde-3-Phosphate Dehydrogenase (G3PDH) was amplified as an internal loading control. The PCR primers used for amplification were as follows:
A1 Anti-Trypsin (AAT): forword. 5’-tctgtttcttggcctcttcggtg-3’, reverse. 5’-cgtaacagctaagtgacagggtc-3’; Albumin: forword.5’-agcggcacagcacttctctaga-3’, reverse. 5’-tccacacggaatgctgccatgg-3’; CD44: forword. 5’-gtgatcaacagtggcaatgga-3’, reverse. 5’-tcaccaaatgcaccatttcct-3’; CD90: forword.5’-ctagtggaccagagccttcg-3’, reverse. 5’-tggagtgcacacgtgtaggt-3’; CCAAT/enhancer binding protein (c/EBPα): forword. 5’-aaccttgtgccttggaaatg-3’, reverse. 5’-ccctatgtttccacccttt-3’; Cytokeratin 7 (CK7): forword. 5’-ggtggatgccctgaatgatg-3’, reverse. 5’- cttggcacgctggttcttgatg-3’; CK8: forword. 5’-ggaggcatcaccgcagttac-3’, reverse. 5’-ggcaatatcctcgtactgtgcc-3’; CK14: forword. 5’-atgattggcagcgtggag-3’, reverse. 5’-gtccagctgtgaagtgctt-3’; CK18: forword. 5’-cacagtctgctgaggttgga-3’, reverse. 5’-gagctgctccatctgtaggg-3’; CK19:forword. 5’-tcccgcgactacagccactactacacgacc-3’, reverse. 5’-cgcgacttgatgtccatgagccgctggtac-3’; CPS1: forword. 5’-attccttggtgtggctgaac-3’, reverse. 5’-ttagggtcggagagaaggta-3’; Cytochrome P450 2B6 (CYP2B6): forword. 5’-cctcttccagtccattaccg-3’, reverse. 5’-tgactgcctctgtgtatggc-3’; CYP3A4: forword. 5’-ccaagctatgctcttcaccg-3’, reverse. 5’-tcaggctccacttacggtgc-3’; Epithelial cell adhesion molecule (EpCAM): forword. 5’-ctggccgtaaactgctttgt-3’, reverse. 5’-agcccatcattgttctggag-3’; G3PDH: forword. 5’-accacagtccatgccatcac-3’, reverse. 5’-tccaccaccctgttgctgta-3’; HNF6: forword. 5’-aaaccaaagacttagctcacctgca-3’, reverse. 5’-gctagacgagcaaaatcacactcc-3’; HNF4α: forword. 5’-ctgctcggagccacaaagagatccatg-3’, reverse. 5’-atcatctgccacgtgatgctctgca-3’; HNF3β: forword. 5’-acggtgaattccagttttcg-3’, reverse. 5’- cctgcaaccagacagggtat-3’; OTC: forword. 5’-accttcaggcagctactcca-3’, reverse. 5’-cctattcgtaccctgctctt-3’; Tryptophan 2,3-Dioxygenase (TO): forword. 5’-gggaactacctgcatttgga-3’, reverse. 5’-cctgactgagcgtggctatt-3’; Vimentin: forword. 5’-ctacatcgacaaggtgcgctt-3’, reverse. 5’-tgccagagacgcattgtcaa-3’.
Densitometric measurements of the bands in RT-PCR analysis were performed using Image J.
Immunocytochemistry
Cells were fixed in 4% neutral buffered paraformaldehyde. Antibodies against α-smooth muscle actin (α- SMA, Dako, Glostrup, Denmark), albumin (Inter-cell Technologies), CK18 (Thermo), CK19 (Thermo), desmin (Dako), EpCAM (Thermo), vimentin (Dako), and Human Leukocyte Antigen (HLA class I, Hokudo, Sapporo, Japan; 1:2000) were used. Immunostaining was carried out using a Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, CA) and a Vector NovaRED substrate kit for peroxidase (Vector Laboratories).
Periodic Acid-Schiff (PAS) staining
Cells were fixed with 10% formaldehyde, oxidized in 0.5% periodic acid for 5 min, and then treated with Schiff’s reagent for 15 min.
Enzyme-Linked Immunosorbent Assay (ELISA)
The conditioned medium obtained from NPC culture was centrifuged (3000 × g, 10 min), and the amount of albumin in the supernatant was determined by ELISA using rabbit anti-human albumin (Inter-cell Technologies) and horseradish peroxidase-conjugated goat anti-human albumin antibodies (MP Biomedicals, Tokyo, Japan).
Cell transplantation
NPCs were cultivated for 21 days in condition I and used for cell transplantation. Animal studies were carried out in compliance with the guidelines of the Kohno Clinical Medicine Research Institute. Female ICR nu/nu mice (4-week old, Charles River, Yokohama, Japan) were divided into two groups and i.p. injection of 100 μl/20 g body weight of olive oil containing 10 μl carbon tetrachloride (CCl4, 10% final concentration) was performed twice a week for 4 weeks in groups 1 (n = 3) and 2 (n = 3). One day after injection of CCl4, 0.2 ml cell suspension containing ~106 cells per mouse was transplanted into the spleen in groups 1; groups 2 did not undergo cell transplantation. Four weeks post-transplantation, mice were bled, and individual serum samples were obtained from the three mice in each group and then stored at −70°C for determination of Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), or lactate dehydrogenase (LD) levels.
Statistical analysis
Statistically significant differences were assessed with the Student’s t-test or Turkey-Kramer test.
Results
Development of SHLCs in NPC culture
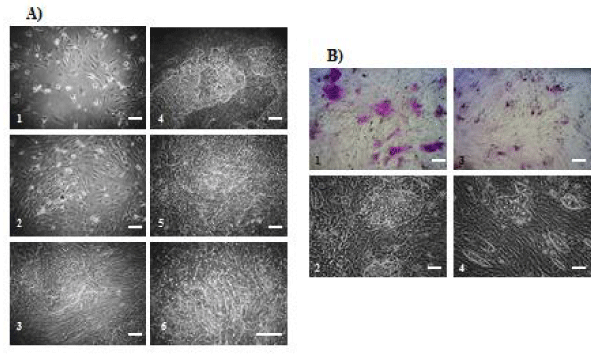
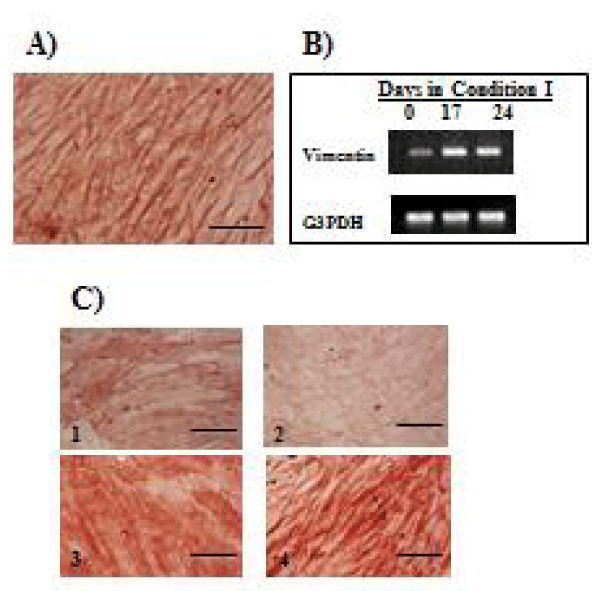
NPCs were plated onto collagen-coated dishes at 105 cells/cm2. Two days later, unattached floating cells were removed, and cultures were switched to one of three conditions (conditions I, II and III). During the early stages of culture, cultures were composed of spindle-shaped or elongated fibroblast-like cells in all conditions. Few epithelial-like cells including mature hepatocytes and SHLCs were observed. After 5 to 7 days, clusters of SHLCs surrounded by fibroblast-like cells had developed in conditions I (patient A, Figures 1A1-5; patient B, Figures 1B,1-2) and II (patient B, Figures. 1B,3-4). SHLCs showed cuboidal morphology and possessed bile canaliculi-like structures that are characteristic of mature hepatocytes in culture in condition I (Figures 1A,5-6). SHLC clusters developed from all NPCs derived from 22 liver specimens when cultivated in condition I or II.
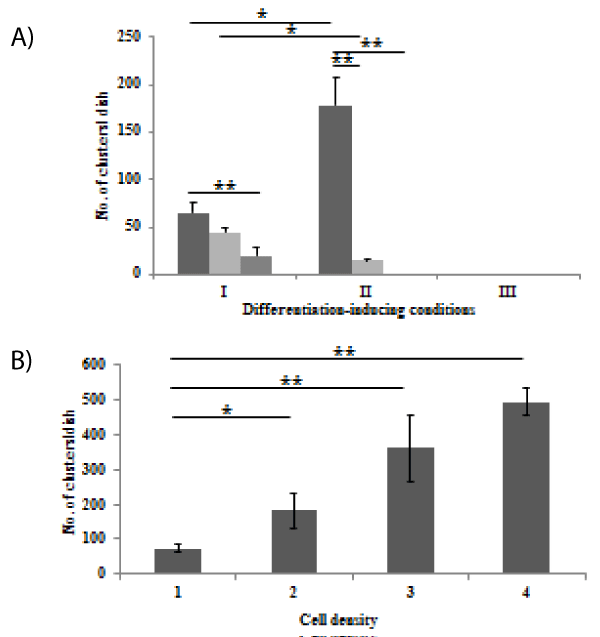
The mean cluster-forming efficiency was 0.004 - 0.006% in condition I. SHLC clusters were also observed in NPCs cultured in condition II, but the sizes (No. of cells/cluster) of SHLC clusters were smaller than those cultured in condition I (Figure 2A). Effect of inoculum cell density on the number of SHLC clusters was examined (Figure 2B). The clusters were formed in a cell number-dependent manner. We observed only fibroblast-like cells in condition III and observed no evidence of development of epithelial-like cells including SHLCs (data not shown). When cultured on plastic dishes, we also observed the development of SHLC clusters. However, there was no significant difference between plastics (103.7 ± 28.5/35-mm dish, n = 3) and collagen substrate (127.9 ± 24.7/35-mm dish, n = 3) in the number of SHLC clusters. In addition, no SHLC clusters appeared from the NPCs derived from the liver specimens of patients with CPS or OTC deficiency even when cultivated in condition I (data not shown). Based on these data, induction of differentiation was carried out only with condition I unless otherwise stated.
Characterization of SHLCs
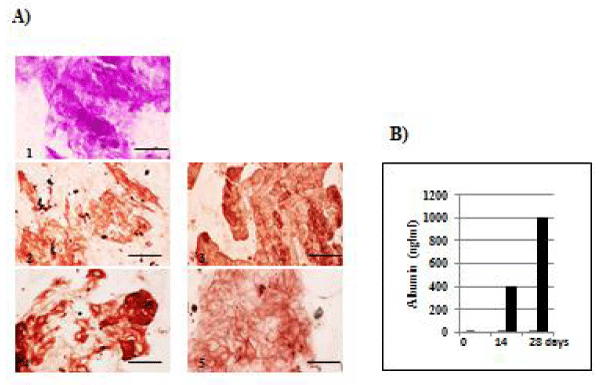
Glycogen storage was shown by PAS staining in SHLCs cultured for 24 days in condition I (Figures 3A,1). The specificity was confirmed using amylase (data not shown). Immunocytochemical study of SHLCs cultured in condition I for 18 days showed that SHLCs are positive for the hepatocytic cell markers albumin and CK18, and the bile duct cell marker CK19 (Figures 3A2-4). SHLCs were also positive for the hepatic stem cell marker EpCA,M, the expression of which was restricted to a membranous pattern (Figures 3A,5). ELISA experiments show that NPCs secreted human albumin after 28 days of culture in condition I (n = 1) (Figure 3B).
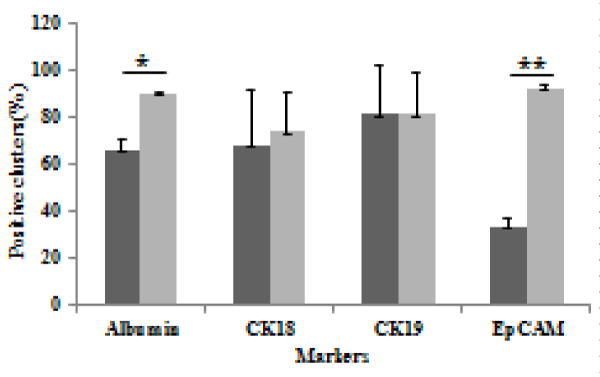
Next, the percentage of albumin-, CK18-, CK19- and EpCAM-positive SHLC clusters was determined by immunocytochemistry in NPCs cultured for 7 or 21 days in condition I (Figure 4). The percentage of albuminand EpCAM-positive SHLC clusters increased in two independent experiments, whereas that of CK18- and CK19-positive SHLC clusters remained unchanged throughout the experiment. Taken together, these observations suggest the differentiation potential of SHLCs into mature hepatocytes.
Properties of the fibroblast-like cells
Immunocytochemical results of mesenchymal markers are shown in Figure 5. Fibroblast-like cells surrounding SHLC clusters were strongly positive for vimentin (Figure 5A). In addition, these fibroblast-like cells expressed vimentin mRNA (Figure 5B). Expression of myofibroblast marker α-SMA and stellate cell marker desmin in NPCs cultured for 14 days in condition I was compared to expression after culturing in condition III. Cells cultured in condition I were positive for α-SMA, but were only weakly positive for desmin (Figures 5C,1-2), whereas those cultured in condition III were positive for both α-SMA and desmin (Figures 5C,3-4). To confirm this finding, two more independent experiments were performed, and essentially the same results were obtained.
Cell transplantation
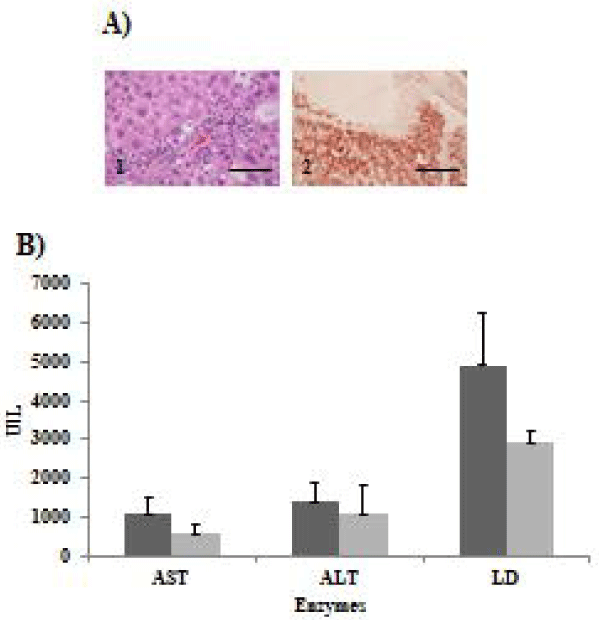
Successful engraftment of cells transplanted into nude mice was evaluated by examining the presence of HLA-positive cells in the liver tissue. Mouse liver sections in group 1 in which transplantation of NPCs containing SHLCs was performed were positive for HLA in two of three nude mice (Figure 6A,2). In group 2 in which cell transplantation was not performed, no HLA-positive cells were observed (data not shown), suggesting successful engraftment of transplanted cells. Next, the effect of cell transplantation on liver injury was examined (Figure 6B). The levels of AST, ALT, and LD in mouse sera in group 1 tended to be lower than those in mouse sera in group 2, although no statistically significant differences were observed.
Cluster isolation
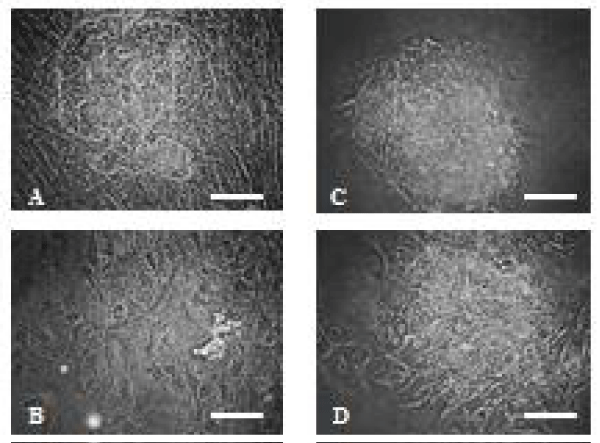
When the Liberase T-Flex, Research Grade Kit was used to isolate SHLC clusters on day 43 in culture, only the cell cluster could be removed from the substrate and the surrounded fibroblast-like cells were left intact. SHLCs thus isolated (These SHLCs will be referred to as isolated SHLCs in this paper) were uniform in size and showed cuboidal morphology on days 1 and 3 (Figures 7A-D).
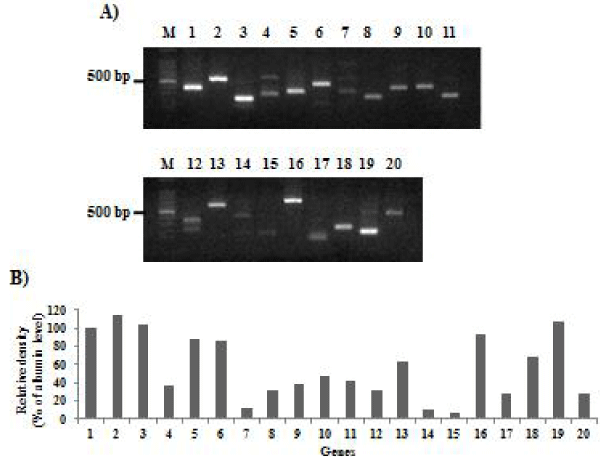
Furthermore, total RNA was extracted from the isolated SHLCs after 3 days in culture and gene expression of several mRNA markers was examined using RT-PCR (Figure 8A). Relative levels (%of albumin) of mRNA were shown in figure 8B. The hepatic marker genes albumin, AAT, TO, CYP3A4, OTC and CK8 exhibited strong expression in the isolated SHLCs, whereas CYP2B6 was expressed at an intermediate level and CPS1 and CK18 were expressed weakly. The bile duct marker gene CK 19 was expressed strongly, but CK7 was expressed at an intermediate level in the isolated SHLCs. The progenitor marker gene CD90 exhibited strong expression in the isolated SHLCs, whereas CK14 was expressed weakly. The hepatic stem cell marker gene EpCAM exhibited strong expression and SH marker gene CD44 exhibited intermediate expression in the isolated SHLCs. Furthermore, the isolated SHLCs expressed the transcriptional genes (HNF3b, HNF4, HNF6 and CEBPα). The fetal hepatocytic marker genes α-fetoprotein (AFP) and delta-like 1 homologue (DLK-1) were not expressed in the isolated SHLCs (data not shown).
Discussion
Characteristics of SHLCs
When mature liver cells are unable to contribute to regeneration because of mitogenic inhibition or replicative exhaustion in human liver diseases, progenitor cells are activated [11]. These cells are referred to as intermediate hepatobiliary cells, defined as larger than 6 microns in diameter, but less than 40 microns, with other features suggesting dual characteristics of both hepatocytes (e.g., albumin, AAT) and cholangiocytes (e.g., CK7, CK19) [12]. In the present study, SHLC clusters appeared from the NPC culture of BA liver, but not from that of CPS or OTC liver that was used as controls. SHLCs surrounded by the fibroblast-like cells were immunocytochemically positive for both hepatocyte (albumin and CK18) and biliary markers (CK19). In addition, the isolated SHLCs showed gene expression profiles and morphologic appearance similar to those of SHs that were separated from the supernatant fractions of adult human livers [5]. Yamasaki et al. reported the passage of colony-forming cells that were also separated from the supernatant fractions of adult human livers [13]. However, the colony-forming cells seem to be distinct from SHLCs in that SHLCs represent the morphology of typical hepatocytes, whereas the colony-forming cells were similar to that of liver epithelial cells. Taken together, these observations suggest that SHLCs are liver progenitor cells with an intermediate hepatobiliary phenotype similar to adult human SHs.
Liver enriched transcription factors (LETFs) including C/EPBα and HNF4α regulate each other and form a transcriptional hierarchy that is involved both in the determination and maintenance of the hepatic phenotype [14]. C/EPBα and HNF4α are strongly expressed in mature hepatocytes and stimulate the transcription of liverspecific genes such as albumin and AAT [14]. In this study, the isolated SHLCs exhibited the expression of not only albumin, AAT and other hepatic marker genes, but also of C/EPBα, HNF4α, and HNF3β in condition I. These data suggest that SHLCs are directed to differentiate toward hepatocyte-like cells in the differentiation inducing condition.
The potential lineage relationship between hepatic oval cells, SH-like progenitor cells (SHPCs), and hepatocytes in liver regeneration has been debated. Chen et al. suggested that hepatic oval cells are an origin of SHPCs in retrorsine exposed rats with chimeric livers [15]. Similar results were also obtained by Mitaka groups in the galactosamine treated rats [16]. We did not observe the attached SHLCs at an early stage of culture, suggesting that NPC populations contain original cells that may be morphologically different from SHLCs. Whether or not the cell origin of SHLCs derived from BA liver is oval cell is currently under investigation.
EpCAM-associated functions are related to maintenance of a pluripotent state, regulation of differentiation, and others [17]. EpCAM is highly expressed in hepatic stem/progenitor cells but is lost during differentiation into hepatocytes [18]. In the present study, immunocytochemistry and RT-PCR analyses showed that SHLCs expressed EpCAM in culture, suggesting their hepatic stem/progenitor cell origin. In addition, during induction of differentiation, the percentage of EpCAM-positive SHLC clusters increased, suggesting a conversion of EpCAM-negative SHLCs to EpCAM-positive SHLCs. Pathological changes in the liver are accompanied by a re-expression of EpCAM [18]. Many hepatocytes in cirrhotic livers express EpCAM [19]. Whether this represents aberrant EpCAM expression in injured hepatocytes was investigated using liver biopsy specimens. The data supported the concept that EpCAM-positive hepatocytes in chronic viral hepatitis are not aberrant, but are recent progeny of the hepatobiliary stem/progenitor cell compartment. The staining pattern of EpCAM is membranous and cytoplasmic in ductal plate cells of fetal livers, whereas human hepatoblasts exhibit only the membranous pattern [20]. EpCAM-positive SHPC clusters show membranous EpCAM-positive/HNF-4α- positive staining and are contiguous with the surrounding cytoplasmic EpCAM-positive/HNF4α-negative ductular oval cells [15]. The significance of the membranous staining pattern of EpCAM in SHLCs remains obscure.
Culture conditions
Culture conditions often affect the cellular behavior. In this study, the appropriate culture conditions for developing SHLC clusters were investigated. Adult human SHs emerge from the hyaluronic acid-coated dishes, but not from the collagen-type I-coated dishes [5]. In the present study, SHLCs appeared after 1-2 weeks in culture even when NPCs are plated on the commonly used substrates such as collagen type I or plastics. These facts suggest that there may be different mechanisms as to the appearance of cells between adult human SHs and SHLCs.
Hepatic differentiation depends on cell density [21]. High cell density could mesenchymal stem cells (MSCs) be induced to form the cells with hepatocyte phenotypes [22]. In this study, many more SHLC clusters developed at a higher cell density in the absence of hepatocyte growth factor (HGF), suggesting a significant role of cell density on the appearance of SHLC clusters. In general, to induce hepatic differentiation, 5-20 ng/ml of HGF is added into the culture medium in nonparenchymal epithelial cells [23] or hepatic progenitor cells [24] derived from adult human livers. Higher doses of HGF (> 100 ng/ml) are added in cultures of adipose tissues-derived MSCs [25]. In this study, we show that NPCs generated SHLCs in the absence of HGF, FGF-4, and/or oncostatin M. SHLC clusters were surrounded by fibroblast-like cells with a mesenchymal phenotype. At a higher cell density, the fibroblast-like cells may produce larger amounts of HGF or other growth factors, which stimulate the appearance of SHLC clusters. We previously showed that conditioned media from primary culture of NPC fractions of BA liver contain a large amount of active HGF [6]. Meanwhile, during liver fibrosis, hepatic stellate cells are activated and differentiated into myofibroblasts [26]. The activation and proliferation of stellate cells correlate with a high expression of desmin, while α-SMA is considered a myofibroblast marker because it is expressed after the differentiation of these cells [27]. In chronic liver injury, stellate cells differentiate into myofibroblast-like cells with marked expression of α-SMA and occasional expression of desmin [27]. The present results show that fibroblast-like cells surrounding SHLC clusters were positive for α- SMA, but were almost negative for desmin when the cells were cultivated in condition I, whereas they were positive for both markers when cultivated in condition III. Whether the expression profiles of desmin and α- SMA in the former, which mimic liver fibrosis, are involved in the appearance of SHLC clusters remains to be determined. Suitable enzymes for subculturing adult human SHs have not yet been reported [5]. To isolate and develop SHLCs we examined the probability of separation of SHLC clusters from the surrounding fibroblast-like cells with several enzymes including trypsin-ethylenediaminetetraacetic acid (EDTA) and Liberase T-Flex. When trypsin EDTA was used, the cell cluster was detached from the plastic wall, but was accompanied by contamination with various fibroblast-like cells. In contrast, only a cell cluster was detached when the cells were incubated with Liberase T-Flex for 2 min. The SHLC cluster thus detached could be easily dissociated with the same enzyme. The Liberase T-Flex kit is intended for use in isolation of pancreatic islets from several animal types, suggesting that this kit is useful for further subcultivations of isolated SHLCs.
Cell transplantation
Previous reports have shown that transplanted hepatocytes or liver stem cells are efficiently transported to CCl4-injured livers [28]. In this study, human HLA-positive cells were observed in CCl4-injured nude mouse livers transplanted with NPCs containing SHLCs, suggesting successful engraftment of NPCs including SHLCs. In addition, CCl4-induced liver injury tended to be attenuated, as demonstrated by a trend toward a decrease in markers of injured liver such as AST, ALT, and LD when NPCs including SHLCs were transplanted into nude mice. This result suggests therapeutic usefulness of differentiated SHLCs, although additional studies are needed.
Conclusions
We observed reproducibly the development of SHLC clusters from BA livers in the commonly used culture conditions. To our knowledge, this is the first investigation of SHLCs derived from BA liver, of which the etiology, pathogenesis, and pathophysiology remain uncertain. This study may facilitate future autologous stem cell therapy to treat this obstinate disease.
- Davenport M, Bezerra JA, Sokol RJ. A challenge on the use of the words embryonic and perinatal in the context of biliary atresis. Hepatology. 2005; 41: 403-405. https://goo.gl/kiY4dr
- Petersen C, Davenport M. Aetiology of biliary atresia: what is actually known? Orphanet J Rare Diseases. 2013; 8: 128-141. https://goo.gl/wpzbz2
- Strom SC, Fisher RA, Rubinstein WS, Barranger JA, Towbin RB, Charron M, et al. Transplantation of human hepatocytes. Transplant Proc. 1997; 29: 2103-2106. https://goo.gl/b8c2jv
- Yamazaki T, Enosawa S, Kasahara M, Fukuda A, Sakamoto S, Shigeta T, et al. Isolation of hepatic progenitor cells from human liver with cirrhosis secondary to biliary atresia using EpCAM or Thy-1 markers. Cell Med. 2012; 3: 121-126. https://goo.gl/xkLTp9
- Sasaki K, Kon J, Miyazaki T, Chen Q, Ooe H, Oshima H, et al. Proliferation of hepatocyte progenitor cells isolated from adult human livers in serum-free medium. Cell Transpl. 2008; 17: 1221-1230. https://goo.gl/Mc8rCE
- Yamazaki T, Wakai M, Enosawa S, Tokiwa T. Analysis of soluble factors in conditioned media derived from primary cultures of cirrhotic liver of biliary atresia. In Vitro Cell Dev Biol Anim. 2017; Doi: 10.1007/s11626-017-0144-3. https://goo.gl/DoJSh4
- Enosawa S. Hepatocyte isolation from human liver tissue. Organ Biology. 2009; 16: 361-370. (In Japanese with English abstract).
- Zhou XD, Tokiwa T, Kano J, Kodama M. Isolation and primary culture of adult pig hepatocytes. Methods in Cell Science. 1998; 19: 277-284. https://goo.gl/HqH9Cz
- Tokiwa T, Yamazaki T, Ono M, Tsukiyama S, Shin Enosawa. Cloning and characterization of liver progenitor cells from the 15 scattered cell clusters in primary culture of porcine livers. Cell Transplantation. 2008; 17: 179-186. https://goo.gl/h8iuYu
- Sigal S, Brill S, Reid LM, Zvibel I, Gupta S, Hixson D, et al. Characterization and enrichment of fetal rat hepatocytes by immunoadsorption (“panning”) and fluorescence activated cell sorting. Hepatology. 1994; 19: 999-1006. https://goo.gl/THoxKX
- Dorrell C, Grompe M. Liver repair by intra- and extra hepatic progenitors. Stem Cell Reviews. 2005; 1: 61-64. https://goo.gl/u8feVx
- Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac Sage P, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004; 39: 1739-1745. https://goo.gl/jkhKuM
- Yamasaki C, Tateno C, Aratani A, Ohnichi C, Katayama S, Kohashi T, et al. Growth and differentiation of colony-forming human hepatocytes in vitro. J Hepatol. 2006; 44: 749-757. https://goo.gl/vGz5HC
- Hayashi Y, Wang W, Ninomiya T, Nagano H, Ohta K, Itoh H. Liver enriched transcription factors and differentiation of hepatocellular carcinoma. Mol Pathol. 1999; 52: 19-24. https://goo.gl/VxzrnT
- Chen YH, Chang MH, Chien CS, Wu SH, Yu CH, Chen HL. Contribution of mature hepatocytes to small hepatocyte-like progenitor cells in retrorsine-exposed rats with chinmeric livers. Hepatology. 2013; 57: 1215-1224. https://goo.gl/bi9trv
- Ichinohe N, Kon J, Mitaka T. Isolation of hepatic progenitor cells from the galactosamine-treated rat liver. Methods Mol. Biol. 2012; 826: 49-58. https://goo.gl/3Yo659
- Dolle L, Theise ND, Schmelzer E, Boulter L, Gires O, van Grunsven LA. EpCAM and the biology of hepatic stem/progenitor cells. Am J Physiol Gastrointest Liver Physiol. 2015; 308: 233-250. https://goo.gl/BJqn2U
- Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor features. Gastroenterology. 2009; 136: 1012-1024. https://goo.gl/p5LY89
- Yoon SM, Gerasimidou D, Kuwabara R, Hytiroglou P, Yoo JE, Park YN, et al. Epithelial adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology. 2011; 53: 964-973. https://goo.gl/avMyTC
- Zhang L, Theise N, Chua M, Reid LM. The stem cell niche of human livers: symmetry between development and regeneration. Hepatology. 2008; 48: 1598-1607. https://goo.gl/QVfCcv
- Nakamura T, Yoshimoto K, Nakayama Y, Tomita Y, Ichihara A. Reciprocal modulation of growth and differentiated functions of mature rat hepatocytes in primary culture by cell--cell contact and cell membranes. Proc Natl Acad Sci USA. 1983; 80: 7229–7233. https://goo.gl/RxvGJM
- Kim DS, Lee MW, Yoo KH, Lee TH, Kim HJ, Jan IK, et al. Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS One. 2014; 9: e83363. https://goo.gl/7g1kbq
- Duret C, Gerbal-Chaloin S, Ramos J, Fabre J-M, Jacquet E, Navarro F, et al. Isolation, characterization, and differentiation to hepatocyte-like cells of nonparenchymal epithelial cells from adult human liver. Stem cells.2007; 25: 1779-1790. https://goo.gl/YNwLB5
- Li J, Xin J, Zhang L,Wu J, Jiang L, Zhou Q, et al. Human hepatic progenitor cells express hematopoietic cell markers CD45 and CD109. Int J Med Sci. 2014; 11: 65-79. https://goo.gl/Vb3Fuk
- Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, QWuinn G, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. 2007; 46: 219-228. https://goo.gl/QRV12Y
- Goncalves JO, Tannuri ACA, Coelho MCM, Bendit I, Tannuri U. Dynamic expression of desmin, α-SMA and TGF-β1 during hepatic fibrogenesis induced by selective bile duct ligation in young rats. Braz J Med Bio Res. 2014; 47: 850-857. https://goo.gl/aJsYMH
- Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 1997; 430: 195-207. https://goo.gl/AZT4kK
- Terai S, Sakaida I, Yamamoto N, Omori K, Watanabe T, Ohata S, et al. An in vivo model for monitoring trans-differentiation of bone marrow cells into functional hepatocytes. J Biochem. 2003; 134: 551-558. https://goo.gl/K7PiqH

Sign up for Article Alerts